S28.3: Endocrinology of aggression in the nonbreeding season
Kiran K. Soma & John C. Wingfield.
Department of Zoology, University of Washington, Seattle, WA, 98195-1800 USA., fax 206 543 3041, e-mail ksoma@u.washington.edu
Soma, K.K. & Wingfield, J.C. 1999. Endocrinology of aggression in the nonbreeding season. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1606-1620. Johannesburg: BirdLife South Africa.Many birds form flocks in the nonbreeding season, but some species maintain winter territories. The Song Sparrow Melospiza melodia morphna of western Washington State, USA is sedentary and males defend territories throughout the year. Territorial aggression in the nonbreeding season (autumn and winter) is high, although plasma testosterone (T) levels are low. Castration of males in autumn does not decrease aggression. Recent experiments, however, indicate that androgen receptor antagonist and aromatase inhibitor treatments decrease territoriality in autumn. These field experiments suggest that sex steroid hormones from non-gonadal sources may regulate territorial aggression during the nonbreeding season. Aggression and dominance in winter flocks may be regulated similarly. Finally, we consider the ecological factors which favour territoriality over flocking in nonbreeding Song Sparrows.
INTRODUCTION
During the nonbreeding season, many avian species are gregarious and form flocks (Crook 1965; Pulliam & Millikan 1982). Some species, however, continue to defend territories in winter. Nonbreeding territoriality has, until recently, received little attention, and indeed was initially considered anomalous or maladaptive behaviour (e.g., Hamilton 1959). The broad analysis of avian species by Crook (1965) suggests that territoriality during the nonbreeding season is more common than generally believed. More specific analyses of neotropical migrant passerines (Greenberg 1983), the genus Parus (Matthysen 1990), and shorebirds (Myers et al. 1979) have also concluded that winter territoriality is not uncommon.
The physiological regulation of breeding territoriality has been the focus of numerous studies (e.g., Wingfield et al. 1990), but the proximate mechanisms underlying nonbreeding territoriality are obscure. Although territorial behaviours in the breeding and nonbreeding seasons may appear similar, they could be regulated in different ways. Also, many of the behaviours used to defend winter territories are similar to behaviours used to establish dominance hierarchies in winter flocks (e.g., threat postures, physical attacks). Similarities and differences in the neuroendocrine regulation of winter territorial aggression and winter dominance interactions also remain to be elucidated.
TERRITORIALITY AND PLASMA TESTOSTERONE IN SONG SPARROWS
The Song Sparrow Melospiza melodia morphna of western Washington State, USA is sedentary and defends territories throughout the year (Wingfield & Hahn 1994). Males in spring (breeding) and autumn (nonbreeding) show similar aggressive responses to a simulated territorial intrusion (live decoy and song playback for 10 min) (Fig. 1). The territorial responses are similar in quality (types of behaviour) and quantity (Wingfield & Hahn 1994). Although we focus on male behaviour here, female Song Sparrows are also territorial, and hormone-behaviour relationships in females are under investigation (Elekonich 1997).
Although males in autumn respond similarly during the simulated intrusion, they are less ‘persistent’ after the intrusion has ended. After the decoy is removed, territorial behaviour is extinguished more rapidly in autumn (Wingfield 1994a). The social context of territoriality also changes seasonally. Spring territories are defended by a breeding pair, but winter territories can be defended by individuals, pairs, or larger groups (Wingfield & Monk 1992).
Although male territorial behaviour remains high in the nonbreeding season, the testes are small and plasma testosterone (T) is low at this time (Fig. 2). Plasma T in wild males is low throughout the nonreproductive season (0.1 ng ml-1 or less) (Wingfield & Hahn 1994). In contrast, plasma T during the breeding season is usually 1 to 8 ng ml-1. Moreover, agonistic interactions between males can increase plasma T in spring, but not in autumn (Wingfield 1985; Wingfield 1994a; Wingfield & Hahn 1994). Thus, plasma T appears consistently low during the nonbreeding season, without transient increases during aggressive encounters. Note that plasma T levels are low, but not zero, in the nonbreeding season, and even completely regressed gonads may secrete hormones which have physiological effects (e.g., Wingfield et al. 1980).
Plasma 17▀-oestradiol (E2, an oestrogen) and 5alpha-dihydrotestosterone (DHT, an androgen) are also basal in males during the nonbreeding season (0.15 ng ml-1 or less) (Fig. 2). Levels of plasma E2 and DHT are significantly lower during the nonbreeding season (t-test, P<0.05 in both cases) and cannot account for winter territoriality. However, not all plasma steroid levels are basal in winter, because plasma corticosterone levels are well above the assay detection limit (Fig. 2). In the future, it will be important to measure other plasma sex steroids in males, such as progesterone and oestrone (Fig. 3).
