S29.1: The anatomy and physics of avian structural colours
Richard O. Prum
Natural History Museum and Department of Systematics & Ecology, University of Kansas, Lawrence, Kansas 66045-2454, USA, e-mail:
prum@ukans.edu Prum, R.O. 1999. The anatomy and physics of avian structural colours. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1633-1653. Johannesburg: BirdLife South Africa.The anatomical and biophysical bases of avian structural colours are reviewed. The physics of light scattering and structural colour production by biological tissues is reviewed in a nontechnical way. Various physical models that have been cited in ornithology are best viewed as variations on incoherent and coherent scattering. Structural colours known from the feather barbules, feather barbs, avian dermis, and eye are described. For each, the evidence concerning the physical basis of the colours of these structures is reviewed. Recent advances indicate that all known avian structural colours are produced by constructive interference among coherently scattered light waves. No known structures produce colours by incoherent scattering alone: i.e. Rayleigh scattering or Mie scattering. An understanding of the anatomical and physical basis of structural colouration is essential to studies of the development, function, and evolution of structural colours in avian species and populations.
INTRODUCTION
The colouration of the avian integument and eyes can be produced by chemical pigments, structures that interact physically with light waves, or by a combination of both of these (Lucas and Stettenheim 1972; Dyck 1976, 1978; Fox 1976; Durrer 1986). Pigments are molecules that differentially absorb and emit wavelengths of visible light. The specifics of the wavelengths absorbed and re-emitted are determined by the molecular structure of the pigment molecule. Pigmentary colours are common in the plumage, egg shells, dermis, and eyes of birds (Lucas and Stettenheim 1972; Dyck 1976, 1978; Fox 1976; Durrer 1986).
Structural colours differ from pigmentary colours in that they are produced by the physical interaction of light with biological structures. Structural colours are important components of colouration of the plumage, skin, and irides of many bird species and are broadly distributed throughout Aves.
The classes, distribution, and production mechanisms of structural colours of bird feathers have been extensively researched over the last century (see Dyck 1971b; Fox 1976 for historical reviews). Despite this body of advanced research, there are still a number of basic questions about the physics of structural colours of feathers that remain to be addressed. Furthermore, relatively few papers have investigated the anatomy and physics of structural colours found in the avian skin (Prum et al. 1994) and iris (Oehme 1969; Ferris and Bagnara 1972; Oliphant 1981, 1987a, 1987b; Oliphant et al. 1992). Recent developments in this field indicate an important new direction for studies of avian colouration.
Since structural colours comprise an important part of the phenotype of many birds, a thorough understanding of the physics of structural colour production is essential to analysing the function, development, and evolution of this component of the phenotype in bird species and populations. Advances in analyses of the physics and anatomy of avian structural colours should provide corroborated biophysical basis for models of the function of these colours in the lives of birds.
Furthermore, ornithologists have recently become interested in the consequences of ultraviolet (UV) vision for avian sensory biology, social signalling, and mate choice (Bennett and Cuthill 1994; Finger and Burkhardt 1994; Andersson 1996). Although some pigmentary colours may produce UV-stimulating reflectance peaks, most of these pigments also have reflectance peaks in the longer wavelength areas of the visible spectrum (Finger and Burkhardt 1994). (Purely UV-coloured pigments are rare in animals for the same chemical reasons that blue pigments are; see Fox 1976). So, in order to focus their studies on the importance of UV stimuli to avian vision, many researchers have turned to research on UV structural colours that are predominantly expressed in the UV range of avian vision. This renewed interest in the biology of these structural colours underscores the importance of having an accurate anatomical and physical understanding of these phenomena.
In this paper, I review the anatomy of avian structurally coloured tissues and the evidence concerning the physical mechanisms by which these tissues produce colours. First, I will briefly discuss the distinction between pigmentary and structural colours. Then, I review and categorise the various physical models of light scattering physics and their applications for structural colour production in birds. Lastly, I present the available data concerning the anatomy and physics of the structural colours of avian feathers, skin, and iris.
STRUCTURAL VS. PIGMENTARY COLOURS
Pigmentary and structural colours both contribute to the colouration of birds. Many of the most striking colours are created by a combination of pigmentary and structural colours. For example, most non-iridescent green plumages are created by a combination of a structural blue hue and a superficial yellow carotenoid pigment in the feather barbs (e.g. Dyck 1978). But what about the brilliantly iridescent hummingbird feather barbs that are composed of matrix of melanin and keratin; are these colours pigmentary or structural?
The complex relationship between structural and pigmentary colours can create some confusion about the distinction between the two. To reiterate the definitions above, pigmentary colours are those created solely as a result of molecular absorbance and emission light. The hue of a pigmentary colour is determined by the molecular structure of the pigment and the density of its distribution in the tissue: i.e. the more densely distributed the pigment is the more saturated the hue. In contrast, structural colours result from the physical interactions of light scattered at the interfaces of biological materials of different refractive indices. Although light scattering may produce colours by various physical mechanisms (discussed below), all mechanisms depend on the nanoscale physical properties of the structures and their refractive indices.
The composition structurally coloured tissues varies extensively, but typically includes a combination of higher and lower refractive index substances: e. g. keratin and air, melanin and keratin, collagen and mucopolysaccaride, purines and cytoplasm. The confusion arises in that some the components of these structurally coloured tissues are themselves pigments, such as melanin or purines. However, the colours produced by these tissues are structural nevertheless because the hues produced are a consequence of the size, spatial distribution, and refractive indices of the pigment granules, not merely their molecular properties. Although the molecular structure of a pigment is important in determining its refractive index, the colours produced by nanometer scale physical structures that are composed partially of pigments are structural colours and are not determined solely by the molecular properties of the pigments. In this regard, the distinction between structural and pigmentary colouration mechanisms is clear. Structural colours are not necessarily ‘ non-pigmentary’ colours; they are hues produced by large-scale physical mechanisms that can some time involve pigments.
So, the iridescent hue of a hummingbird feather is a structural colour even though it is created by a matrix of melanin and keratin in the feather barbule (see below). In complex systems where a combination of structural and pigmentary colours interact to create the observed hue (such as most noniridescent green birds), it is best to analyse and discuss the structural and pigmentary components of the colour separately.
LIGHT SCATTERING PHYSICS AND STRUCTURAL COLOUR
A long list of physical mechanisms have been cited as responsible for the production of various avian structural colours, including Rayleigh scattering, Tyndall scattering, Mie scattering, constructive interference, thin-film reflexion, and Bragg reflexion. An exact, electromagnetic model of the physical mechanism of structural colour production has never been attempted for any biological structure. All the hypothesised physical mechanisms of structural colour production are theoretical models of how light waves interact with optically heterogeneous tissues (i.e. tissues that vary in refractive index) to produce the reflectance spectra humans and birds observe as colours.
The most physically sensible way to view the diversity of proposed mechanisms is as various models of light scattering (van de Hulst 1981; Bohren and Huffman 1983). Whenever a light wave travels from one medium to another medium with a different refractive index, some of the total energy of the light wave is absorbed as heat and the remainder is scattered as light. Some of the scattered light waves may travel forward and further propagate the wave, while others scatter in all directions. The absorbance and scattering behaviour of light waves in a optically heterogeneous tissue is determined by the size, shape, and spatial distribution of the scattering objects and their refractive indices.
