S25.2: Avian nectarivores that breed in winter: Balancing energy and water
Susan Jackson
Department of Human and Animal Physiology, University of Stellenbosch, Private Bag X1, Stellenbosch 760, South Africa, e-mail sjack@land.sun.ac.za
Jackson, S. 1999. Avian nectarivores that breed in winter: Balancing energy and water. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1427-1436. Johannesburg: BirdLife South Africa.Osmoregulation and energetics are inextricably linked in nectarivorous birds, which rely chiefly on aqueous sugar solutions for energy. Some African sunbirds (Nectarinia violacea and N. chalybea) and sugarbirds (Promerops cafer), and Australian honeyeaters (e.g. Phylidonyris novaehollandiae) breed in winter when rainfall, hence nectar availability, is highest, but when ambient temperatures are low. Low ambient temperatures necessitate increased nest attendance in incubating birds, which must nonetheless meet their energy needs by drinking large volumes of dilute nectar. Water influx rates are thus above those predicted by allometric equations, but are comparable with rates in hummingbirds. Ratios of field to basal metabolic rates (FMR/BMR) for Orange-breasted Sunbird suggest that incubating adults are close to their energetic limits. Among sunbirds, sugarbirds and honeyeaters, physiological responses to the rigours described above are probably similar to those reported for hummingbirds, their better-studied New World counterparts. Energetic parsimony is accomplished by, inter alia, nocturnal torpor and highly efficient intestinal absorption of the three major nectar sugars (> 98% for fructose, glucose and sucrose).
INTRODUCTION
During the 1970's and 1980's avian nectarivores, particularly hummingbirds, were used as models with which to test optimal foraging theory. Laboratory and field studies confirmed that both hummingbirds and their Old World counterparts, sunbirds and honeyeaters, closely regulate their energy intake by adjusting the volume of nectar that they consume (e.g. Collins & Cary 1981, Mitchell & Paton 1990, Roberts 1995, Downs 1997). More than 20 years ago, researchers recognised the intimate links between osmoregulation and energy balance in nectarivorous birds, whose chief energy source is flower nectar i.e: dilute sugar solutions (Calder 1979). The importance of research on water and energy balance in hummingbirds was highlighted twelve years ago at the 19th IOC in Ottawa (Hiebert & Calder 1986), and a comprehensive review published by Beuchat et al. (1990).
Passerine nectarivores of the Southern Hemisphere have received less attention until recently, except for the seminal work of Brian Collins, David Paton and their colleagues in Australia. The development and refinement of the doubly-labelled water technique for measuring field metabolic rate and of the osmotic mini-pump technique for quantifying renal variables in vivo (e.g.: Goldstein and Bradshaw 1998a) herald an increase in the number of studies on honeyeater, sunbird and sugarbird energetics and osmoregulation, both in the laboratory and in the field (Weathers et al. 1996, Goldstein and Bradshaw 1998b). Passerine nectarivores are larger than are hummingbirds and their mass-specific metabolic rates are probably lower than those of hummingbirds, because passerines are not specialised to hover while foraging.
This review summarises currently available data from published studies of energy and water balance for a subset of passerine nectarivores which inhabit the winter rainfall areas of southern Australia and southern Africa. I have selected four species which breed in winter, early spring or late autumn, when nectar availability is high but ambient temperatures low and day length, hence available foraging time, short. Moreover, females of these species incubate the eggs without assistance from the male and are therefore exposed to periods of severe energetic and osmoregulatory stress as they must drink and process large volumes of dilute nectar to meet their energy needs while they are incubating (Williams 1991, 1993). Weathers et al. (1996) and Goldstein and Bradshaw (1998b) studied field metabolic rate and water balance in New Holland Honeyeaters Phylidonyris novaehollandiae during the austral summer and winter, respectively. Williams (1993) measured field metabolic rate and water flux in breeding Orangebreasted Sunbirds Nectarinia violacea in winter, and Leon and Nicolson (1997) measured metabolic rate of Lesser Doublecollared Sunbirds N. chalybea exposed to a range of temperatures in the laboratory. Collins (1983) used energy and time budgets in the field to estimate energy balance in Cape Sugarbirds Promerops cafer in spring. Taken together, these studies suggest further directions for comparative research into the physiological mechanisms that enable nectarivores to excrete several times their body mass in water every day while maintaining energy and osmotic balance. The literature on the physiology of Australian and southern African nectarivores is not extensive, and one of the main aims of this review is to show how these studies highlight future research directions and gaps in current knowledge.