ENDOCRINE MANIPULATIONS IN NONBREEDING MALES
The effects of hormone manipulations on nonbreeding aggression have been examined in the field. Free-living animals are often more aggressive and have different hormone levels than captive animals (e.g., Wingfield et al. 1990; Schwabl & Kriner 1991), which emphasizes the value of field experiments. In one experiment, nonbreeding Song Sparrow males were either castrated or sham operated and released back onto their territories. Territorial behaviour was measured a week later. Interestingly, castration of nonbreeding males had no effect on territorial behaviour (Wingfield 1994a). This suggests that small amounts of testicular T do not support autumnal aggression, and other testicular hormones are also not necessary. In lizards and mammals, castration also has no effect on nonbreeding territoriality (Caldwell et al. 1984; Moore & Marler 1987). The effects of castration on breeding Song Sparrows have not been fully assessed, but studies of other avian species (including closely related sparrows) show that castration of breeding males decreases aggression (Wingfield & Ramenofsky 1985). Taken together with the low plasma sex steroid levels in winter, these data raise the hypothesis that nonbreeding territoriality is independent of sex steroid hormones (Wingfield 1994b).
This hypothesis was tested in several field experiments. First, male Song Sparrows in autumn received subcutaneous T implants designed to produce high spring levels of plasma T (8 to 10 ng/ml) (Wingfield 1994a). Relative to controls, T-treated males were more aggressive during the simulated intrusion and also after the intrusion (‘persistence’). The effects of T treatment on aggression appeared more pronounced after the intrusion. These data indicate that nonbreeding aggression can be regulated by exogenous T, but do not address the role of endogenous sex steroids.
The next studies examined the possible function of endogenous sex steroids by using hormone receptor antagonists and hormone synthesis inhibitors. Testosterone can regulate behaviour by binding to androgen receptors, or after conversion to E2 by the enzyme aromatase (Fig. 3) (e.g., Wingfield et al. 1997). Aromatization occurs within the avian brain and can regulate male aggressive behaviour in the breeding season (e.g., Schlinger & Callard 1990). In one experiment, nonbreeding Song Sparrow males received subcutaneous implants of an androgen receptor antagonist (flutamide) and an aromatase inhibitor (ATD) in combination. Flutamide inhibits T and DHT from binding to intracellular androgen receptors (Labrie 1993), and ATD inhibits aromatase activity (Foidart et al. 1995). Relative to controls, ATD+flutamide treatment (for 30 days) significantly reduced aggressive behaviours in nonbreeding males (K. Soma, K. Sullivan & J. Wingfield, unpub. results). However, ATD+flutamide treatment had no effect on body mass or fat reserves, suggesting the experimental birds were healthy and foraging normally. These data suggest that nonbreeding territoriality may be regulated by endogenous sex steroids. Interestingly, flutamide alone does not decrease nonbreeding territoriality in male European Robins Erithacus rubecula (Schwabl & Kriner 1991), suggesting either a species difference or the importance of aromatase (Schlinger et al. 1992).
The role of aromatase was tested with fadrozole, an aromatase inhibitor more specific and potent than ATD (Wade et al. 1994). Nonbreeding Song Sparrow males received either fadrozole or saline (vehicle) via subcutaneous osmotic minipumps (Alzet Model 1002) (Soma et al. 1998). Relative to controls, fadrozole treatment (for 9 days) significantly reduced territorial aggression. A third group received fadrozole pumps along with a subcutaneous E2 implant. The E2 implant completely restored aggression in fadrozole-treated animals, indicating that fadrozole’s effect on aggression was specifically mediated through aromatase. Also, fadrozole had no effect on body mass or fat reserves, suggesting all birds were healthy. These results strongly suggest that endogenous estrogens regulate nonbreeding territoriality in male Song Sparrows.
Clearly, endocrine manipulations in the nonbreeding season suggest that sex steroids regulate winter territoriality, contrary to earlier evidence (Wingfield 1994b). Data do not support the long-standing hypothesis that nonbreeding territoriality is independent of sex steroids in Song Sparrows. The same may be true for other species which defend winter territories (e.g., Caldwell et al. 1984; Moore & Marler 1987; Schwabl & Kriner 1991; Gwinner et al. 1994). Furthermore, endocrine manipulations in autumn (especially fadrozole treatment) caused relatively rapid changes in aggression (Soma et al. 1998). Thus, these data indicate an activational effect of sex steroids, although organizational (Schwabl 1993) and trans-seasonal effects (Crews 1991) cannot be excluded. If sex steroids have activational effects on nonbreeding territoriality, why are plasma levels of sex steroids low during winter, and why does castration have no effect in this season? Clearly, new hypotheses are necessary to reconcile these apparently contradictory lines of evidence.
HORMONES AND NONBREEDING TERRITORIALITY: NEW HYPOTHESES
Several hypotheses can account for the seemingly conflicting results described above. First, during the nonbreeding season, there may be increased neural sensitivity to plasma sex steroids of non-gonadal origin. Second, during the nonbreeding season, the brain itself may synthesise T and E2 de novo from cholesterol (‘neurosteroids’) (Robel & Baulieu 1995). Third, during the nonbreeding season, a peripheral organ (e.g., adrenal glands) may synthesize a sex steroid precursor, which is converted to T and E2 in the brain (‘intracrinology’) (Labrie et al. 1995). These 3 hypotheses are not mutually exclusive.