Incoherent vs. Coherent scattering
Structural colours of avian tissues are produced by light scattering from the interfaces between structures of different refractive indices. The observed colour of a tissue is the result of scattering by numerous scattering structures in the tissue which comprise planes or arrays in the tissues. Light scattering models differ in whether these scatterers are hypothesised to be incoherent or coherent (van de Hulst 1981; Bohren and Huffman 1983). Incoherent scattering models assume that the phase relationships among the scattered waves are random so that they can be ignored in describing the behaviour of light scattered by the array. Consequently, an incoherent array of scatterers is characterised by spatial independence of the scatterers. Spatially independent scatterers are separated by distances that are larger than the wavelength of light.
In contrast, coherent scattering models account for the phase relationships of waves scattered by the array. A coherent array is characterised by scatterers that are closer together than the wavelength so that the waves scattered from nearby scatterers are nonrandom in phase. The interactions of the waves scattered by adjacent scatterers produces coherent scattering, or selective reinforcement of a subset of visible wavelengths.
The various physical models hypothesised to explain colour production by various avian tissues include both incoherent and coherent scattering models. The distinction between them can be complex. For example, an array may be appropriately sized to be an incoherent array at visible wavelengths but coherent for far infrared radiation (e.g. snow; Bohren 1987). However, understanding the basic differences between these models can provide a better understanding of the testable predictions and explanatory limits of the models.
Incoherent Scattering Models
Incoherent scattering can result in structural colour production because individual scatterers can differentially scatterer and absorb visible wavelengths of light. The most commonly cited incoherent scattering models are Rayleigh and Tyndall scattering. Rayleigh scattering is an approximate model of scattering by objects that are smaller than wavelength of visible light, including small spheres or molecules (van de Hulst 1981; Bohren and Huffman 1983). The term Rayleigh scattering has been used confusingly by physicists to refer to a number of different light scattering phenomena, but is now generally agreed to refer to light scattering by small particles (Young 1982). The Rayleigh scattering model predicts that the scattering efficiency of a particle of a given size will be inversely related to the fourth power of the wavelength. As a consequence of Rayleigh's Inverse Fourth Power Law, particles of a given size will scatterer smaller wavelengths of light much more efficiently than longer wavelengths. The result should be a distinct blue, violet, or UV colour, depending on the size of the scatterer. Rayleigh scattering can only produce colours from the smaller end of the visible spectrum since larger particles would scatter all wavelengths of light equivalently producing a white appearance.
Tyndall scattering is often synonymized with Rayleigh scattering, but actually Tyndall scattering refers to scattering by particles smaller than visible light wavelengths but larger than molecules; Rayleigh scattering explicitly includes molecular scattering (Young 1982). In one sense, Tyndall scattering may be accurately applied to organismal structural colours since light scattering by biological tissues is hypothesised to be produced by particles that are much larger than molecules. However, Tyndall scattering does not include the Inverse Fourth Power Law (Young 1982), and use of the term in biology should probably be eliminated in favour of the more predictive Rayleigh scattering model.
Mie scattering is an exact, exhaustive electromagnetic model of incoherent scattering of light by spherical particles of a given size and refractive index (van de Hulst 1981; Bohren and Huffman 1983). Rayleigh scattering is essentially a simplified approximation of Mie scattering for particles that are smaller than the wavelengths of visible light (van de Hulst 1981; Bohren and Huffman 1983). Mie theory is complex and cumbersome, but computer programs are available at various websites to calculate scattering distributions for particles of given sizes and refractive indices.
Coherent Scattering Models
Coherent scattering models can produce differential propagation of visible light waves as a result of the interactions among the waves scattered by different scatterers (Benedek 1971; Dyck 1971a; Land 1972). The light observed after scattering by a tissue is the summation of the waves scattered by a larger number of elements in the tissue. Light waves scattered by different scatterers travel different distances before observation, and so have the potential to shift in phase before summation. The difference in the distance travelled by waves scattered by adjacent scatterers is called the path length addition. In general, the path length addition depends on the distance between the scatterers and the angle of observation.
Scattered waves that are out-of-phase will destructively interfere with one another and cancel out. For a given path length addition, most wavelengths will be substantially out-of-phase and will therefore cancel out. In contrast, scattered waves that are near to the path length addition in wavelength (or an integer multiple of the path length addition) will be in-phase at summation. This limited subset of wavelengths will constructively reinforce one another, and be coherently scattered by the array.
The phase relationships among scattered waves are determined by the size and spatial distribution of the scatterers. Periodic spatial relationships among scatterers will produce predictable path length differences among scattered waves, and reinforcement of a limited set of wavelengths. Genuinely random spatial relationships among scatterers will not result in reinforcement of a specific subset of wavelengths and will result in incoherent scattering (see above).
In biological tissues, coherent scattering is produced by the predictable distribution of substances of different refractive indices at spatial scales near the size of the wavelengths of visible light. So, biological scatterers are structures of a different refractive index that are immersed in another medium with a different refractive index. The best known coherent scattering model is scattering by thin-film reflectors (Land 1972; Macleod 1986). A thin-film system is characterized by single layers or repeated pairs of layers of materials of different refractive indices. An 'ideal' thin-film system is characterized by having layers of both media of same optical thickness: n (the refractive index) x d (the actual thickness). In an ideal thin-film system (Land 1972), the wavelength of maximum reflection (
lmax) is given by lmax = 4nada = 4nbdb , where na and nb are the refractive indices of the two media, and da and db are the actual thicknesses of the layers of the two media. Thus, one simple prediction of the thin-film optics is that ideal thin-film systems should have layers that are approximately lmax/4 in thickness. Such systems are frequently referred to as l/4 or ‘ quarter wave’ reflectors. A 'non-ideal' thin film is characterized by layers of different optical thicknesses (nada ¹nbdb) (Land 1972). Similarly, calculating lmax for a nonideal system is given by lmax = 2(nada +nbdb).Land (1972) demonstrates that, in addition to the primary reflectance peak centered on
lmax, thin-film systems can give rise to second and third order reflection peaks. Land presents the optical theory establishing the height and breadth of the first, second, and third order reflectance peaks based on the refractive indices, thicknesses, and number of layers in the system. Theoretically in ideal systems, the second order and third order reflectance peaks are given by lmax/3 and lmax/5. Non-ideal thin film systems should yield lower but narrower primary reflectance peaks around lmax (perhaps creating structural colours with purer hues). But the second and third order reflectance peaks of nonideal systems are given by lmax/2 and lmax/3, which means that they should be closer in wavelength to lmax, and would be more likely to be within the visible spectrum.Coherent scattering by thin-films is common in arthropod cuticles and elsewhere in animals (Fox 1976; Neville 1993), and even plants (Lee 1991, 1997). But, except for the structurally coloured, iridescent feather barbules of hummingbirds (Trochilidae) and quetzals (Pharomachros, Trogonidae; see below), few of the colour producing tissues of birds can be accurately modelled as thin-films reflectors (Land 1972). Instead of being organised as parallel planes, many avian colour producing structures are composed of square, hexagonal, or quasirandom arrays of bars, tubes, fibers, or bubbles. For the most regular or squarely-oriented of these structures, thin-film optics may provide an approximation of the colour production mechanism, but for most structures more detailed physical models are required.