ENERGY BALANCE
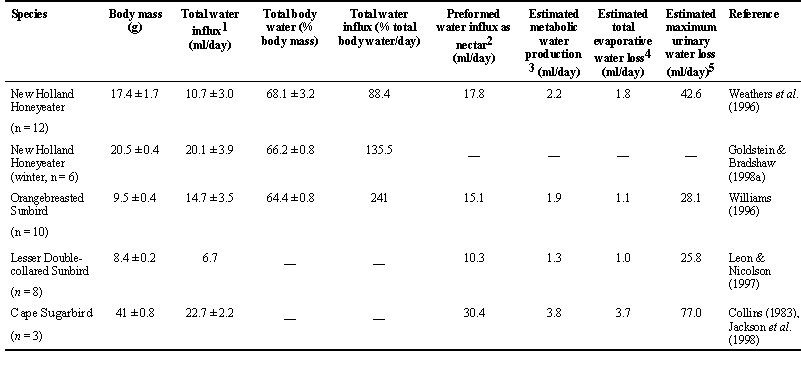
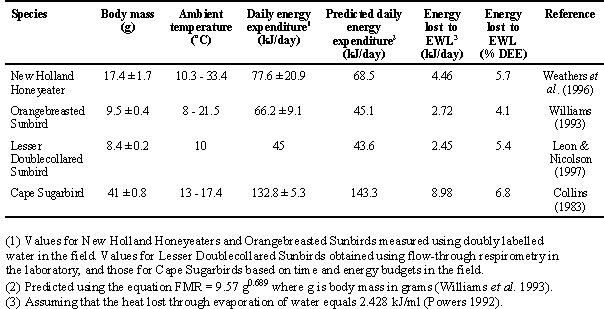
Field metabolic rates (FMRs) of New Holland Honeyeaters and Orangebreasted Sunbirds were measured using doubly labelled water (Weathers et al. 1996, Williams 1993). Metabolic rates of Lesser Doublecollared Sunbirds were measured using flow-through respirometry, and for Cape Sugarbirds were estimated from time and energy budget data (Collins 1983) (Table 1). Laboratory and time-budget measurements of energy expenditure are not comparable with measurements on free-living birds, and are presented here because they are currently the only published data for these species. I therefore restrict comparisons based upon different techniques for measuring metabolic rate to intraspecific comparisons of energy intake and expenditure. Interspecific comparisons are made between the only two species for which comparable data have been published, the New Holland Honeyeater and the Orangebreasted Sunbird.
To predict FMRs, I used an equation for 62 species of passerine and non-passerine birds (FMR = 9.57 g0.689where g is body mass in g, Williams et al. 1993). The greatest disparity between measured and predicted FMRs was for the Orangebreasted Sunbird, the only species studied while incubating. Use of an equation calculated exclusively for nectarivores (FMR = 4.56 g0.421, MacMillen & Carpenter 1977) yielded values which closely agreed with those from the Williams et al. (1993) equation for the two sunbird species, but which underestimated measured FMRs for the two larger species by a greater margin than did the general equation, yielding values of 63.4 kJ/day for the New Holland Honeyeater and 91 kJ/day for the Cape Sugarbird. All discussion that follows therefore uses predicted values based on the Williams et al. (1993) equation. The measured values of daily energy expenditure for Cape Sugarbirds that were estimated using time budgets (Collins 1983) were considerably higher than an estimate of 99.4 ± 9.8 kJ/day based on consumption of a 20% (w:w) sucrose solution by 10 captive birds (Jackson et al. 1998), but this is not surprising in view of the relative inactivity of the captive birds.