The first hypothesis states that the brain is more sensitive to plasma sex steroids during the nonbreeding season, although these plasma sex steroids must be of non-gonadal origin because castration has no effect. If true, low levels of plasma T could support high levels of aggression. This scenario is unlikely for several reasons. In Northwestern Song Sparrows, T implants in autumn increased aggression, but not to levels higher than in the spring (Wingfield 1994a). Also, in captive Eastern Song Sparrows M. m. melodia, birds under short day (winter) conditions took longer to sing in response to T implants than long day subjects (Nowicki & Ball 1989). This suggests decreased sensitivity to T in the winter. Finally, neurobiological studies of other species suggest nonbreeding birds have fewer androgen receptors (Soma et al. 1997), fewer estrogen receptors (Gahr & Metzdorf 1997), and less hypothalamic aromatase (Silverin & Deviche 1991). All these data suggest that nonbreeding birds are less, not more, sensitive to T.
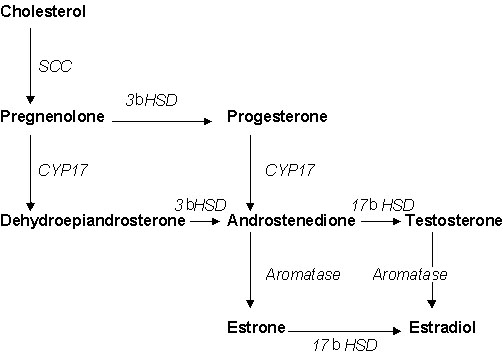
The second hypothesis states that the brain makes its own T and E2 de novo from cholesterol (‘neurosteroids,’ Robel & Baulieu 1995) in the nonbreeding season. This local synthesis would provide high T and E2 levels within the appropriate brain regions, without greatly increasing T and E2 levels in the general circulation. This hypothesis is possible, but the data currently available give mixed support. In favour of this hypothesis, there is evidence that the avian brain can create several neurosteroids, such as pregnenolone and progesterone (Fig. 3) (Tsutsui & Yamakazi 1995; Vanson et al. 1996). However, studies in mammals and birds generally indicate that the brain cannot make T de novo (Robel & Baulieu 1995; Lane et al. 1996, but see Mensah-Nyagan et al. 1996). To produce T from cholesterol, the enzyme cytochrome P450 17alpha-hydroxylase/C17-20 lyase (CYP17) is critical (Fig. 3), and this enzyme has not been found in the brain in most cases, including in adult songbirds (Lane et al. 1996). It is unknown whether nonbreeding Song Sparrows contain CYP17 in the brain.
The third hypothesis states that a peripheral organ produces a sex steroid precursor, which travels to the brain via the blood, and is then converted to T and E2 within the brain (‘intracrinology’) (Labrie et al. 1995). Potential sex steroid precursors include androstendione (AE) and dehydroepiandrosterone (DHEA) (Fig. 3). Plasma AE has been measured in several avian species, and is generally lower than plasma T (e.g., Ramenofsky 1984; Schlinger & Arnold 1992). For this reason, DHEA, and its sulphated form DHEA-S, are more likely candidates. Plasma DHEA and DHEA-S have been detected in breeding male quail Coturnix japonica (Tsutsui & Yamakazi 1995). In several primate species, the adrenals secrete DHEA and DHEA-S into the blood (Labrie 1993). The enzyme CYP17, which is critical for DHEA production (Fig. 3), appears to be present in the avian adrenals (Nakamura et al. 1978; Lane et al. 1996), and the songbird brain may be able to convert plasma DHEA into active androgens and estrogens (Vanson et al. 1996). It is important to note that DHEA itself is generally thought to be biologically inert, unless target cells have the necessary enzymes to convert DHEA into active steroids (Labrie 1995). Thus, this third hypothesis is supported by several forms of indirect evidence.
TESTS OF HYPOTHESES
These hypotheses are testable using current neurobiological, endocrine and molecular techniques. Again, note that these hypotheses are not mutually exclusive. To test the first hypothesis, it is possible to measure seasonal changes in brain androgen receptors (AR), estrogen receptors (ER), or aromatase in Song Sparrows using immunocytochemical or molecular probes (e.g., Soma et al. 1997). Aromatase can also be quantified with an in vitro product formation assay that measures the conversion of 3H-T to 3H-E2 by brain homogenates (Schlinger et al. 1992). If AR, ER or aromatase in relevant brain regions increase in nonbreeding birds, that would support the first hypothesis.
The second hypothesis can be tested by looking for CYP17 in the brain. Conversion of 3H-pregnenolone to 3H-DHEA (or 3H-progesterone to 3H-androstendione) (Fig. 3) by brain homogenates would support the second hypothesis. Furthermore, molecular probes to avian CYP17 mRNA are now available (Abinawanto et al. 1996). These probes can be used to look for CYP17 expression in the brains of nonbreeding Song Sparrows.
The third hypothesis can be tested by looking for CYP17 in peripheral organs and by measuring plasma DHEA (and DHEA-S). If the adrenals are secreting DHEA, they should express CYP17 mRNA, and plasma DHEA levels should be elevated in nonbreeding birds. If only the brain expresses CYP17 (second hypothesis), then plasma DHEA should be low or nondetectable.