Bragg reflexion was first developed as a model of X-ray scattering by atoms in elemental crystals (Bragg and Bragg 1915). As modified for biological systems, Bragg's Law provides an expression for the relationship between angle of incident light, the size, spacing, and refractive index of a lattice-like array of scatterers and the maximum wavelength of reflexion,
lmax (Durrer 1962; Durrer and Villiger 1966, 1970). According the Bragg's Law, the wavelength of maximum reflectance, lmax is given by: lmax = n 2d sin a ; where n is the refractive index of the medium between the reflectors, d is the space lattice dimension (the minimum distance between parallel planes of reflection), and a is the angle of incident light.The application of Bragg's Law is limited to anatomical systems that are highly regular or nearly crystal-like in spatial organisation. However, there are also less ordered, quasirandom arrays of scatterers in other structurally coloured tissues. In these instances, a more detailed theory which accounts for the variation in size and spacing of the scatterers is required. An appropriate theory has been developed for analysis of the optical transparency of the cornea, and this theory has only recently been applied to structural colour production. The cornea is composed of a quasirandom array of parallel collagen fibers (Maurice 1984). The cornea is optically transparent because the collagen fibers that compose it are very small and close together, resulting in destructive interference among all scattered wavelengths of visible light (Benedek 1971; Maurice 1984; Vaezy and Clark 1991, 1993). Previous research on structural colouration by collagen arrays in the avian skin (Prum et al. 1994) led me to consider applying Benedek's theory of corneal transparency to structural colour production by the spongy medullary keratin matrix of feather barbsû an optically similar phenomenon on a different spatial scale.
According to Benedek's theory, in a quasirandom array of scatterers (i.e. a less than perfect lattice), significant reinforcement is predicted only for those light waves in the medium that are twice the size of the largest components of the Fourier transform of the spatial variation in refractive index (Benedek 1971). The discrete Fourier transform is a basic mathematical tool used to describe how a signal or image is composed of different periodic components (Briggs and Henson 1995). Discrete data are transformed into a sum of component sine waves of different amplitudes and frequencies (Briggs and Henson 1995). The relative squared amplitudes of these component waves, called the Fourier power spectrum, express the contributions of each frequency of variation to the original data, and indicate which frequencies carry the most energy (Briggs and Henson 1995).
Benedek's theory predicts that the Fourier power spectrum of spatial variation in refractive index of a tissue will predict which wavelengths will be constructively reflected by that tissue. This is because the distribution of the Fourier coefficients of the spatial variation in refractive index is directly related to the distribution of the path length additions experienced by light waves scattered by the tissue.
Both one-dimensional and two-dimensional (2-D) Fourier Analysis have been used to analyse spatial variation in optical density in optically transparent, opaque, and white tissues of the mammalian eye (Gisselberg et al. 1991; Vaezy and Clark 1991, 1993). Currently, my colleagues and I are applying 2D Fourier analysis to a number of avian tissues that are characterised by imperfect lattice structure. This technique should also prove interesting for the analysis of complex multilayer systems composed of multiple scattering media (e.g. keratin, melanin, and air).
Role of a Basal Pigment Layers in the Production of Structural Colours
All structurally coloured tissues are some how connected to the rest of the organism. If the number of scatterers is extremely large (e.g. many white contour feathers in a body plumage), then the colour producing structure can be essentially infinitely optically thick (Bohren 1987), and no light can scatter from the tissue below. However, in most cases the number of scattering layers or arrays is limited. Typically, the tissue below the colour producing tissue cannot also be structured for the purpose of colour production, and could produce incoherent scattering of visible wavelengths (i.e. white light scattering up toward the observer). Such incoherent white scattering would greatly reduce the efficiency of any wave-length specific structurally coloured tissue.
To prevent this, most structurally coloured tissues that have a limited number of scatterers are anatomically underlain by a layer of pigment granules which can absorb incident light that has penetrated the entire colour producing tissue and prevent incoherent scattering from the tissue below (Fox 1976). Most avian structural colour systems are underlain by a layer of melanin granules, but in some cases the absorbing pigment is a carotenoid (e.g. feather barbs of Cotinga).
STRUCTURAL COLOURS OF FEATHERS
There are three classes of colour producing structures in feathers. The first is unspecialised, unpigmented feather keratin, which produces white by incoherent scattering of all visible light waves, perhaps by randomly distributed air vacuoles in the feather.
The second class includes structurally coloured feather barbules, which produce iridescent structural colours by scattering from arrays of melanin granules and/or air vacuoles suspended in the barbule keratin. Here, iridescence refers only to colours that change hue with the angle of observation.
The third class of colour-producing feather structures includes the specialised, spongy, medullary layer of feather barbs. Structural barb colours include generally noniridescent blue, violet, green, and UV hues, which are broadly distributed among bird species and must have arisen numerous times independently within the phylogenetic history of birds. In combination with yellow carotenoid pigments in the feather barb cortex, structural barb colours are an essential component of almost all green bird plumages.
White of Unpigmented Feathers
The white appearance of unpigmented feathers is generally recognised as a structural ‘ colour’ (Fox 1976). White is probably the result of incoherent scattering from unpigmented feather keratin, perhaps by the surfaces of the feather structures and air vacuoles within unpigmented feather keratin. All parts of the feather can be white, though the barbules are often so thin that they can appear transparent. White feathers appear in a broad diversity of avian families and have clearly evolved many times independently.
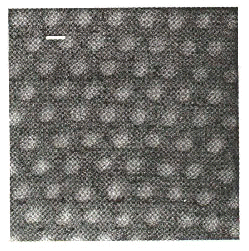
In a detailed analysis of the brilliantly white Winter plumage of the Rock Ptarmigan Lagopus mutus, Dyck (1979) demonstrated that white Lagopus feathers are whiter than unpigmented feathers of other species. Single Winter plumage Lagopus feathers reflect almost 50% of visible light of all wavelengths, compared to 15-18% reflectance of visible wavelengths for single feathers of white Domestic Hen Gallus gallus. Using transmission electron microscopy, Dyck demonstrated that this white colour is produced by large, randomly organized air vacuoles in the barbules of the Lagopus feathers (Fig. 1), and that these vacuoles are absent from white feathers of other species. Dyck hypothesised that many feathers overlapping in the plumage of the bird would eventually approach the highly efficient 80% reflectance of fallen snow. Furthermore, the reflectance spectra of white Lagopus feathers are slightly bluish (scattering smaller wavelengths more efficiently) even further approaching the genuinely ‘ bluish’ reflectance spectrum of snow. This white colour is probably produced by incoherent scattering by the irregularly shaped, randomly distributed air vacuoles in the Lagopus feather barbules. Whether the slightly bluish hue of the feathers is due to differential scattering (Rayleigh scattering) or differential absorption of longer visible wavelengths is unknown.
Dyck's (1979) fascinating analysis of white Lagopus feathers indicates convincingly that structural colours can function as cryptic plumages in certain environments, and that this colour likely evolved by natural selection as an adaptation against detection by visual predators.