Sustained metabolic scope (SusMS), the ratio of FMR to basal metabolic rate (BMR), was 2.8 for the New Holland Honeyeater, 6.5 for the Orangebreasted Sunbird, 2.1 for the Lesser Doublecollared Sunbird, and 2.6 for the Cape Sugarbird. For each species, BMR was estimated using the equation given in Aschoff and Pohl (1970). Not surprisingly, the species which was breeding (the Orangebreasted Sunbird) showed the most elevated FMR. This is the highest such ratio ever reported for birds. It exceeds the theoretical ceiling of six proposed by Kirkwood (1983) and Peterson et al. (1990), and the measured ceilings to SusMS of 4.2 for captive House Wrens Troglodytes aedon forced to exercise in the cold (Dykstra and Karasov 1992), and of 4.3 estimated for Tour De France cyclists (Hammond and Diamond 1997). It is only exceeded by that of lactating mice rearing 14 pups in the cold (Hammond and Diamond 1997). The value of 6.5 should be regarded with caution because BMR was estimated rather than measured. Ricklefs et al. (1996) found no relationship between BMR and FMR in birds, therefore there is no reason to suppose that the allometric equation of Aschoff and Pohl (1970) substantially underestimates the BMR of Orangebreasted Sunbirds. Regarded with caution, this evidence nonetheless suggests that winter-breeding nectarivores are close to their physiological limits. Further research on birds while they are breeding is most likely to indicate which physiological processes or combinations thereof limit metabolic rate. However, such research is difficult for both birds and researchers.
Lesser Doublecollared Sunbirds reduce their potential daily energy costs by approximately 23% as a result of the diel rhythm in metabolic rate and body temperature (Leon & Nicolson 1997). This rhythm is shared by other sunbird species (Prinzinger et al. 1989), and by honeyeaters (Collins et al. 1980a), but incubating birds must maintain egg temperature above 35ºC overnight (Williams 1993) and therefore cannot substantially reduce their own body temperature. This fact probably contributes to the high FMRs of Orangebreasted Sunbirds.
WATER BALANCE
Measured water influx rates (WIR) relative to predicted values
I estimated water influx rates (WIR) on the basis of body mass using the equation WIR = 1.52 g 0.689 (n = 46 species, Williams et al. 1993), and compared these with rates measured in the field for New Holland Honeyeaters in summer (Weathers et al. 1996) and winter (Goldstein and Bradshaw 1998b), and for Orangebreasted Sunbirds in winter (Williams 1993). Weathers et al. and Williams used doubly labelled water, and Goldstein and Bradshaw used tritiated water. Metabolic water production is estimated as 0.029 ml H2O yielded per kJ (Williams et al. 1993) and is a component of both measured and estimated WIR, both of which are based on whole body water turnover estimated using labelled water. This technique does not distinguish between ingested (preformed) water and metabolic water. Water influx rates have not been directly measured for Lesser Doublecollared Sunbirds or Cape Sugarbirds in the field. Laboratory measurements for Lesser Doublecollared Sunbirds are reported by C.N. Lotz elsewhere in this publication. Ten captive Cape Sugarbirds at an ambient temperature of 25° C consumed 26.3 ± 2.6 ml/day of a 20% (w:w) sucrose solution, yielding 22.7 ± 2.2 ml of preformed H2O/day (Jackson et al. 1998). This is not substantially higher than the value of 20 ml/day predicted on the basis of interspecific allometry (Table 2, Williams et al. 1993), but allometric predictions include metabolic water whereas measured intakes do not. Allometric predictions may underestimate actual water intakes for nectarivorous birds.