HORMONES AND WINTER TERRITORIES IN OTHER BIRDS
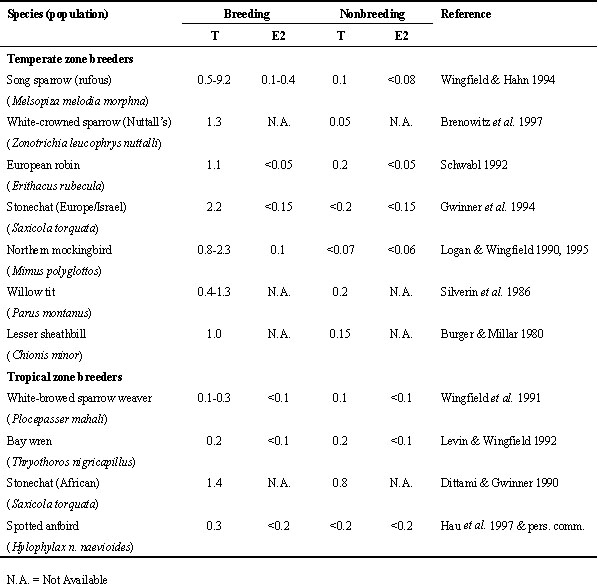
Are the mechanisms underlying nonbreeding territoriality in male Song Sparrows similar in other species? Plasma hormones have been measured in several avian species which are territorial in the breeding and nonbreeding seasons (Table 1), and the data allow at least 2 generalisations. First, plasma T and E2 are generally low in nonbreeding adult males that defend winter territories. Second, plasma T and E2 are higher during the breeding season in birds that breed in the temperate zone. In contrast, birds that breed in the tropics have low plasma sex steroids throughout the year. One possible exception to these generalisations is the African Stonechat Saxicola torquata (Dittami & Gwinner 1990).
Another factor to consider when making comparisons across species is whether the nonbreeding territory is ‘sexual’ in context, or solely related to feeding (Wingfield et al. 1997). That is, if the winter territory can become the breeding territory, or if future mates overwinter together, plasma sex steroids may show transient elevations in winter (Wingfield et al. 1997). However, determining whether nonbreeding territories are ‘sexual’ in context is not always straightforward. For example, even if a male and female jointly defend a winter territory, they may not breed together (Wingfield & Monk 1992; Gwinner et al. 1994). And even in sedentary birds, the winter and spring territories do not always overlap (Wingfield & Monk 1992).
In several species, females also defend winter territories. For example, in European Robins, females have basal plasma T during breeding, but elevated plasma T during winter, when they defend individual feeding territories (Schwabl 1992). This peak of plasma T may support winter territoriality in females (Kriner & Schwabl 1991). This suggests a sex difference because males have low plasma T in winter (Schwabl 1992). In other species, however, females that defend winter territories do not have increased plasma T (Wingfield et al. 1991; Levin & Wingfield 1992; Gwinner et al. 1994). The main point here is that females can also display territorial aggression in the nonbreeding season, and endocrine mechanisms may differ between the sexes (Elekonich 1997).
In general, there is still too little data to assess whether common physiological mechanisms underlie nonbreeding territoriality in different animals. In most cases, only plasma hormones have been measured. Among birds, endocrine manipulations have only been performed in Song Sparrows and European Robins (Schwabl & Kriner 1991; Soma et al. 1998).
HORMONES AND DOMINANCE HIERARCHIES IN WINTER FLOCKS
Aggressive behaviours used for territorial defence often resemble behaviours used for establishing and maintaining dominance hierarchies in winter flocks. In many cases, the postures and vocalisations are outwardly similar (e.g., wing flutters, beak gapes, calls, chases). This similarity is particularly clear when different populations within a single species vary in winter social organisation. For example, some Song Sparrow populations form dominance hierarchies in loose winter associations (10 to 20 juveniles) (Knapton & Krebs 1976). Rather than viewing territoriality and flocking as dichotomous, it may be better to consider them as the ends of a behavioural continuum (Pulliam & Millikan 1982).
The endocrine regulation of dominance status in winter flocks has been examined in a variety of species. In general, plasma T is not correlated with social status (Rohwer & Wingfield 1981; Holberton et al. 1989; Ramenofsky et al. 1992). In fact, sometimes the subordinate individuals (females) had higher plasma T (e.g., Ramenofsky et al. 1992). These data suggest that plasma T does not promote high status. However, T treatment can increase the status of low-ranking birds, especially if plumage signals of status are simultaneously manipulated (Rohwer & Rohwer 1978; Baptista et al. 1987). These apparently contradictory results resemble the initial studies on winter territoriality in Song Sparrows described above.
How the physiology of dominance hierarchies in winter flocks is similar to and different from the physiology of winter territoriality remains to be seen. As a first step, it will be informative to treat dominant individuals in winter flocks with aromatase inhibitors and androgen receptor antagonists. If these treatments cause a decrease in status, this would suggest winter dominance is regulated by endogenous sex steroids.
ECOLOGY OF WINTER TERRITORIALITY AND FLOCKING
The function of winter territoriality in Northwestern Song Sparrows is not entirely clear. Several ecological factors have been hypothesised to favour territoriality over flocking in the nonbreeding season (Table 2). In the case of these Song Sparrows, winter territoriality is probably most related to benefits in the future breeding season and to food.
Northwestern Song Sparrows are sedentary, and thus winter territories can become breeding territories. If a shortage of good habitat for territories exists, it may be advantageous to secure a territory for breeding well in advance. Territory holders generally have a strong advantage in territory disputes (‘prior residency effect’) (Arcese 1987). However, Song Sparrow males often move in the spring to establish breeding territories in a different location (generally <1 km away) (Wingfield & Monk 1992), which suggests that this is not the only factor.