Structural Colours of Feather Barbules
Structural colours of avian feather barbules are produced by scattering from arrays of granules of melanin, or melanin and air, that are distributed in the keratin of the feather barbules. Although some aspects of the nature of these arrays were determined experimentally by researchers early in this century (Häcker and Meyer 1902; Mason 1922), the description and analysis of these structures advanced tremendously with the invention of transmission electron microscopy (Greenewalt et al. 1960; Schmidt and Ruska 1961; Durrer 1962; Schmidt and Ruska 1962; Durrer and Villiger 1966; Rutschke 1966; Durrer and Villiger 1970; Dorst et al. 1974; Dyck 1987). These colour-producing structures are found in many avian orders and families, and have evolved numerous times independently within groups of birds that vary widely in size, behaviour, and ecology (Dyck 1976; Durrer 1986).
Given the diversity of avian taxa in which structural barbule colours have evolved, it is not surprising that there is a tremendous diversity in the composition, size, and shape of these structures. The diversity of light scattering arrays in avian barbules has been described and categorised by Durrer (1986). They can be roughly categorised in the following classes (Fig. 2):
Type 1: a thin layer of keratin above a layer of large spherical melanocytes and air vacuoles
Type 2: single or multiple layers of adjacent rod-like melanin granules (1-2¦m long, 0.2 ¦m diameter),
Type 3: single or multiple layers of thinner rods of melanin (1 ¦m long, 0.1 ¦m diameter) that are arranged in nearly square or hexagonal arrays, or in tight packed single or double layers
Type 4: melanin platelets or lozenges (1.5-2.5 ¦m long; 0.25-0.4 ¦m wide, and 0.25-0.4 ¦m thick) arranged in single or multiple layers;
Type 5: air-filled melanin tubes (1.2-1.6 ¦m long; 0.12-0.27 ¦m in diameter) arranged in single or multiple layers or in a hexagonal array;
Type 6: air-filled melanin platelets (2.5 x 1 ¦m in area, and 0.3 ¦m thick) arranged in single or multiple layers, which may be immediately adjacent or separated by an additional keratin layer.
Each of these six types of light scattering structures has convergently evolved in multiple families and orders of birds. For example, all six classes are found in some passerine and non-passerine families.
In general, colour production by these structures is hypothesized to be produced by coherent scattering, or constructive interference, of light by the interfaces between the keratin, melanin, and air. Many of these systems have been successfully modeled using thin-film scattering (Greenewalt et al. 1960; Dyck 1976), or Bragg's Law (Durrer 1962; Durrer and Villiger 1966, 1970). In many cases, nanometer-scale variation in the size and spatial distribution of these structures has been accurately correlated with the observed hue or the reflectance spectrum of the barbules.
In all of these types, the single layer or array of scatterers is usually oriented parallel to the flattened surface of the feather barbs. Typically, some portion or all of the distal barbules (the distally directed barbules which lie superficially on the obverse, or outer surface, of the feather vane) is elongated, flattened, and rotated to create a planar surface for the efficient presentation of the colour-producing arrays (Durrer 1986). This arrangement creates iridescence, or potential changes in hue with the angle of observation, which is such a striking feature of most structural colours of barbules. The change in hue occurs because the path length addition (or difference in distance traveled) between light waves scattered by adjacent scatterers or layers increases with decreasing angle of observation. So, theoretically, the hue of iridescent feathers should shift toward longer visible wavelengths with decreasing angles of observation.
Several lineages of birds have evolved vivid barbule colours that are not iridescent by evolving specialized barbule surfaces which are not planar. Dyck (1987) has elegantly demonstrated that the non-iridescent structural greens of the barbules of Ptilinopus fruit pigeons and related genera (Columbidae) are produced by arranging the layers of contiguous melanin granules in concentric circular arrays within specialised rounded ridges on the barbule surface. Because the array is circular, light from any angle is essentially directly incident on the array, and the hue of the colour does not change with angle of observation. An amazingly similar arrangement has evolved convergently in Chrysococcyx (Cuculidae).
Scattering theory predicts a strong positive relationship between number of scattering layers or the depth of a scattering array and the intensity of the colour produced. Dyck documented this relationship very well for Ptilinopus and relatives. Furthermore, scattering theory predicts that the efficiency of scattering increases with the magnitude of the difference in refractive index between the scattering media. Thus, the structural colours produced by air-filled melanin granules (Type 6 of Durrer 1986) should be more intense than solid melanin arrays of similar size and depth. The brilliance of structural colours produced by barbules with multiple layers of air-filled melanin granules or platelets has been empirically demonstrated for several species (Greenewalt 1960, Dyck 1976). However, the precise relationship between the striking diversity of array designs and scatterer efficiency has not been quantified. For example, it may be that air-filled granules make it possible to create more intense, saturated structurally colours in a smaller space (e.g. Land 1972).
Scattering theory also predicts the existence of second order and third order reflectance peaks. These peaks are due to reinforcement among wavelengths that are close to one half, one third, twice, or three times the path length addition. If the primary reflectance peak is at either extreme of the visual spectrum, then it is possible for the secondary peak to be at the other end of the visual spectrum producing complex, combined hues (e.g. purple). Dyck (1976) reports the presence of visible first and second order reflectance peaks in iridescent feathers of Pica which has a air-filled melanin granules in what appear to be quasi-ordered layers. The first order peak is near 600 nm and the second order peak is in the near UV. Recent development of fiber optic concave gradient diode spectrophotometers has allowed for convenient measurement of avian reflectance spectra into the UV (e.g. Andersson 1996). This advance will enable researchers to measure more broadly in the electromagnetic spectrum and identify more cases second and third order reflectance peaks that are visible to birds.
Thin-film scattering theory predicts that second and third order peaks will be closer in wavelength to
lmax in non-ideal systems, where the optical thickness of the layers of media are not equal, than in ideal systems (Land 1972). Although the application of thin-film theory to arrays of rods is not exact, it is interesting that the barbule arrays of Pica appear to be less ordered than in other some other taxa (e.g. Trogon) which do not exhibit visible second order peaks. Perhaps application of a more exact scattering theory to the multimedia arrays will yield a more precise understanding of array order and the presence of visible second order reflectance peaks. This is particularly important to analyses of signal evolution, since second order peaks could greatly influence the perceived hue of a structural colour.Structural Colours of Feather Barbs
Structural colours of avian feather barbs are created by light scattering from specialised ‘ boxy’ cells or ‘ spongy’ cells in the medullary layer of the barbs (Auber 1957, 1971/72; Dyck 1971b, 1971a, 1978; Lucas and Stettenheim 1972; Fox 1976). The spongy medullary layer is composed of a matrix of keratin rods and air vacuoles of varying shapes, sizes, and complexity. To prevent incoherent backscattering from underlying feather structures or the large nuclear vacuole at the center of most spongy medullary cells, most species have a layer of eumelanin granules that surrounds the large air-filled nuclear vacuoles or in the layer of cells below the medullary layer. A few species use phaeomelanin (‘ brown’ melanin) or carotenoids to prevent backscattering (Auber 1957; Dyck 1971a). The spongy, medullary keratin is absent in noncolour-producing feather barbs (Dyck 1978).
Auber (1957) described and classified the variation in shape, size, position, and pigmentary composition of the spongy medullary tissue of many families of birds with structurally coloured barbs. In general, the medullary cells can form a ring around the surface of the barb, a central core, a basal layer, or a even a divided pair of obverse and reverse cell bundles. Auber (1971/72) also described the development of the medullary cells using histology and light microscopy. He documented that during the development of the feather barbs, the medullary keranitocytes first expand in size to create the box-like, polygonal, keratin cell boundaries. Then, the medullary cells themselves gradually shrink in volume as they create the spongy medullary keratin matrix on the inner surface of the original polygonal cell boundaries. Eventually, the cells cease to produce keratin, detaches from the inner walls of the nuclear vacuole (creating the central vacuole), and then die.
