S25.3: Fat and protein utilisation during migratory flight
Lukas Jenni & Susanne Jenni-Eiermann
Swiss Ornithological Institute, CH-6204 Sempach, Switzerland, f
ax 41 41 462 97 10, e-mail jennil@orninst.ch Jenni, L. & Jenni-Eiermann, S. 1999. Fat and protein utilisation during migratory flight. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1437-1449. Johannesburg: BirdLife South Africa.Non-stop endurance flapping flight in migrant birds may be limited by the amount of stored fuel or water that they carry. To maximise flight duration, most of the energy is derived from lipid stores. In order to adequately supply the active muscles with adipose tissue derived lipids during endurance exercise, small passerines utilise a specific metabolic pathway, and may have other unique means of fatty acid delivery. However, as in inactive birds during long-term fasting, flying birds also catabolise protein, which may also limit flight duration. Since amino acids have no special storage form (such as glycogen for carbohydrates and triglycerides for fatty acids), but serve specific functions, protein utilisation results in some functional loss. The few available data suggest that the relative contribution of energy derived from protein as a proportion of the total energy expenditure of birds during endurance flight is as low as that of inactive fasting birds. This means that the absolute amount of protein catabolism is elevated during flight, possibly in proportion to metabolic rate. The level of protein utilisation attained is negatively related to initial fat stores. These findings suggest that the ratio of fat to protein stored before, and used during endurance flight is important for maximising flight duration and should be included in models of optimal bird migration. Predictions are given about the ratio of fat to protein catabolised during flight as a function of the duration of non-stop flight and the risk of dehydration.
INTRODUCTION
Migratory birds are adapted to exploit several geographically separated habitats during their annual cycle which in some species takes place on a worldwide scale. In order to reach distant localities within the limited time provided by the other annual events, migrants must cover large distances rapidly. For instance, birds exploiting both the arctic and subantarctic summers (e.g., Arctic Tern Sterna arctica, Cramp 1985; some Red Knot Calidris canutus populations, Piersma & Davidson 1992) must reach the other side of the globe within 2-3 months. This demands a mean travel speed of several hundred kilometers per day. As a most extreme example, Bar-tailed Godwits Limosa lapponica baueri fly from New Zealand to their East-Siberian and Alaskan breeding grounds (a great circle distance of 11000 km). Some cover the distance between Australia and China in a non-stop trans-Pacific flight of at least 8000 km (Barter in Piersma & Gill 1998), a flight duration of more than 100 h (depending on wind assistance).
This suggests that the ecological options of a migratory bird, i.e., the ability to exploit widely separated places within the annual cycle, critically depend on the ability of efficient endurance locomotion. Compared with other activities in a bird’s life and with endurance locomotion performances by other animal taxa, migratory endurance flight is outstanding in at least three respects. First, flight is a rapid form of locomotion with specific costs of transport per kilometer travelled that are lower than those of walking or running (Schmidt-Nielsen 1997). Nevertheless, even when flying at the speed of minimum energy expenditure, the metabolic rate of small passerines is twice the maximum rate of exercising small mammals (Butler & Woakes 1990). Therefore, during endurance flight birds have rates of energy consumption among the highest in the animal kingdom. Second, most birds do not forage or drink during flight and, hence, must rely exclusively on body energy stores and on body and metabolic water. Third, endurance locomotion of migrant birds can be of extremely long duration (example see above).
In the light of the above, an efficient fuel budget is crucial, and adaptations for endurance flight influence which sites may be selected for wintering or breeding. Until recently, most attention was paid to energy and water as the limiting factors of flight range and migratory performance. Several authors have estimated maximum ranges in relation to energy stores and water balance (e.g., Biebach 1992; Carmi et al. 1992; Klaassen 1995, 1996; Weber & Houston 1997). However, the types of fuel and mechanisms of fuel delivery from stores to active tissues was largely neglected (Weber 1988, 1992; Klaassen 1996). Yet, the type of fuel used during flight can affect endurance performance and different types of fuel impose different metabolic constraints in supply and oxidation that, in turn, limit the flexibility of the energy budget.
It follows that the physiological and biochemical mechanisms and constraints operating in the utilisation of the different fuel types are important for the understanding of ecological and behavioural aspects of migrants and, ultimately, for the understanding of the ecological options open to migrants. The aim of this paper is to review (1) the metabolic constraints on use of different fuel types during flight, and (2) mechanisms of efficient fuel use and their ecological significance.