Song Sparrows may also choose future mates in autumn. If females prefer territory holders to ‘floaters,’ this would favour winter territoriality in males (Smith & Arcese 1989). This factor may have some role in Song Sparrows, but again, it cannot be the sole explanation for winter territoriality, because females and males on winter territories do not always breed together in the following spring (Wingfield & Monk 1992, 1994). Furthermore, some winter territories are defended by individuals, same-sex pairs, or ‘alliances’ of 3 to 5 birds (Wingfield & Monk 1992).
Food may be another important factor in Song Sparrow winter territoriality (Smith et al. 1980; Arcese 1989). Territoriality is positively correlated with an even distribution of food in the habitat and high predictability of food availability (Orians 1971). Territoriality is also positively correlated with a narrow diet and foraging which requires skill (Crook 1965). In western Washington State, where winters are relatively mild and snow is uncommon, we hypothesise that winter food for Song Sparrows is not patchy and food availability is highly predictable. Furthermore, mild temperatures may allow a relatively high arthropod abundance in winter. Foraging for arthropods could, in turn, favour social organisations which reduce interference from conspecifics (Davies 1976). To test these hypotheses, current studies are measuring temperature, snowfall, arthropod abundance (using sweep nets), and Song Sparrow diets (using stomach contents) in winter. Experimental manipulations of food distribution and abundance may also affect social organisation (Zahavi 1970, but see Smith et al. 1980).
Most likely, no single factor will explain why some Song Sparrows are territorial in the winter, while others form flocks. The entire combination of ecological factors (Table 2) for a given population will determine whether winter territoriality is an advantageous strategy or not (Brown 1964). Some have also argued that winter territoriality is a non-adaptive carryover from the breeding season (e.g., Hamilton 1959). However, this seems highly unlikely in this particular case, because some Song Sparrows are not territorial in the winter. Comparisons between territorial and flocking Song Sparrow populations should reveal the most important ecological factors and generate specific, testable hypotheses.
ACKNOWLEDGMENTS
Kim Sullivan and Anthony Tramontin provided comments on the manuscript and were invaluable collaborators in field experiments involving androgen receptor antagonist and aromatase inhibitor treatments. Tom Hahn assisted in collection of behavioural and hormonal data presented in Fig. 1 and Fig. 2. Lynn Erckmann provided assistance with radioimmunoassays. Kiran Soma is a Howard Hughes Medical Institute predoctoral fellow. The National Science Foundation (IBN-9408013 to John Wingfield) supported many of the studies reported here.
REFERENCES
Abinawanto, Shimada, K., Yoshida, K. & Saito, N. 1996. Effects of aromatase inhibitor on sexual differentiation and levels of P45017alpha and P450arom messenger ribonucleic acid of gonads in chicken embryos. General and Comparative Endocrinology 102: 241-246.
Arcese, P. 1987. Age, intrusion pressure and defense against floaters by territorial male song sparrows. Animal Behaviour 35: 773-784.
Arcese, P. 1989. Intrasexual competition and the mating system in primarily monogamous birds: the case of the song sparrow. Animal Behaviour 38: 96-111.
Baptista, L.F., DeWolfe, B.B. & Avery-Beausoleil, L. 1987. Testosterone, aggression, and dominance in Gambel’s White-crowned Sparrows. Wilson Bulletin 99: 86-91.
Brenowitz, E., Baptista, L., Lent, K. & Wingfield, J.C. 1997. Seasonal plasticity of the song control system in wild Nuttall’s white-crowned sparrows. Journal of Neurobiology 33: 69-82.
Brown, C.R. & Brown, M.B. 1986. Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67: 1206-1218.
Brown, J.L. 1964. The evolution of diversity in avian territorial systems. Wilson Bulletin 76: 160-169.
Brown, J.L. & Orians, G.H. 1970. Spacing patterns in mobile animals. Annual Review of Ecology and Systematics 1: 239-262.
Burger, A.E. & Millar, R.P. 1980. Seasonal changes of sexual and territorial behaviour and plasma testosterone levels in male Lesser Sheathbills (Chionis minor). Zeitschrift fur Tierpsychologie 52: 397-406.
Caldwell, G.S., Glickman, S.E. & Smith, E.R. 1984. Seasonal aggression independent of seasonal testosterone in wood rats. Proceedings of the National Academy of Sciences USA 81: 5255-5257.
Caraco, T., Martindale, S. & Pulliam, H.R. 1980. Avian flocking in the presence of a predator. Nature 285: 400-401.
Crook, J.H. 1965. The adaptive significance of avian social organizations. Symp. Zool. Soc. London 14: 181-218.
Cuadrado, M. 1997. Why are migrant Robins (Erithacus rubecula) territorial in winter?: the importance of the anti-predator behaviour. Ethology, Ecology and Evolution 9: 77-88.
Crews, D. 1991. Trans-seasonal action of androgen in the control of spring courtship behavior in male red-sided garter snakes. Proceedings of the National Academy of Sciences USA 88: 3545-3548.
Davies, N.B. Food, flocking and territorial behaviour of the pied wagtail (Motacilla alba yarrelli Gould) in winter. Journal of Animal Ecology 45: 235-254.