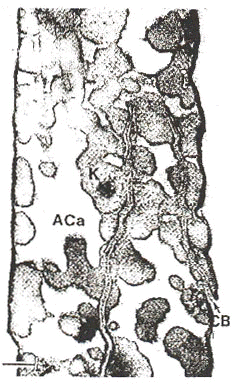
The colour-producing medullary keratin matrix varies in structure among taxa. One general form is characterized by a series of nearly circular, equivalently sized air-filled tubes separated by keratin bridges (Fig. 3). Avian taxa known to demonstrate this type of structure include Cotinga (Cotingidae), Megalaima (Ramphastidae), and Poephila (Estrildidae) (Dyck 1976; R. O. Prum pers. obs.). The other structural type is characterized by air-filled channels of more variable diameter separated by essentially equivalently sized keratin bars (Fig. 4). This type is known from many psittacids (Psittacidae), Coracias (Coraciidae), Chloropsis (Irenidae), and others (Dyck 1976; R. O. Prum pers. obs.).
Despite a more than a century of research (reviewed in Dyck 1971a; Fox 1976), the physical mechanism of colour production by feather barbs is still debated (Dyck 1971a, 1971b, 1976; Fox 1976; Finger et al. 1992; Finger 1995; Andersson 1996). Raleigh scattering, or Tyndall scattering, has been the predominant explanation of structural colours of feather barbs for nearly a century (see reviews in Dyck 1971a, and Fox 1976). The support for this hypothesis first came from reflectance spectra of blue feather barbs by Häcker and Meyer (1902) which appeared to show adherence to Rayleigh's predicted inverse fourth power relationship for a limited set of visible wavelengths (480-650 nm). Subsequently, Dyck (1971a, 1976) demonstrated that many structurally coloured feather barbs have distinct peaks in the visible spectrum. These distinct peaks falsified a major prediction of Rayleigh scattering theory which is that reflectance should continue to increase in value into the UV, following the inverse fourth power law. Häcker and Meyer (1902) probably did not measure a broad enough section of the visible spectrum to detect a peak in the lower portion of the human-visible spectrum, and erroneously interpreted their data as support for Rayleigh scattering. Despite the compelling evidence against Raleigh scattering that has been available for more than 25 years, Rayleigh scattering is currently cited as the exclusive or primary explanation of feather barb structural colours in most current ornithology texts and references (e.g. Brooke and Birkhead 1991; Campbell and Lack 1985; Gill 1995)
.
Finger (1995) has recently hypothesised that Mie scattering theory, an exact electromagnetic theory of incoherent scattering by a particle, is an accurately explanation of structural colour production by feather barbs. Finger argues that medullary keratin-air matrix is appropriately sized to scatter the smaller wavelengths of visible light and that cortical filtering, or absorption of light by the keratin cortex, is responsible for creating the distinct peaks in the visible spectrum.
Dyck proposed a coherent scattering, constructive interference, ‘ hollow cylinder’ hypothesis as an alternative to Raleigh scattering. The hollow cylinder model hypothesised that the sizes of the keratin bars and air vacuoles in the spongy medullary keratin create opportunities for constructive interference and vivid structural colour production. Although this hypothesis was apparently accepted by Auber (1970/1971) and Durrer (1986), it has received limited general acceptance in ornithology and zoology, perhaps because of the lack of evidence demonstrating that the spongy medullary keratin was sufficiently ordered to produce coherent phase relationships among scattered waves.
Advances in the understanding of the scattering physics of the optically transparent cornea have demonstrated that tissues need not have a precise, lattice-like structure to produce predictable phase relationships among scattered light waves (Benedek 1971, Vaezy and Clark 1991, 1993; Gisselberg et al. 1991). Recently, Prum, Dyck and colleagues have applied 2-D discrete Fourier analysis of spatial variation in refractive index to investigate the nanostructural periodicity of the spongy medullary keratin matrix. Our results demonstrate that the spongy medullary keratin is highly nanostructured and is appropriately scaled to produce the observed hues by coherent scattering, or constructive interference, alone (Prum et al. 1998; Prum et al. 1999).
Furthermore, these Fourier analyses demonstrate that the spongy medullary keratin matrices are spatially ordered at scales that are smaller than visible light. This result is direct evidence against the independence of scatterers that is assumed by the incoherent scattering models such as Rayleigh and Mie scattering. Incoherent scattering of visible light requires that scatterers be separated by distances that are greater than the wavelengths of visible light. The scatterers in the medullary keratin matrices of all bird species known are generally much closer together than 350 - 800 nm, the general limits of the avian-visible spectrum. Given direct evidence that the scatterers in the spongy medullary tissues of structurally coloured barbs are coherent and not spatially independent, incoherent scattering models that do not take into account the phase relationships among the scattered light waves, such as Rayleigh and Mie scattering, cannot be considered to be complete or accurate descriptions of the light scattering behaviour of these tissues.
These Fourier analysis results strongly support Dyck's original ‘ hollow cylinder’ hypothesis (Dyck 1971a, b) that feather barb structural colours are produced by coherent scattering, or constructive interference. Furthermore, these results document convincingly that the conditions for incoherent scattering required by Rayleigh and Mie theory are not met by any feathers, and that these mechanistic hypotheses provide incomplete explanations of the phenomenon.
This finding indicates that structural colours of the feather barbs and barbules are produced by the same physical mechanism, but differ in the spatial position, organisation, and physical composition of the scatterers. The result is vivid structural colour production but without obvious, macroscopic iridescence. The lack of iridescence arises because the medullary keratin is appropriately structured on a nanoscale to produce constructive interference, but is structurally random at higher spatial scales. The observed colour is the summation of many scatterers that are randomly oriented to the surface, but a change in angle of observation does not change the average path length addition to waves scattered by the array.
STRUCTURAL COLOURS OF THE AVIAN DERMIS
Given the intricate structural beauty, variation, and frequently striking colours of feathers, it is perhaps understandable that ornithologists have almost completely neglected to investigate the biology and physics of the colours of the avian skin. However, it is clear that pigmentary and structural colours of the avian skin can play an important, even critical role in social communication of many species of birds. Obviously, an understanding of the biology and biophysics of dermal structural colours is essential to understanding the evolution of this component of avian phenotype and social signalling.
There have been several cursory investigations of structural blue colours in bird skin. All previous authors have hypothesized that these colours are produced by Rayleigh scattering from melanin granules distributed in the dermis, but the anatomical structures responsible for these colours were never described (Rawles 1960; Lucas and Stettenheim 1972; Fox 1976; Durrer 1986). Prum et al. (1994) were the first to use both light and transmission electron microscopy to investigate the anatomy and biophysics of apparent structural colour production in the vividly green and blue caruncles of male Philepitta castanea (Eurylaimidae).