New Holland Honeyeaters in winter and incubating Orangebreasted Sunbirds showed water influx rates that were considerably higher than values predicted on the basis of the general allometric equation (measured: 20.5 ml/day, predicted: 12.5 ml/day for honeyeaters; and 14.7 and 7.3 ml/day respectively for sunbirds). However, there was close agreement between measured (10.7 ml/day) and predicted (11.2 ml/day) WIR for New Holland Honeyeaters in summer (Weathers et al. 1996).
New Holland Honeyeaters in summer ingested 88% of their total body water (TBW) per day, whereas the same species ingested 135% of TBW in winter (Table 2). Orangebreasted Sunbirds ingested 241% of TBW per day. These values are comparable with those of approximately 120% - 214% of TBW for hummingbirds (Beuchat et al. 1990, Powers & Conley 1994), and illustrate the high water load in nectarivorous birds.
Metabolic water comprises 20% of the total WIR for New Holland Honeyeaters in summer (Table 2), and 13% of total WIR for Orangebreasted Sunbirds. Goldstein and Bradshaw did not measure metabolic rates for New Holland Honeyeaters in winter, so I did not attempt to estimate metabolic water production in these individuals.
Water loss rates relative to water influx rates
Goldstein and Bradshaw (1998a) used osmotic mini-pumps to measure Urine Flow Rates (UFR) in captive Red Wattlebirds Anthochaera carunculata fed different concentrations of sucrose solution. They reported maximum flow rates in the afternoon after a day of feeding, and I used their value (corrected for body mass0.689, see Table 2) for birds fed the lowest concentration of sucrose (250 mmol/l) to estimate maximum possible rates of urine flow in the four species of winter breeding nectarivore discussed here. I stress that these are maximum values and are unlikely to have predictive value: they are currently the only measures of urine (rather than mixed gut and kidney excreta) flow rate available for passerine nectarivores. In the context of this paper, these values are presented purely to establish a theoretical maximum possible rate of urine production. (Elsewhere in this publication, C.N. Lotz will present data on cloacal fluid output by captive Lesser Doublecollared Sunbirds.) I estimated Total Evaporative Water Loss (TEWL) on the basis of body mass using the equation TEWL = 0.177 g.0.819 This equation was obtained from data for passerines only, controlled for phylogenetic effects using independent contrasts (Williams 1996).
Comparison of total predicted water loss (the sum of UFL and TEWL) with measured water influx rates (WIR, see above) suggests that WIR in all four species are well below maximum possible renal water output rates. Moreover, this comparison does not take water excretion by the gut into account. If water passes unabsorbed through the intestines, potential water efflux rates may be substantially increased without additional loading of the kidney. Collins et al. (1980) reported that TEWL in Brown Honeyeaters Lichmera indistincta (body mass 9 g) and Singing Honeyeaters Meliphaga virescens (body mass 25 g) in the laboratory was 2.6 and 3.6 ml/day at 10° C for the two species respectively, and 4.3 and 7.2 ml/day at 30° C. Comparison of these values with estimated figures for nectarivores of similar body mass in Table 2 suggests that TEWL in passerine nectarivores is likely to be substantially greater than that predicted by allometric equations. These birds are probably well able to offload the water that they must ingest to meet their energy needs, even in winter while they are breeding.
The permeability of sunbird, sugarbird and honeyeater intestines to water is unknown. Karasov and Cork (1994) reported that most glucose transport across the intestines of nectarivorous Rainbow Lorikeets Trichoglossus haematodus is passive, whereas passive permeability of hummingbird intestines to glucose is thought to be very low (Karasov et al. 1986). Passively absorbed glucose would be accompanied by water pulled from the lumen into the interstitial spaces of the mucosa by ‘solvent drag’. The implications of different mechanisms of glucose uptake by nectarivore intestines for water balance, need to be explored.