Dittami, J.P. & Gwinner, E. 1990. Endocrine correlates of seasonal reproduction and territorial behavior in some tropical passerines. In: Wada, M. et al. (eds) Endocrinology of birds: molecular to behavioral. Berlin; Springer-Verlag: 225-233.
Elekonich, M. 1997. Female-female territorial aggression and its hormonal control in the song sparrow. PhD Thesis, University of Washington, Seattle, USA.
Foidart, A., Tlemcani, O., Harada, N., Abe-Dohmae, S. & Balthazart, J. 1995. Pre- and post-translational regulation of aromatase by steroidal and non-steroidal aromatase inhibitors. Brain Research 701: 267-278.
Gahr, M. & Metzdorf, R. 1997. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Research Bulletin 44: 509-517.
Goss-Custard, J.D. 1976. Variation in the dispersion of redshank (Tringa totanus) on their winter feeding grounds. Ibis 118: 257-263.
Greenberg, R. 1983. Competition in migrant birds in the nonbreeding season. In: Johnston, R. (ed) Current Ornithology. Vol. 3; New York; Plenum Press: 281-307.
Gwinner, E., R÷dl, T. & Schwabl, H. 1994. Pair territoriality of wintering stonechats: behaviour, function and hormones. Behavioral Ecology and Sociobiology 34: 321-327.
Hamilton, W. 1959. Aggressive behavior in migrant Pectoral Sandpipers. Condor 61: 161-179.
Hau, M., Wikelski, M. & Wingfield, J.C. 1998. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proceedings of the Royal Society London B 265: 89-95.
Holberton, R.L., Able, K.P. & Wingfield, J.C. 1989. Status signalling in dark-eyed juncos, Junco hyemalis: plumage manipulations and hormonal correlates of dominance. Animal Behaviour 37: 681-689.
Knapton, R.W. & Krebs, J.R. 1976. Dominance hierarchies in winter Song Sparrows. Condor 78: 567-569.
Kriner, E. & Schwabl, H. 1991. Control of winter song and territorial aggression of female robins (Erithacus rubecula) by testosterone. Ethology 87: 37-44.
Labrie, F. 1993. Mechanism of action and pure antiandrogenic properties of flutamide. Cancer (supplement) 72: 3816-3827.
Labrie, F., Belanger, A., Simard, J., Luu-the, V. & Labrie, C. 1995. DHEA and peripheral androgen and estrogen formation: intracrinology. Annals of the New York Academy of Sciences 774: 16-28.
Lane, N., Grisham, W., Brown, S., Thompson, L., Arnold, A. & Schlinger, B. 1996. Plasma testosterone and tissue 17alpha-hydroxylase activity in castrated and fadrozole-treated male zebra finches. Society for Neuroscience Abstracts 22: 156.
Levin, R.N. & Wingfield, J.C. 1992. The hormonal control of territorial aggression in tropical birds. Ornis Scandinavica 23: 284-291.
Logan, C.A. 1992. Testosterone and reproductive adaptations in the autumnal territoriality of Northern Mockingbirds Mimus polyglottos. Ornis Scandinavica 23: 277-283.
Logan, C.A. & Wingfield, J.C. 1990. Autumnal territorial aggression is independent of plasma testosterone in mockingbirds. Hormones and Behavior 24: 568-581.
Logan, C.A. & Wingfield, J.C. 1995. Hormonal correlates of breeding status, nest construction, and parental care in multiple-brooded Northern Mockingbirds, Mimus polyglottos. Hormones and Behavior 29: 12-30.
Matthysen, E. 1990. Nonbreeding social organization in Parus. In: Power, D. (ed) Current Ornithology. Vol. 7; New York; Plenum Press: 209-249.
Mensah-Nyagan, A.G., Feuilloley, M., Do-Rego, J.L., Marcual, A., Lange, C., Tonon, M.C., Pelletier, G. & Vaudry, H. 1996. Localization of 17▀-hydroxysteroid dehydrogenase and characterization of testosterone in the brain of the male frog. Proceedings of the National Academy of Sciences USA 93: 1423-1428.
Moore, M.C. & Marler, C.A. 1987. Effects of testosterone manipulations on nonbreeding season territorial aggression in free-living male lizards, Sceloporous jarrovi. General and Comparative Endocrinology 65: 225-232.
Myers, J.P., Connors, P.G. & Pitelka, F.A. 1979. Territoriality in non-breeding shorebirds. Studies in Avian Biology 2: 231-246.
Nakamura, T., Tanabe, Y. & Hirano, H. 1978. Evidence for the in vitro formation of cortisol by the adrenal gland of embryonic and young chickens (Gallus domesticus). General and Comparative Endocrinology 35: 302-308.
Nice, M.M. 1943. Studies in the life history of the song sparrow II. The behavior of the song sparrow and other passerines. Transactions of the Linnaean Society of New York 6: 1-388.
Nowicki, S. & Ball, G.F. 1989. Testosterone induction of song in photosensitive and photorefractory male sparrows. Hormones and Behavior 23: 514-525.
Orians, G. 1971. Ecological aspects of behavior. In: Farner, D.S. & King, J.R. (eds) Avian Biology. Vol. 1; New York; Academic Press: 513-546.