Prum et al. (1994) found that the colour of the caruncle of Philepitta was produced by extensive, nearly perfectly hexagonal arrays of parallel collagen fibers in the dermis (Fig. 5). The hexagonal arrays are organised into large macrofibrils that are as wide as dozens of the smallest hexagonal fiber units. These macrofibrils are chaotically or randomly organized within cone-shaped papillae on the surface of the skin that are covered with an epidermal keratinous cone. The dermal collagen macrofibrils are underlain by a thick layer of melanocytes which prevent incoherent back scattering from the optically disorganized tissue below.
Prum et al. (1994) demonstrated that the colour of the blue preserved caruncles of adult male Philepitta castanea conformed well to the wavelengths predicted by the application of the Bragg equation to the dimensions of collagen arrays in the caruncle papillae. The colour produced by the caruncles of Philepitta castanea is the summation of constructive reflections from thousands of collagen arrays arranged in macrofibrils within each papilla. This paper was based on formalin-fixed caruncle tissues that had changed substantially in size and colour. In subsequent research on glutaraldehyde preserved caruncle tissues that retained the original colour almost exactly, I have documented that the difference between the green and blue portions of the caruncle can be accurately described as a result of nanoscale differences in the size and spacing of the collagen fibers in the arrays (Prum et al. In Prep.).
This mechanism of structural colour production had never been described before in any organisms. However, similar collagen-based dermal colours have been implicated in primates. This study was also the first detailed microscopic study of any structurally-coloured, avian dermal tissue. These exciting results demonstrate the possibilities for future structural colour research in birds. A general survey of structural colouration in birds is required to further document the distribution of this and other colour-production mechanisms in the avian dermis.
There are additional structural colours of other dermal derivatives that have been very poorly studied. The ramphotheca and scutellated hindlimbs of many bird species are obviously structurally coloured (i.e. blue or green colours that are unlikely to be purely pigmentary). Further, many of these tissues that are colourful in life turn black in museum specimens, indicating that they are underlain with melanin and may have a structural basis. Among the few papers specifically addressing the nature of a structural colour of a dermal derivative is a paper on the bright light blue bill of the Ruddy Duck Oxyura jamaicensis (Anatidae; Hays and Habermann 1969). Hays and Habermann (1969) demonstrate that the colour is produced by a spongy layer beneath the ramphotheca that is underlain with melanin. They did not examine the tissues with transmission electron microscopy, though it is a distinct possibility that this spongy layer could be composed of collagen arrays, as in Philepitta (Prum et al. 1994). Currently, I know of no other studies of structural colours of non-feather dermal derivatives of birds.
STRUCTURAL COLOURS OF THE AVIAN EYE
Traditionally, the colour of the avian iris has been hypothesised to be produced by melanin and carotenoid pigments. Although there are obviously many iris colours that could not be caused merely these pigments, little research has been done on the nature of avian iris colours.
Oehme (1969) produced an extensive treatment of the pigments of avian iris. Although he did not separate the pigments chromotographically, Oehme identified many bird species with carotenoid, pteridine, and purine pigments in their irides. The carotenoids were present within intracellular lipid droplets. The pteridine or purine pigments were found in crystalline form in some species, but they were not chemically identified.
The detection of crystalline pigments in the avian iris by Oehme (1969) implied the presence of iridiphores or leucophores: special pigment cells, commonly distributed in poikilothermic vertebrates, that produce structural colours by constructive interference (i.e. coherent scattering ) from arrays of purine or pteridine pigments. Purines are only present in pigment cells in crystalline form, but colour producing pteridines can be found in both crystalline and non-crystalline form.
The first detailed anatomical analysis of iridphores in the avian iris was by Ferris and Bagnara (1972). They documented that the irides of Inca Dove Columbina inca and the Common Ground-Dove Columbina passerina (Columbidae) include two structurally distinct types of iridiphores. One type of iridiphore included melanin granules and abundant, spherical guanine crystals, and the second type included many thinner, rod-shaped guanine crystals. The two types of purine-containing pigment cells were distributed in different parts of the iris.
Subsequently, Oliphant (1981, 1987a, 1987b; Oliphant et al. 1992) has identified numerous species of birds from many families that have crystalline purines and pteridines in the iris. Oliphant (1981) identified pteridine-containing leucophores in the iris of the Great Horned Owl Bubo virginianus (Strigidae). Subsequently, in a sample of 28 species from 11 families, Oliphant identified crystalline purines in the irides of 20 species in 11 families and crystalline pteridines in 3 species. In all species with purine crystals, Oliphant identified the presence of non-crystalline pteridines. He hypothesised that these pteridine pigments were superficial to the purine crystal-containing iridiphores and were modifying their structural colour. Similar structural-pigmentary colour combinations are found widely in poikilothermic vertebrates.
Obviously, there is much more to be learned about pigmentary and structural colours of avian iris. However, based even on the small sample of current data, it is clear that the colouration of the iris is based on entirely different pigmentary and cellular mechanisms than the avian integument. To date, no purine- or pteridine-containing pigment cells have been identified in the dermis of birds (Oliphant et al. 1992). Currently, nothing is known about the ontogeny or evolution of these structures in the avian iris.
The other structural colouration of the avian eye is the well-known phenomenon of eye-shine: the reflection of incident light off of the retina of the eye. Although eye-shine is well-known from a wide variety of nocturnal and even diurnal species, its mechanistic basis remains a mystery (Walls 1963). In mammals, eye-shine is created by the tapetum lucidum : a specially structured layer of the choroid membrane within the retina (Land 1972). In birds, the tapetum lucidum is apparently absent although a choroid is present (Walls 1963). Apparently, no one has microscopically examined the eye of a nocturnal bird to try to identify the anatomical source of this well known phenomenon. One can predict that it should be made of a nearly lattice-like array of reflecting crystal or fibers, given the iridescent quality of the eye-shine of some species.
DISCUSSION
Structural colours are present in all parts of the avian integument and eyes, and they doubtless play an important role in the social communication and cryptic colouration of birds. Our understanding of the anatomical basis of structural colours has expanded tremendously in the last 50 years with the application of electron microscopy. However, there are still woefully few published studies of structural colours of the avian dermis, dermal derivatives, and eyes. The fact that the anatomical basis of the structural colours of the toucans bill and the eye-shine of nightjars have not be determined anatomically is an unfortunate indictment of ornithological curiosity!
Our understanding of the physics of structural colour has been consistently advancing as well. The hypothesis that the iridescent structural colours of feather barbules are caused by constructive interference among coherently scattered light waves has been accepted for over a century (Fox 1976). However, recent advances in anatomy and physics imply that the noniridescent colours of feather barbs and dermis are produced by the same mechanism.
Early in this century, ornithologists assumed that iridescent colours must be caused by constructive interference, whereas non-iridescent colours must be caused by incoherent scattering by small particles, such as Rayleigh scattering (e.g. Mason 1922). Now, several different coherently scattering structures have been described in birds that produce non-iridescent colours by constructive interference: blue barbs of Cotinga (Prum et al. 1998), and the dermal collagen arrays in Philepitta (Prum et al. 1994). The constructive interference is the result of structural organisation at a nanoscale, but the lack of iridescence is a consequence of the lack of higher scale spatial organisation. It appears likely that constructive interference of coherently scattered light waves is the sole physical explanation of all avian structural colours. It appears that evolution has created tissues with the appropriate nanostructured periodicity in refractive index to produce visible colours by constructive interference. However, there are no clear examples in birds where a tissue has evolved to include appropriately sized and distributed incoherent scatterers. That is, there are no avian tissues for which Rayleigh or Mie scattering are accurate descriptions of the colours created. Any description of the physics of structural colour produced by known avian tissues must include an analysis of the phase relationships among the scattered waves.