Measured cloacal fluid output for captive Cape Sugarbirds fed 20% sucrose at an ambient temperature of 25°C was 20.3 ± 1.9 ml/day (Jackson et al. 1998). This value is very close to the difference between total WIR and estimated TEWL for the same individuals, which is 19 ml/day. For captive birds such as these which are not under energetic stress, allometric predictions of TEWL are likely to be close to real values. Departures from the allometric predictions are inevitable in water-loaded (energy-stressed) birds, and warrant further investigation.
Urine flow rate in nectarivores shows strong diel rhythms which reflect the daily fluctuations in water intake. Notably, Red Wattlebirds appeared to exert more marked short-term control of Urine Flow Rate than of Glomerular Filtration Rate (Goldstein & Bradshaw 1998a). These authors described considerable variation in fractional water reabsorption (recovery of water) rates in the distal tubule which appeared to be associated with reduced circulating levels of Arginine Vasotocin. The urine osmolarity reported for Red Wattlebirds on a diet of 250 mmol/l sucrose (175 - 413 mmol/l) is considerably higher than that for captive Lesser Doublecollared Sunbirds on a 300mmol/l sucrose diet (34 mmol/l, S. Nicolson personal communication). Urine osmolalities in hummingbirds range from 41 - 89 mOsm/kg (Beuchat et al. 1990). These data suggest that sunbirds and hummingbirds may be capable of excreting more dilute urine that are honeyeaters, but such speculation must be supported with data for more than the handful of species for which urine osmolarities have been published to date. Variation in fractional water reabsorption in the kidney implies short-term control of water permeability in the distal tubule, by a mechanism which has yet to be described. The potential loss of electrolytes in the copious and dilute urine excreted by nectarivores will be discussed in detail by other authors elsewhere in this publication, but it is worth noting here that Lesser Doublecollared Sunbirds recover sodium and potassium from their urine with extreme efficiency (S. Nicolson, pers. comm.).
The relative importance of Urinary and Total Evaporative Water Loss (TEWL)
For all four species of winter-breeding nectarivores, estimated TEWL is between 6 and 8% of Urine Flow Rate, suggesting that the latter is a much more important route for offloading water. However, both these measures are estimates, based on either allometry (TEWL), or on a single value obtained from Red Wattlebirds (UFR), and it is premature to attach undue significance to their relative importance.
Speculation about evaporative water loss (EWL) as a route for offloading water must take the energy lost through evaporation into account. One can estimate the potential heat loss from the above predicted TEWL rates by assuming that 2.428 kJ are lost for every ml of H2O that evaporates (Powers 1992). The heat losses associated with the TEWL rates shown in Table 2 represent between 4 and 7% of FMR (Table 1) for the four species of nectarivores under discussion. Increases in EWL rates would carry substantial energy costs because EWL requires transformation of the water lost from the liquid to the gaseous phase, which requires energy, whereas an equivalent volume of water could be excreted via the kidneys or GI tract with far lower energy loss. The efficacy of EWL also depends on ambient humidity, whereas renal and gastrointestinal excretion may be regulated independently of ambient humidity and temperature. Speculation about the relative merits of these avenues of water loss would be better informed by more data, including direct measurements of water loss in the laboratory (Collins et al. 1980a) and in the field (e.g. Adams et al. 1997). It must be remembered that TEWL predictions based on allometric equations do not take into account the fluctuations in ambient temperature and humidity which profoundly affect rates of evaporation.