TYPES OF FUEL: THEIR STORAGE, SUPPLY AND OXIDATION DURING ENDURANCE FLIGHT
One of the most important ‘decisions’ of a migrating bird is to select the types and proportions of fuels used during flight. Below, we evaluate the three main types of oxidative fuels, carbohydrates, lipids and proteins, with regard to their storage, energy density, supply to muscles, and oxidation during endurance exercise.
Carbohydrates
Carbohydrates are stored mainly in the form of glycogen in the liver and skeletal muscles. The total amount of glycogen stored by birds preparing for migration is small (e.g., Farner et al. 1961; Dawson et al. 1983; Marsh 1983) and is not quantitatively a major fuel source for endurance flight. The energy density of glycogen is comparatively low, because of the high proportion of bound water (Table 1).
Glycogen can be readily mobilised at the onset of flight. It is suggested that at the onset of flight, glycogen contributes substantially to energy expenditure before other fuels are fully available (Rothe et al. 1987; Schwilch et al. 1996). Bursts of flight are mainly fuelled by anaerobic catabolism of glycogen (e.g., George & Berger 1966; Rothe et al. 1987). Therefore, a migrant should conserve some glycogen because the need for a burst of flight may arise during flight or after landing at a stopover site (e.g., predator escape, prey catching). Hence, glycogen may play a role at the very beginning of flight, possibly during flight for predator escape, and after landing at stopover sites for escape and foraging, but should be spared during endurance flight.
Lipids
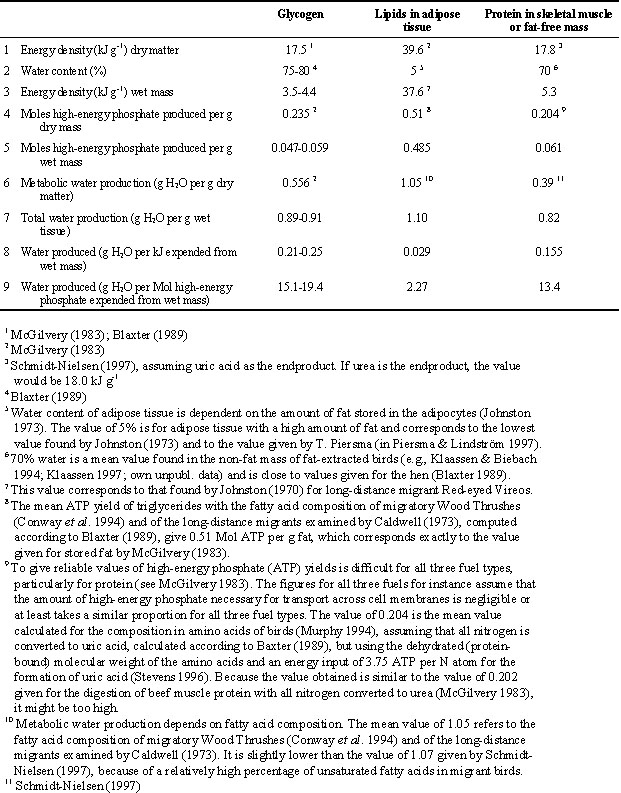
Fat is the main fuel stored for long-distance flight and lipids are sequestered mainly as triglycerides in adipose tissues, but also in small quantities in muscle and other organs. The amount of fat stored may equal or slightly exceed lean wet body mass (e.g., Piersma & Gill 1998). The energy density of stored lipids is more than seven times higher than that of glycogen and protein (Table 1). In terms of high-energy phosphate (e.g., ATP) produced, fat from adipose tissue yields eight times more chemical energy than wet protein, and 8.2 – 10.3 times more than glycogen (Table 1). This is chiefly because fat stored in adipose tissue contains only about 5% water (Piersma & Lindström 1997), compared with 70% or more for muscle tissue or stored glycogen. Another advantage of adipose tissue is that it is comparatively cheap to maintain (e.g., Scott & Evans 1992).
Extra-muscular lipids are not readily available at the onset of flight. In homing pigeons, fatty acids reach a steady-state contribution to energy expenditure after about 1 - 2 h of flight (Rothe et al. 1987; Schwilch et al. 1996). Once fatty acid delivery is in full swing, its supply is constrained not only by membrane transport, but also by its insolubility in plasma and by perfusion limitations in adipose tissue (Weber 1988). For these reasons, mammals, for instance, are unable to use a high proportion of fatty acids during moderate endurance exercise (Weber 1988, 1992; Vock et al. 1996). During high endurance exercise in humans, the relative contribution of energy derived from fat per total energy expenditure is even lower (Romijn et al. 1993, 1995), and marathon runners draw no more than 40 - 50% of their energy from lipids (Callow et al. 1986).