Pulliam, H.R. & Millikan, G.C. 1982. Social organization in the nonreproductive season. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology. Vol. 6; New York; Academic Press: 169-197.
Ramenofsky, M. 1984. Agonistic behavior and endogenous plasma hormones in male Japanese quail. Animal Behaviour 32: 698-708.
Ramenofsky, M., Gray, J.M. & Johnson, R.B. 1992. Behavioral and physiological adjustments of birds living in winter flocks. Ornis Scandinavica 23: 371-380.
Robel, P. & Baulieu, E. 1995. Dehydroepiandrosterone (DHEA) is a neuroactive neurosteroid. Annals of the New York Academy of Sciences 774: 82-110.
Rohwer, S. & Rohwer, F.C. 1978. Status signalling in Harris sparrows: experimental deceptions achieved. Animal Behaviour 26: 1012-1022.
Rohwer, S. & Wingfield, J. C. 1981. A field study of social dominance, plasma levels of luteinizing hormone and steroid hormones in wintering Harris’ Sparrows. Zeitschrift fur Tierpsychologie 57: 173-183.
Schlinger, B.A. & Arnold, A.P. 1992. Plasma sex steroids and tissue aromatization in hatchling zebra finches: implications for the sexual differentiation of singing behavior. Endocrinology 130: 289-299.
Schlinger, B.A. & Callard, G.V. 1990. Aromatization mediates aggressive behavior in quail. General and Comparative Endocrinology 79: 39-53.
Schlinger, B.A., Slotow, R.H. & Arnold, A.P. 1992. Plasma estrogens and brain aromatase in winter White-crowned sparrows. Ornis Scandinavica 23: 292-297.
Schwabl, H. 1992. Winter and breeding territorial behaviour and levels of reproductive hormones of migratory European Robins. Ornis Scandinavica 23: 271-276.
Schwabl, H. 1993. Yolk is a source of maternal testosterone for developing birds. Proceedings of the National Academy of Sciences USA 90: 11446-11450.
Schwabl, H. & Kriner, E. 1991. Territorial aggression and song of male European robins (Erithacus rubecula) in autumn and spring: effects of antiandrogen treatment. Hormones and Behavior 25: 180-194.
Silverin, B. & Deviche, P. 1991. Biochemical characterization and seasonal changes in the concentration of testosterone-metabolizing enzymes in the European great tit (Parus major) brain. General and Comparative Endocrinology 81: 146-159.
Silverin, B., Viebke, P. & Westin, J. 1986. Seasonal changes in plasma levels of LH and gonadal steroids in free-living Willow Tits Parus montanus. Ornis Scandinavica 17: 230-236.
Smith, J.N.M. & Arcese, P. 1989. How fit are floaters? Consequences of alternative territorial behaviors in a nonmigratory song sparrow. American Naturalist 133: 830-845.
Smith, J.N.M., Montgomerie, R.D., Taitt, M.J. & Yom-Tov, Y. 1980. A winter feeding experiment on an island Song Sparrows population. Oecologia 47: 164-170.
Soma, K.K., Hartman, V., Brenowitz, E.A. & Wingfield, J.C. 1997. Seasonal plasticity of the avian song nucleus HVc as indicated by androgen receptor immunocytochemistry in a wild songbird. Society for Neuroscience Abstracts 23: 1328.
Soma, K.K., Tramontin, A.D. & Wingfield, J.C. 1998. Aromatase regulates song and aggression in a wild non-breeding bird. Society for Neuroscience Abstracts: in press.
Tsutsui, K. & Yamakazi, T. 1995. Avian neurosteroids. I. Pregnenolone synthesis in the quail brain. Brain Research 678: 1-9.
Vanson, A., Arnold, A.P. & Schlinger, B.A. 1996. 3▀-hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: dehydroepiandrosterone as substrate for synthesis of androstendione and estrogens. General and Comparative Endocrinology 102: 342-350.
Wade, J., Schlinger, B.A., Hodges, L. & Arnold, A.P. 1994. Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. General and Comparative Endocrinology 94: 53-61.
Wingfield, J.C. 1985. Short-term changes in plasma levels of hormones during establishment and defense of a breeding territory in male song sparrows, Melospiza melodia. Hormones and Behavior 19: 174-187.
Wingfield, J.C. 1994a. Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Hormones and Behavior 28: 1-15.
Wingfield, J.C. 1994b. Control of territorial aggression in a changing environment. Psychoneuroendocrinology 19: 709-721.
Wingfield, J.C., Follett, B.K., Matt, K.S. & Farner, D.S. 1980. Effect of day length on plasma FSH and LH in castrated and intact white-crowned sparrows. General and Comparative Endocrinology 42: 464-470.
Wingfield, J.C. & Hahn, T.P. 1994. Testosterone and territorial behaviour in sedentary and migratory sparrows. Animal Behaviour 47: 77-89.
Wingfield, J.C., Hegner, R.E., Dufty, A.M.Jr. & Ball, G.F. 1990. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist 136: 829-846.
Wingfield, J.C., Hegner, R.E. & Lewis, D.M. 1991. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Journal of Zoology, London 225: 43-58.