However, there is the distinct possibility that an exact description of the light scattering behaviour by a tissue will include both coherent scattering by particles that are best described by Rayleigh or Mie scattering models. For example, Mie scattering could give an accurate description of the differential scattering by an individual medullary air vacuole in a feather barb, but a coherent scattering model may be required to describe the subsequent interactions among the scattered waves from different scatterers. A complete, exact description of scattering and absorption of light by any of these tissues would be an enormous but very interesting undertaking.
Several basic, and important questions remain in the physics of avian structural colours. First, why are feather barb structural colours limited to the lower end of the visible spectrum? Second, what is the anatomical basis of structural colours of the dermis and dermal derivatives in other birds? Lastly, why have no structurally coloured pigment cells (i.e. iridiphores or leucophores) been found in the avian dermis when they are close to ubiquitous in the irides of birds with brightly coloured eyes? These questions should receive top priority in subsequent investigations.
A review of the implications of structural colours for the evolution of signal function in birds is too large to accomplish in this paper. However, there is an expanding literature on the evolution of carotenoid pigmentation as an honest indicator of quality (e.g. Hill 1991, 1992, 1993, 1994; Andersson 1994). Some of this literature reflects an ignorance of structural colouration in birds. For example, Price et al. (1993) hypothesised a model of sexual selection in which the male trait varied with the amount of exogenous carotenoid pigments deposited in the feathers; high quality males with high concentrations of carotenoids were hypothesised to be bright green, whereas low quality males with low concentrations of carotenoids were hypothesised to be drab brown. Unfortunately, these authors did not realise that the absence of carotenoids in a bright green plumage would produce bright blue. Obviously, further discussions on the quality information of avian colouration should be based on an accurate understanding of the differences between exogenous pigments, endogenous pigments, and structural colours.
Most important among the questions regarding the evolution of structural colours for social signaling is the question of condition dependence. Do structural colours vary within populations in relation to the quality or condition of the individuals? This question needs to be addressed experimentally with each class of structural colours. However, I would predict that the fine levels of developmental control required to create colour producing structures of the correct size are likely to be under tight genetic control. I would expect that experimental studies will not find a high degree of quality correlated variation in avian structural colours. This result would have a fascinating impact on sexual selection theory. The Peacock's tail Pavo cristatus (Phasiandiae) is a famous example of a secondary sexual character. The question of whether the brilliant colours of the tail evolved because they have quality information or because they are merely preferred by females remains entirely unanswered.
REFERENCES
Andersson, M. 1994. Sexual Selection. Princeton University Press: Princeton, NJ.
Andersson, S. 1996. Bright ultraviolet colouration in the Asian whistling-thrushes (Myiophonus spp.). Proceedings of the Royal Society of London. Series B. 263: 843-848.
Auber, L. 1957. The structures producing ‘ non-iridescent’ blue color in bird-feathers. Proc. Zool. Soc. London 129: 455-486.
Auber, L. 1971/72. Formation of 'polyhedral' cell cavities in cloudy media of bird feathers. Proceedings of the Royal Society, Edinborough 74: 27-41.
Benedek, G.B. 1971. Theory of transparency of the eye. Appl. Optics 10: 459-473.
Bennett, A.T.D. & Cuthill, I.C. 1994. Ultraviolet Vision in birds: what is its function? Vision Research 34:.
Bohren, C.F. 1987. Multiple scattering of light and some of its observable consequences. American Journal of Physics 55: 524-533.
Bohren, C.F. & Huffman, D.R. 1983. Absorption and Scattering of Light by Small Particles. John Wiley and Sons.: New York.
Bragg, W.H. & Bragg, W.L. 1915. X-rays and Crystal Structure. G. Bell: London.
Briggs, W.L. & Henson, V.E. 1995. The DFT. Society for Industrial and Applied Mathematics: Philadelphia, Penn.
Brooke, M. & Birkhead, T. 1991. The Cambridge Encyclopedia of Ornithology. Cambridge University Press: Cambridge.
Campbell, B. & Lack, E 1985. A Dictionary of Birds. T. A. & D. Poyser: London.
Dorst, J., Gastaldi, G., Hagege, R. & Jacquemart, J. 1974. Different aspects des barbules de quelques Paradisaeid
Ts sur coupes en microscopie Tlectronique. Comptes Rendus de l'Academie des Sciences, Paris 278: 285-290.Durrer, H. 1962. Schillerfarben beim Pfau (Pavo cristatus L.). Verhandl. Naturf. Ges Basel 73: 204-224.
Durrer, H. 1986. The Skin of Birds: Colouration. In: Bereiter-Hahn, J., Matoltsky, A.G. & Richards, K.S. (eds) Biology of the Integument: 2 Vertebrates. J. Springer-Verlag: Berlin: 239-247.
Durrer, H. & Villiger, W. 1966. Schillerfarben der Trogoniden. Journal für Ornithologie 107: 1-26.
Durrer, H. & Villiger, W. 1970. Shilleradien des Goldkuckucks. Zeitschrift für Zellforschung 109: 407-413.
Dyck, J. 1971a. Structure and colour-production of the blue barbs of Agapornis roseicollis and Cotinga maynana. Z. Zellforsch. 115: 17-29.
Dyck, J. 1971b. Structure and spetral reflectance of green and blue feathers of the Lovebird (Agapornis roseicollis). Biol. Skrift. 18: 1-67.
Dyck, J. 1976. Structural colours. Proc. Internat. Ornithol. Congr 16: 426-437.
Dyck, J. 1978. Olive green feathers: Reflection of light from the rami and their structure. Anser, Supplement 3: 57-75.
Dyck, J. 1979. Winter plumage of the Rock Ptarmigan: structure of the air-filled barbules and function of the white colour. Dansk. Orn. Foren. Tidsskr. 73: 41-58.
Dyck, J. 1987. Structure and light reflection of green feathers of fruit doves (Ptilinopus spp.) and an Imperial Pigeon (Ducula concinna). Biol. Skrift. (Copenhagen) 30: 2-43.
Ferris, W. & Bagnara, J.T. 1972. Reflecting pigment cells in the dove iris. In: Riley, V. (ed.) Pigmentation: Its Genesis and Biological Control. Appleton-Century-Crofts; New York: 181-192.
Finger, E. 1995. Visible and UV Coloration in Birds: Mie Scattering as the Basis of Color Production in Many Bird Feathers. Naturwissenschaften 82: 570-573.
Finger, E., & Burkhardt, D. 1994. Biological aspects of bird coloration and avian vision including the ultraviolet range. Vision Research 34: 1509-1514.
Finger, E., Burkhardt, D. & Dyck, J. 1992. Avian Plumage Colors: Origin of UV Reflection in a Black Parrot. Naturwissenschaften 79: 187-188.
Fox, D.L. 1976. Animal Biochromes and Structural Colors. Univ. California Press: Berkeley,CA.
Gill, F.B. 1995. Ornithology. Second Edition ed., W. H. Freeman and Co.: New York.
Gisselberg, M., Clark, J.I., Vaezy, S. & Osgood, T. 1991. A quantitative evaluation of Fourier components in transparent and opaque calf cornea. American Journal of Anatomy 191: 408-418.