Lipids can be used as an energy substrate by most tissues. However, the central nervous system cannot oxidise fatty acids. During fasting, ketone bodies produced from fatty acids can substitute part of the glucose needs, the remainder being supplied by glycogen or via gluconeogenesis in the form of amino acids or glycerol. However, glycerol supplies less than 20% of the glucose precursors in exercising mammals (Shaw et al. 1975; Weber et al. 1993).
Fatty acids are oxidised in the citric acid cycle. Intermediates of the citric acid cycle are constantly drained away and need to be replaced (Lee & Davis 1979; Dohm 1986; Sahlin et al. 1990). This anaplerotic flux is fed from carbohydrates or certain amino acids (Dohm 1986). Therefore, the oxidation of fatty acids requires a certain quantity of protein in migrating birds.
The oxidation of 1 g of lipid from adipose tissue produces slightly more water than the oxidation of 1 g of wet protein or glycogen (Table 1). However, if one considers these quantities in relation to the energy produced, lipids yield 5.3 times less water per unit energy produced than protein and 7.2 - 8.6 times less than glycogen. In terms of ATP produced, lipid catabolism produces 5.9 times less water than protein and 6.6 - 8.5 times less water than glycogen oxidation (Table 1). Therefore, theoretically net water loss during endurance flight may be altered by changing the proportions of fuel types used, but whether this occurs in birds has not been studied so far.
Proteins
Unlike fats and glycogen, proteins have no special storage form. Apart from a small free amino acid pool, all proteins serve specific functions in the body. Therefore, an appreciable amount of protein catabolism inevitably results in functional loss. Proteins can be catabolised to amino acids and used as gluconeogenic precursors or transformed into intermediates of the citric acid cycle. One product of amino acid catabolism is the highly toxic ammonia which requires removal or incorporation into less toxic compounds.
There are now a number of studies demonstrating protein catabolism during endurance flight (Piersma & Jukema 1990; Jenni-Eiermann & Jenni 1991; Åkesson et al. 1992; Gauthier et al. 1992). Protein can be catabolised from any tissue including the working muscles. For example, endurance flight results in a decrease of flight muscle lean mass (essentially protein), other muscles and digestive organs (Piersma & Jukema 1990).
Energy consumption for maintaining skeletal muscle is an order of magnitude higher than that of adipose tissue (Scott & Evans 1992). Maintenance costs for liver and digestive tract tissue are even higher (ca. four times that of skeletal muscle) (e.g., Field et al. 1939; Scott & Evans 1992). Therefore, transporting these proteinous tissues over long distances incurs not only high costs of transport, but also high costs of maintenance. In migrants with extremely long non-stop flights, a reduction in metabolically costly tissues (liver and gut) was observed even prior to the onset of flight (Piersma & Gill 1998; Piersma 1998).
Conclusions
Lipids are believed to be the best fuel to be stored for migratory flight in birds, primarily because of their high energy density. However, as outlined above, lipids as a fuel also have a number of disadvantages: (a) lipids from adipose tissue are not readily available at the onset of flight; (b) fatty acid delivery to the working muscles is constrained; (c) fatty acids cannot be used as a fuel for all organs; (d) the oxidation of fatty acids requires amino acids to maintain citric acid cycle intermediates; (e) oxidation of lipids might incur a higher net water loss during flight than oxidation of the other two fuel types.
In mammals, limitations on mobilisation and delivery of fatty acids to the working muscles are believed to be the reason for the concurrent use of all three fuel types during exercise (Weber 1988). However, birds must give priority to minimise the extra mass of energy stores to be carried on migration, because a high load reduces the travel range to a much greater extent than in walking or swimming animals (Pennycuick 1989; Schmidt-Nielsen 1997). Only lipids have an energy density high enough to enable the long non-stop flights of birds. Indeed, despite the problems of lipid utilisation outlined above, birds during endurance flight are able to provide more than 90% of the energy from lipids (see below) while working at twice the maximum aerobic rate of small mammals. It thus appears that birds have developed special mechanisms to overcome the constraints of fatty acid supply found in mammals. These adaptations will be examined in the following section.
METABOLIC ADAPTATIONS TO ENDURANCE FLIGHT IN MIGRANTS
Lipid supply
In order to be released into the blood, triglycerides stored in adipocytes need to be hydrolysed into free fatty acids and glycerol. The rate of lipolysis in adipose tissue is more than sufficient for endurance exercise and does not limit the supply of fatty acids (Weber 1988; Weber et al. 1993). In fact a large fraction of the fatty acids does not even leave the adipocyte and is re-esterified (Wolfe et al. 1990).