Wingfield, J.C., Jacobs, J. & Hillgarth, N. 1997. Ecological constraints and the evolution of hormone-behavior interrelationships. In: Carter, C.S., Lederhendler, I.I. & Kirkpatrick, B. (eds) The integrative neurobiology of affiliation. Vol. 807; New York; Annals of the New York Academy of Sciences: 22-41.
Wingfield, J.C. & Monk, D. 1992. Control and context of year-round territorial aggression in the non-migratory Song Sparrow Zonotrichia melodia morphna. Ornis Scandinavica 23: 298-303.
Wingfield, J.C. & Monk, D. 1994. Behavioral and hormonal responses of male song sparrows to estradiol-treated females during the non-breeding season. Hormones and Behavior 28: 146-154.
Wingfield, J.C. &Ramenofsky, M. 1985. Testosterone and aggressive behaviour during the reproductive cycle of male birds. In: Gilles, R. & Balthazart, J. (eds) Neurobiology. Berlin; Springer-Verlag: 92-104.
Zahavi, A. The social behaviour of the White Wagtail Motacilla alba alba wintering in Israel. Ibis 113: 203-211.
Table 1. Plasma levels (ng ml-1) of testosterone (T) and 17▀-estradiol (E2) in wild adult males that defend territories year-round.

Table 2. Factors hypothesized to favour territoriality over flocking in the nonbreeding season.
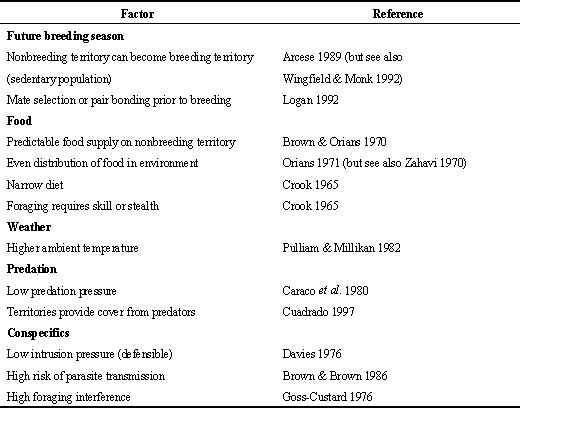
Fig. 1. Territorial behaviour of wild male Song Sparrows in the breeding season (spring) and nonbreeding season (autumn). Males were challenged with a simulated territorial intrusion (live decoy and song playback) for 10 min. Rapid flights, singing, close approaches, and time spent near the decoy are typical aggressive responses in this species (Nice 1943). Territorial responses (mean▒SE) are not different between seasons (P>0.50 in all 4 cases). Sample sizes are in parentheses. Redrawn from data in Wingfield & Hahn (1994).
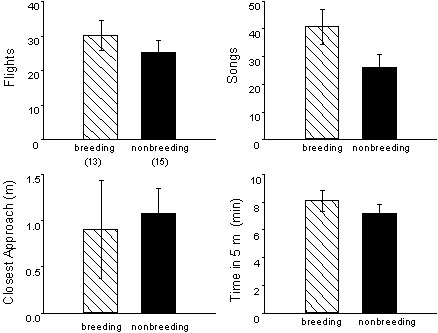
Fig. 2. Plasma hormone levels (mean▒SE) of wild male Song Sparrows in the breeding season (March to July) and nonbreeding season (August to February). Blood samples were collected within 10 min of capture and hormones were measured using radioimmunoassay. Plasma sex steroids (T, E2, DHT) are low or nondetectable during the nonbreeding season, and levels increase during the breeding season (P<0.05 in all 3 cases). Plasma corticosterone, in contrast, remains detectable in the nonbreeding season. Note the Y-axes have different scales and the X-axis is divided by stage in the breeding season. Sample size is approximately 6 at each time point (for details, see Wingfield & Hahn 1994). Samples with nondetectable hormone levels were assigned the assay detection limit. Plasma T data redrawn from Wingfield & Hahn (1994).
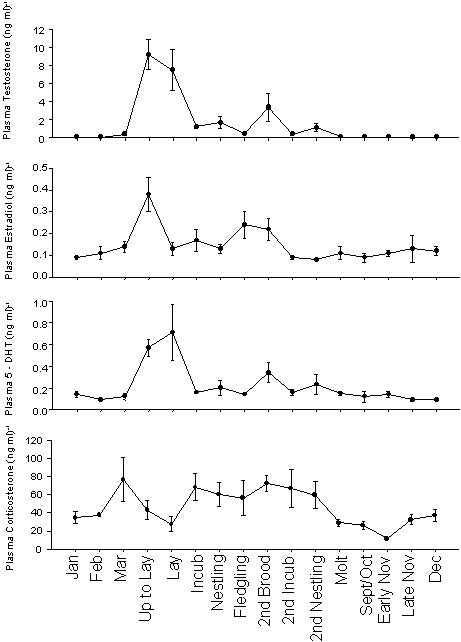
Fig. 3. Simplified diagram of the production of sex steroid hormones from cholesterol. Hormones are in bold, and enzymes that catalyze conversions are in italics. SCC=cytochrome P450 side chain cleavage, CYP17=cytochrome P450 17alpha-hydroxylase/C17-20 lyase, 3▀HSD=3▀-hydroxysteroid dehydrogenase/isomerase, 17▀HSD=17▀-hydroxysteroid dehydrogenase. After Vanson et al. (1996).