Greenewalt, C.H., Brandt, W. & Friel, D. 1960. The iridescent colors of hummingbird feathers. Journal of the Optical Society of America 50: 1005-1013.
Häcker, V. & Meyer, G. 1902. Die blaue Farbe der Voelfedern. Zool. Jb., Abt. Syst. Geog. Biol. Tiere 15: 267-294.
Hays, H., & Habermann, H. 1969. Note on bill color of the Ruddy Duck, Oxyura jamaicensis rubida. Auk 86: 765-766.
Hill, G. 1991. Plumage coloration is a sexually selected indicator of male quality. Nature 350: 337-339.
Hill, G.E. 1992. Proximate basis of variation in carotenoid pigmentation in male House Finches. Auk 109: 1-15.
Hill, G.E. 1993. Geographic variation in the carotenoid plumage pigmentation of male house finches (Carpodacus mexicanus). Biol. J. Linn. Soc 49: 63-86.
Hill, G.E. 1994. Geographic variation in male ornamentation and female mate preference in the House Finch: a comparative test of models of sexual selection. Behavioral Ecology 5: 64-73.
Land, M.F. 1972. The physics and biology of animal reflectors. Progress in Biophysics and Molecular Biology 24: 77-106.
Lee, D.W. 1991. Ultrastructural basis and function of iridescent blue colour of fruits in Elaeocarpus. Nature 349: 260-262.
Lee, D.W. 1997. Iridescent Blue Plants. American Scientist 85:56-63.
Lucas, A.M. & Stettenheim, P.R. 1972. Avian Anatomy- Integument. U.S. Dept. Agricult. Handbook: Washington, DC.
Macleod, H.A. 1986. Thin-film Optical Filters. 2nd ed., Adam Hilger, Ltd.: Bristol.
Mason, C.W. 1922. Structural Colors of Feathers. I. Journal of Physical Chemistry 27: 201-251.
Maurice, D.M. 1984. The cornea and sclera. In: Davson, H. (ed.) The Eye. Academic Press: New York 1-158.
Neville, A.C. 1993. Biology of Fibrous Composites. Cambridge University Press: Cambridge.
Oehme, H. 1969. Vergleichende Untersuchungen nber die Farbung der Vogeliris. Biologische Zentralblatt 88: 3-35.
Oliphant, L.W. 1981. Crystalline Pteridines in the stromal pigment cells of the iris of the Great Horned Owl. Cell and Tissue Research 217: 387-395.
Oliphant, L.W. 1987a. Observations on the pigmentation of the pigeon iris. Pigment Cell Research 1: 202-208.
Oliphant, L.W. 1987b. Pteridines and purines as major pigments of the avian iris. Pigment Cell Research 1: 129-131.
Oliphant, L.W., Hudon, J. & Bagnara, J.T. 1992. Pigment cell refugia in homeothermsû the unique evolutionary position of the iris. Pigment Cell Research 5: 367-371.
Price, T. Schluter, D. & Heckman, N.E. 1993. Sexual selection when the female directly benefits. Biological Journal of the Linnean Society 49: 187-211.
Prum, R.O., Morrison, R.L. & Ten Eyck, G.R. 1994. Structural color production by constructive reflection from ordered collagen arrays in a bird (Philepitta castanea: Eurylaimidae). Journal of Morphology 222: 61-72.
Prum, R.O., Torres, R.H., Williamson, S & Dyck, J. 1998. Coherent light scattering by blue bird feather barbs. Nature 396: 28-29.
Prum, R.O., Torres, R.H., Williamson, S & Dyck, J. 1999. Two-dimensional Fourier analysis of the spongy medullary keratin of structurally coloured feather barbs. Proceedings of the Royal Society: Biological Sciences (B) 266: 13-22.
Rawles, M.E. 1960. The integumentary system. 189-240. In: Marshall, A.J. (ed.) Biology and Comparative Physiology of Birds. 1. Academic Press; New York: 189-240.
Rutschke, E. 1966. Die submikroskopische struktur schillernder federn von entenvögeln. Zeitschrift für Zellforschung 73: 432-443.
Schmidt, W.J. & Ruska, H. 1961. Elektronenmikroskopische Untersuchung der Pigmentgranula in den Shillerenden Federstahlen der Taube Columba trocaz H. Zeitschrift für Zellforschung 55: 379-388.
Schmidt, W.J. & Ruska, H. 1962. Über das schillernde federmelanin bei Heliangelus und Lophophorus. Zeitschrift fnr Zellforschung 57: 1-36.
Vaezy, S. & Clark, J.I. 1991. A quantitative analysis of transparency in the human sclera and cornea using Fourier methods. Journal of Microsopy 163: 85-94.
Vaezy, S. & Clark, J.I. 1993. Quantitative analysis of the microstructure of the human cornea and sclera using 2-D Fourier methods. Journal of Microscopy 175: 93-99.
van de Hulst, H.C. 1981. Light Scattering by Small Particles. Dover: New York.
Walls, G.L. 1963. The Vertebrate Eye. Hafner Pub. Co.: New York.
Young, A.T. 1982. Rayleigh Scattering. Physics Today 35: 42-48.
Fig. 1. Cross section of a white feather barbule of a winter plumage Rock Ptarmigan Lagopus mutus (from Dyck 1979). ACa = air filled channels which travel down the length of the feather barbule. K = keratin. CB = cell boundary between neighboring barbule cells.

Fig. 2. Diagrammatic representation of the six types of colour producing arrays in avian feather barbules (based on Durrer 1986). See text for details. Examples illustrated are: Type 1: Columba livia (Columbidae); Type 2: various starlings (Sturnidae); Type 3: Meleagris gallipavo (Meleagridae); Type 4: Nectarinia (Nectariniidae); Type 5: Galbula (Galbulidae); Type 6: various hummingbirds (Trochilidae).
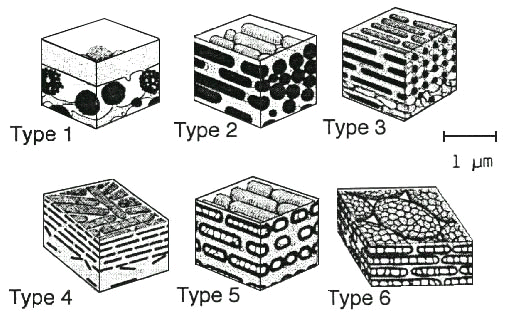
Fig. 3. Transmission electron micrograph of the spongy medullary keratin of purple breast feathers of a male Gouldian Finch Poephila guttata (Estrildidae). Magnification X30k Scale bar is 200 nm.
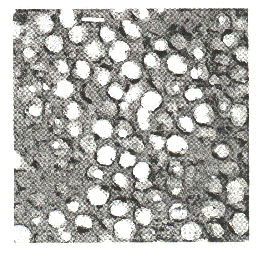
Fig. 4. Transmission electron micrograph of the spongy medullary keratin of a blue feather barb of the Lilac-breasted Roller Coracias caudata (Coraciidae). Magnification X50k. Scale bar is 100 nm.

Fig. 5. Transmission electron micrograph of a blue portion of the supraorbital caruncle of the Velvet Asity Philepitta castanea (Eurylaimidae). Magnification X50k. Scale bar is 100 nm.