Free fatty acids are insoluble in the blood and are transported bound to albumin. Plasma concentration of albumin, the molar ratio of fatty acids to albumin in the blood, and blood flow through the tissues likely limit fatty acid delivery to working muscles in small passerines. Thus, to increase fatty acid delivery small passerines apparently re-esterify fatty acids into very low density lipoproteins in the liver and release them into the plasma from where they are taken up by the muscle (Jenni-Eiermann & Jenni 1992).
The metabolism of fatty acids may also be constrained by other steps in the chain of the transport system, e.g., transport within the cell and across cell membranes (Weber 1988, 1992). For example, the inability of humans to oxidise large quantities of fatty acids during exercise is probably due to an inhibition of the entry of long-chain fatty acids into mitochondria, particularly at high rates of energy use (Sidossis et al. 1997). However, no studies on birds have been done. It is likely that additional adaptations of migrant birds in their fatty acid delivery system will be revealed. Recently, the highest fatty acid binding protein concentration in muscle in any vertebrate was found in a shorebird (Guglielmo et al. 1998).
Fatty acid oxidation and the proportion of protein catabolism
As described above, during periods of fasting, oxidation of fatty acids from adipose tissue and catabolism of body protein are interrelated through the need to replace citric acid cycle intermediates. The use of fat as a proportion of the total energy expenditure during endurance flight has an upper limit, determined by the needs of tissues and processes which are unable to use fatty acids, glycerol or ketone bodies. Because glycogen stores are low, protein is the main alternative to lipids. Amino acids from protein can be transformed into gluconeogenic precursors, intermediates of the citric acid cycle, and most other metabolites needed for maintenance and repair.
The question that remains is what is the lower limit of protein utilisation during endurance flight and does it depend, as in humans, on the composition of stored fuels (Forbes & Drenick 1979) and on the level of energy expenditure (Romijn et al. 1993, 1995). These authors showed that the proportion of energy derived from protein decreases with increasing initial fat stores and with decreasing energy expenditure. In the following discussion, the relative contribution of energy derived from protein as a proportion of the total energy expenditure is referred to as RPC. As reviewed elsewhere (Jenni & Jenni-Eiermann 1998), RPC in fasting inactive birds is higher in birds with low initial fat stores than in birds with high initial fat stores. As shown with different avian species, RPC drops from about 20% in birds with an initial fat content of 5% (fat on total body mass) to about 5% in birds with an initial fat content of 25-30%, and remains constant at this level for birds with initial fat stores above 30%. This has also been observed within species (Cherel et al. 1992; Lindgård et al. 1992). When energy expenditure is increased by keeping birds in the cold (-10°C), there is no change in RPC, as shown for caged Thrush Nightingales Luscinia luscinia during the migration season (Klaassen & Biebach 1994, reanalysed in Klaassen et al. 1997). There are only a few data available for migratory birds during flight. Birds flying with initial fat stores in excess of 25% spend around 5 - 7% RPC (Piersma & Jukema 1990, reanalysed in Lindström & Piersma 1993; Gauthier et al. 1992), about the same as in inactive fasting birds. European Robins Erithacus rubecula with initial fat stores of only 11% have a higher RPC of 16% during flight (Åkesson et al. 1992) which again accords with inactively fasting birds with corresponding initial fat stores. In summary, although flying birds and birds kept at low temperatures have a metabolic rate several times higher than inactive fasting birds, RPC remains virtually unchanged. Because their metabolic rate is several times higher than in inactive fasting birds, the absolute amount of protein catabolised, however, must increase in proportion to metabolic rate. Contrary to mammals (Romijn et al. 1993, 1995), birds during endurance flight appear to be able to keep RPC comparatively low, at 5-7%, provided that their initial fat stores are above about 25%.
Proteins may be used for tissue maintenance under the high demands of flight (as suggested by Lindström & Piersma 1993), but the two main reasons for protein use, according to studies in exercising mammals, are gluconeogenesis and the maintenance of citric acid cycle intermediates (e.g., Dohm 1986). Both processes are interrelated and involve the conversion of amino acids into pyruvate (Dohm 1986). If protein catabolism in birds during endurance flight mainly feeds the anaplerotic flux, protein breakdown is expected to be proportional to fatty acid oxidation and, in fasting birds, metabolic rate. This fits exactly with the findings reported above that protein utilisation increases in proportion with metabolic rate.
In mammals, maximum aerobic capacity (VO2 max) has been shown to be another factor affecting RPC. As mammals approach their maximum aerobic capacity, the relative contribution of energy derived from non-lipids as a proportion of total energy expenditure increases (Weber 1992; Weber et al. 1993; Romijn et al. 1993, 1995). However, increasing VO2 max enhances the relative contribution of energy derived from lipids for a given total energy expenditure. For example in humans, endurance training increases number and size of mitochondria, myoglobin content and activity of enzymes involved in fatty acid oxidation, all of which contribute to increased lipid utilisation (Fox et al. 1993). Migrant birds have presumably a higher aerobic capacity during the migratory season than during the non-migratory season and than non-migrant species because they increase the mass of their flight muscles (e.g., Fry et al. 1972; Marsh 1984; Driedzic et al. 1993) and the activity of catabolic enzymes (Marsh 1981; Lundgren & Kiessling 1985; Driedzic et al. 1993). In the European Robin, the composition of fibre types in flight muscle also changes in preparation for migration (Lundgren & Kiessling 1988). Hence, the very high aerobic capacity of birds during migration may explain their outstanding ability to use lipids as a fuel.
CONCLUSIONS AND PRESPECTIVES
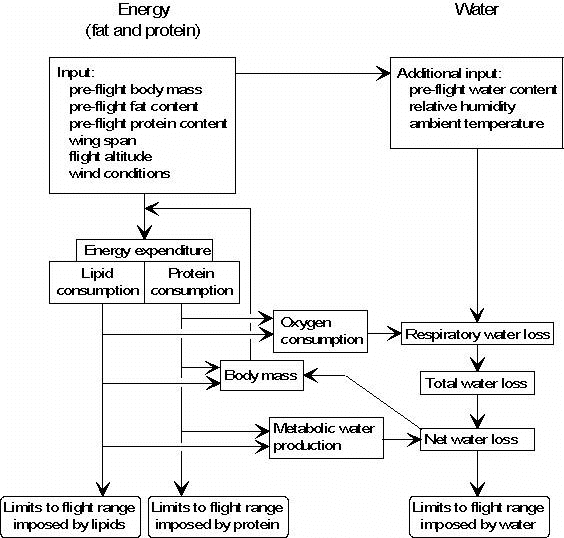
Until now, limits to flight range were evaluated on the basis of energy and water (Carmi et al. 1992; Klaassen 1995, 1996). Because there is a certain amount of protein loss during endurance flight, limits to flight range imposed by energy can be subdivided into limits imposed by lipids and protein. Hence, I refined the original model to estimate flight range of migrating birds to account for these two main fuel types in Fig. 1.
Because of their mode of locomotion, birds have high energy demands and must give priority to the most energy dense fuel - fat (Table 1). The consequence is that birds need to derive a particularly high proportion of their total energy from fat. Flying birds have a very high, perhaps the highest possible, contribution of energy derived from lipid oxidation, a level similar to that of resting birds adapted to long-term fasting, such as penguins (Cherel et al. 1988; Robin et al. 1988). Until now adaptations in the transport system of fatty acids to flight muscles, high initial fat stores and an increase in maximum aerobic capacity have been demonstrated at the biochemical and physiological level. However, all these adaptations entail costs which are at least partly responsible for an increase in basal metabolic rate (Piersma et al. 1995; Weber & Piersma 1996).
Because of these costs, it is expected that the optimum ratio of fat to protein stored before, and used during, migratory flight depends on the length of the non-stop flight (Table 2). In birds flying only short distances non-stop, it may not be worthwhile to build up mechanisms to maximise lipid use. A substantial contribution of protein, or even glycogen, might do. The longer the flight, the higher the relative amount of energy derived from fat in the total energy expenditure should be and, consequently, the higher the investment in adaptations to increase lipid oxidation. However, birds flying in conditions where water loss is excessive may alleviate water stress by increasing the relative contribution of energy derived from protein per total energy expenditure (Table 1).
Species covering very long non-stop flights probably push maximising lipid use to the limits and may in addition reduce the mass of organs which are not needed for flight (Table 2). Indeed, a reduction in mass of nutritional organs (stomach, intestine and liver) has been demonstrated (Piersma & Gill 1998; Piersma 1998). It remains to be shown how widespread this phenomenon is, under what ecological circumstances it occurs and what are the consequences during subsequent refuelling in the wild. A reduction of the nutritional organs before or during the fasting period may help birds to increase their flight range by reducing the mass to be transported and the maintenance costs (basal metabolic rate), which also contribute to maintain water balance (Klaassen & Biebach 1994). However, detailed energy accounting which would take into account the costs of a slower increase in body mass during the first day of stopover due to reduced digestive tract functioning (Klaassen & Biebach 1994; Piersma & Lindström 1997) is still to be addressed. Also, birds reducing their protein content before flight would probably not be able to counterbalance much dehydration (Table 2).
In order to further unravel the endurance performance of migrating birds, investigation of the delivery mechanisms of fatty acids to the flight muscle cells would seem promising. Furthermore, more information on the proportion of energy derived from lipid and protein as a proportion of the total energy expenditure during endurance flight is needed to test the hypothesis of a minimum ratio which is independent of energy expenditure (see above). Data are also required to elucidate whether all migrants incur the costs associated with increasing the relative contribution of energy derived from fat or whether under certain circumstances (e.g., short flight bouts) such costs are bypassed and other fuels have a more important share in energy expenditure.
ACKNOWLEDGMENTS
We benefited from a sabbatical stay at the Department of Zoology at the University of Washington in Seattle and critical comments by Nigel Adams, Meta Landys, Berry Pinshow and Marilyn Ramenofsky.
REFERENCES
Åkesson, S., Karlsson, L., Pettersson, J. & Walinder, G. 1992. Body composition and migration strategies: a comparison between Robins (Erithacus rubecula) from two stop-over sites in Sweden. Vogelwarte 36: 188-195.
Biebach, H. 1992. Flight-range estimates for small trans-Sahara migrants. Ibis 134 suppl. 1: 47-54.
Blaxter, K. 1989. Energy metabolism in animals and man. Cambridge, Cambridge University Press, 336 pp.
Butler, P.J. & Woakes, A.J. 1990. The physiology of bird flights. In: Gwinner, E. (ed.). Bird migration. New York; Springer: pp. 300-318.
Callow, M., Morton, A. & Guppy, M. 1986. Marathon fatigue: the role of plasma fatty acids, muscle glycogen and blood glucose. European Journal of Applied Physiology 55: 654-661.
Carmi, N., Pinshow, B., Porter, P. & Jaeger, J. 1992. Water and energy limitations on flight duration in small migrating birds. Auk 109: 268-276.
Cherel, Y., Robin, J.-P., Heitz, A, Calgari, C. & Le Maho Y. 1992. Relationships between lipid availability and protein utilisation during prolonged fasting. Journal of Comparative Physiology B 162: 305-313.
Cherel, Y.,Robin, J.-P. & Le Maho Y. 1988. Physiology and biochemistry of long-term fasting in birds. Canadian Journal of Zoology 66:159-166.
Cramp, S. (ed.) 1985. The birds of the western Palearctic. Vol. 4. Oxford; Oxford University Press: 960 pp.
Dawson, W.R., Marsh, R.L. & Yacoe, M.E. 1983. Metabolic adjustments of small passerine birds for migration and cold. American Journal of Physiology 245: R755-R767.
Dohm, G.L. 1986. Protein as a fuel for endurance exercise. Exercise and Sport Science Review 14:143-173.
Driedzic, W.R., Crowe, H.L., Hicklin, P.W. & Sephton, D.H. 1993. Adaptations in pectoralis muscle, heart mass, and energy metabolism during premigratory fattening in Semipalmated Sandpipers (Calidris pusilla). Canadian Journal of Zoology 71: 1602-1608.
Farner, D.S., Oksche, A., Kamemoto, F.I., King, J.R. & Cheyney, H.E. 1961. A comparison of the effect of long daily photoperiods on the pattern of energy storage in migratory and non-migratory finches. Comparative Biochemistry and Physiology 2: 125-142.
Field, J., Belding, H.S. & Martin, A.W. 1939. An analysis of the relation between basal metabolism and summated tissue respiration in the rat. Journal of Cellular and Comparative Physiology 14: 143-157.
Forbes, G.B. & Drenick, E.J. 1979. Loss of body nitrogen on fasting. American Journal of Clinical Nutrition 32: 1570-1574.
Fox, E.L., Bowers, R.W. & Foss, M.L. 1993. The physiological basis for exercise and sport, 5th ed. Brown and Benchmark, Madison.
Fry, C. H., Ferguson-Lees, I.J. & Dowsett, R.J. 1972. Flight muscle hypertrophy and ecophysiological variation of Yellow Wagtail Motacilla flava races at Lake Chad. Journal of Zoology, London 167: 293-306.
Gauthier, G., Giroux, J.-F. & Bédard, J. 1992. Dynamics of fat and protein reserves during winter and spring migration in Greater Snow Geese. Canadian Journal of Zoology 70: 2077-2087.
George, J.C. & Berger, J.C. 1966. Avian myology. Academic Press, London.
Guglielmo, C.G., Haunerland, N.H. & Williams, T.D. 1998. Fatty acid binding protein, a major protein in the flight muscle of the Western Sandpiper. Comparative Biochemistry and Physiology B 119: 549-555.
Jenni, L. & Jenni-Eiermann, S. 1998. Fuel supply and metabolic constraints in migrating birds. Journal of Avian Biology. 29: 521-528.
Jenni-Eiermann, S. & Jenni, L. 1991. Metabolic responses to flight and fasting in night migrating passerines. Journal of Comparative Physiology B 161: 465-474.
Jenni-Eiermann, S. & Jenni, L. 1992. High plasma triglyceride levels in small birds during migratory flight: a new pathway for fuel supply during endurance locomotion at very high mass-specific metabolic rates? Physiological Zoology 65: 112-123.
Johnston, D.W. 1970. Caloric density of avian adipose tissue. Comparative Biochemistry and Physiology 34: 827-832.
Klaassen, M. & Biebach, H. 1994. Energetics of fattening and starvation in the long-distance migratory Garden Warbler, Sylvia borin, during the migratory phase. Journal of Comparative Physiology B 164: 362-371.
Klaassen, M. 1995. Water and energy limitations on flight range. Auk 112: 260-262.
Klaassen, M. 1996. Metabolic constraints on long-distance migration in birds. Journal of Experimental Biology 199: 57-64.
Klaassen, M., Lindström, Å. & Zijlstra, R. 1997. Composition of fuel stores and digestive limitations to fuel deposition rate in the long-distance migratory Thrush Nightingale, Luscinia luscinia. Physiological Zoology 70: 125-133.
Lee, S.-H. & Davis, E.J. 1979. Carboxylation and decarboxylation reactions. Journal of Biological Chemistry 254:420-430.
Lindgård, K., Stokkan, K.A., Le Maho, Y. & Groscolas, R. 1992. Protein utilization during starvation in fat and lean Svalbard Ptarmigan (Lagopus mutus hyperboreus). Journal of Comparative Physiology B 162: 607-613.
Lindström, Å. & Piersma, T. 1993. Mass changes in migrating birds: the evidence for fat and protein storage re-examined. Ibis 135: 70-78.
Lundgren, B.O. & Kiessling, K.-H. 1985. Seasonal variation in catabolic enzyme activities in breast muscle of some migratory birds. Oecologia 66: 468-471.
Lundgren, B.O. & Kiessling, K.-H. 1988. Comparative aspects of fibre types, areas, and capillary supply in the pectoralis muscle of some passerine birds with differing migratory behaviour. Journal of Comparative Physiology 158: 165-173.
Marsh, R.L. 1981. Catabolic enzyme activities in relation to premigratory fattening and muscle hypertrophy in the Grey Catbird (Dumetella carolinensis). Journal of Comparative Physiology 141: 417-423.
Marsh, R.L. 1983. Adaptations of the Gray Catbird Dumentella carolinensis to long distance migration: energy stores and substrate concentrations in plasma. Auk 100: 170-179.
Marsh, R.L. 1984. Adaptations of the Grey Catbird Dumetella carolinensis to long-distance migration: flight muscle hypertrophy associated with elevated body mass. Physiological Zoology 57: 105-117.
McGilvery, R.W. 1983. Biochemistry, a functional approach. Saunders, Philadelphia.
Murphy, M. 1994. Amino acid composition of avian eggs and tissues: nutritional implications. Journal of Avian Biology 25: 27-38.
Pennycuick, C.J. 1989. Bird flight performance. Oxford University Press. Oxford New York Tokyo.
Piersma, T. & Davidson, N.C. 1992. The migration of Knots. Wader Study Group Bulletin 64, Suppl., 209 pp.
Piersma, T. & Gill Jr, R.E. 1998. Guts don’t fly: small digestive organs in obese Bar-tailed Godwits. Auk 115: 196-203.
Piersma, T. & Lindström, Å. 1997. Rapid reversible changes in organ size as a component of adaptive behaviour. Trends in Ecology and Evolution 12: 134-138.
Piersma, T. 1998. Phenotypic flexibility during migration: optimization of organ size contingent on the risks and rewards of fueling and flight? Journal of Avian Biology 29: 511-520.
Piersma, T., Cadée, N. & Daan, S. 1995. Seasonality in basal metabolic rate and thermal conductance in a long-distance migrant, the Knot (Calidris canutus). Journal of Comparative Physiology B 165: 37-45.
Piersma. T. & Jukema, J. 1990. Budgeting the flight of a long-distance migrant: changes in nutrient reserve levels of Bar-tailed Godwits at succesive spring staging sites. Ardea 78: 315-337.
Robin, J.-P., Frain, M., Sardet, C., Groscolas, R. & Le Maho, Y. 1988. Protein and lipid utilisation during long-term fasting in Emperor Penguins. American Journal of Physiology 254: R61-R68.
Romijn, J.A., Coyle, E.F., Sidossis, L.S., Gastaldelli, A., Horowitz, J.F., Endert, E. & Wolfe, R.R. 1993. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. American Journal of Physiology 265: E380-E391.
Romijn, J.A., Coyle, E.F., Sidossis, L.S., Zhang, X.-J. & Wolfe, R.R. 1995. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. Journal of Applied Physiology 79: 1939-1945.
Rothe, H.-J., Biesel, W. & Nachtigall, W. 1987. Pigeon flight in a wind tunnel. II Gas exchange and power requirements. Journal of Comparative Physiology B 157: 99-109.
Sahlin, K., Katz, A. & Broberg, S. 1990. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. American Journal of Physiology 259: C834-C841.
Schmidt-Nielsen, K. 1997. Animal Physiology: Adaptation and environment. 5th ed. Cambridge University Press. Cambridge.
Schwilch, R., Jenni, L. & Jenni-Eiermann, S. 1996. Metabolic responses of homing pigeons to flight and subsequent recovery. Journal of Comparative Physiology B 166: 77-87.
Scott, I. & Evans, P. R. 1992. The metabolic output of avian (Sturnus vulgaris, Calidris alpina) adipose tissue, liver and skeletal muscle: implications for BMR/body mass relations. Comparative Biochemistry and Physiology 103A: 329-332.
Shaw, W.A.S., Issekutz, T.B. & Issekutz Jr., B. 1975. Interrelationship of FFA and glycerol turnovers in resting and exercising dogs. Journal of Applied Physiology 39: 30-36.
Sidossis, L.S., Gastaldelli, A., Klein, S. & Wolfe, R.R. 1997. Regulation of plasma fatty acid oxidation during low- and high-intensity exercise. American Journal of Physiology 272: E1065-E1070.
Stevens, L. 1996. Avian Biochemistry and Molecular Biology. Cambridge: Cambridge University Press, 272 pp.
Vock, R., Weibel, E.R., Hoppeler, Ordway, G., Weber, J.-M. & Taylor, C.R. 1996. Design of the oxygen and substrate pathways. V. Structural basis of vascular substrate supply to muscle cells. Journal of Experimental Biology 199: 1675-1688.
Weber, J.-M. 1988. Design of exogenous fuel supply systems: adaptive strategies for endurance locomotion. Canadian Journal of Zoology 66: 1116-1121.
Weber, J.-M. 1992. Pathways for oxidative fuel provision to working muscles: ecological consequences of maximal supply limitations. Experientia 48: 557-564.
Weber, J.-M., Roberts, T.J. & Taylor, C.R. 1993. Mismatch between lipid mobilization and oxidation: glycerol kinetics in running African goats. American Journal of Physiology 264: R797-R803.
Weber, T. & Houston, A.I. 1997. Flight costs, flight range and the stopover ecology of migrating birds. Journal of Animal Ecology 66: 297-306.
Weber, T.P., Houston, A. I. & Ens, B.J. 1994. Optimal departure fat loads and stopover site use in avian migration: an analytical model. Proceedings of the Royal Society London B 258:29-34.
Weber, T.P. & Piersma, T. 1996. Basal metabolic rate and the mass of tissues differing in metabolic scope: migration-related covariation between individual Knots Calidris canutus. Journal of Avian Biology 27: 215-224.
Wolfe, R.R., Klein, S., Carraro, F. & Weber, J.-M. 1990. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. American Journal of Physiology 258E. 382-389.
Table 1. Energy and water yield of the three main fuel types: stored glycogen, triglycerides in adipose tissue and protein in skeletal muscle or fat free mass. The calculations are based on the values given in lines 1, 2, 4 and 6 (for references see footnotes), from which the values given in the other lines follow
.
Table 2. Schematic outline of proposed strategies of fuel utilisation during migratory flight in relation to the length of non-stop flight and dehydration.
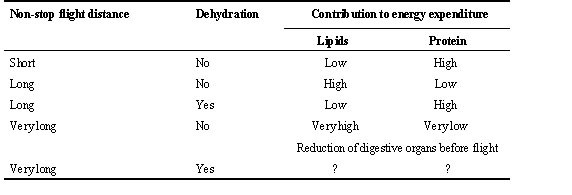
Fig. 1. Model for estimating the flight range of migratory birds on the basis of fuel stores (left hand side) and water (right hand side). The model was developed by Carmi et al. (1992), refined by Klaassen (1995, 1996) and here complemented by considering fat and protein as separate types of fuels. This results in lipids, protein or water as limiting flight range, instead of energy or water as in the original model.