Estimation of preformed water ingested in nectar
I assumed that birds in the field studies by Weathers et al. (1996) and Williams (1993) were consuming nectar that contained 20% sugar (w:w) (Pyke 1980, Barnes et al. 1995, Mostert et al. 1980), and that the birds met all their daily energy requirements with nectar which they absorbed with an efficiency of 100% (Collins et al. 1980b, Lotz & Nicolson 1996, Jackson et al. 1998). Table 2 shows that preformed water influx rates estimated in this fashion were close to measured influx rates only for Orangebreasted Sunbirds. New Holland Honeyeaters may have been consuming nectar more concentrated than 20%, and supplementing their diets with insects. The disparity between estimated water influx as nectar and measured water influx in Cape Sugarbirds is probably because nectar consumption was estimated on the basis of a energy budgets for birds in the field (Collins 1983), whereas measured water influx was calculated from captive birds (Jackson et al. 1998). Recall that the daily energy expenditure of the captive birds was substantially below that estimated for birds in the field.
CONCLUSIONS
Energy balance:
FMRs for the two species for which direct measurements have been made, the New Holland Honeyeater and the Orangebreasted Sunbird, are higher than values predicted on the basis of allometry. This probably reflects the time of year that the measurements were taken, during the winter breeding season. Sustained Metabolic Scope is likewise probably high for these two species, but calculation of this awaits laboratory measurements of BMR. Lastly, heat loss during EWL may account for a substantial fraction of daily energy expenditure (FMR), suggesting that direct measurements of this variable, too, would be instructive. The energy costs of EWL must be taken into account in studies of nectarivore energetics.
Water balance:
For all four species discussed here, urine output is more than adequate to offload the water ingested as birds meet their energy needs. Ion recovery may be a more physiologically important consideration for nectarivores.
FUTURE RESEARCH DIRECTIONS
Field Studies
(1) FMR has currently been measured in very few of the nectarivorous passerine species which breed during winter in the southern Hemisphere. Cape Sugarbirds and more species of winter-breeding honeyeater are notable candidates for future study.
(2) Measurements of urine and plasma osmolarity such as those reported by Goldstein and Bradshaw (1998a) are needed for more species, to establish the ability of nectarivore kidneys to balance high water influx rates with high excretion rates.
(3) The need for repeated sampling of plasma and urine may preclude the use of osmotic mini-pumps to quantify GFR and UFR in the field, but this technique would be extremely informative if it could be applied in the field. Regulation of urine flow rates of passerine nectarivores may be more important than is regulation of glomerular filtration rates in the birds’ responses to water loading. Extremely efficient recovery of electrolytes by active transport across the epithelium lining the distal tubules suggests that permeability of these epithelia to water must be well controlled.
Laboratory Studies
(1) Quantification and partitioning of whole-body water loss is essential to our understanding of the mechanisms whereby nectarivores achieve energy and water balance, and may well suggest which physiological processes limit this balance. The relative magnitudes of TEWL, renal water loss, and water loss from the gastro-intestinal tract and are currently being measured in laboratories in southern Africa, Australia, the USA and Israel.
(2) The thermoregulatory costs of the different pathways of evaporative water loss must be remembered in any consideration of the relative costs and benefits of these pathways in birds subject to different environmental conditions. At low nectar concentrations, heat lost through EWL might constitute a substantial fraction (5 - 40%) of FMR.
(3) Hitherto undescribed mechanisms of short-term control of cutaneous EWL are likely to prove extremely interesting in birds, nectarivorous and otherwise.
(4) The Heat Increment of Feeding (HIF or SDA) has been measured for insectivorous and piscivorous birds, but not for nectarivores. Complete energy budgets for nectarivores should include estimates of the contribution of HIF to their thermoregulatory costs, because ingestion of large volumes at ambient temperatures necessitates warming of the nectar, which may carry a substantial energetic cost. Controlled experiments measuring postprandial metabolic rate in birds in the laboratory will supply such data.
(5) The mechanism of glucose uptake by the nectarivore gut (passive paracellular transport or active, carrier-mediated transport through the mucosal cells) must influence water uptake by the gut. Elucidation of mechanism of glucose transport would therefore contribute to our understanding of the routes of water excretion: is water absorbed from the gut and excreted by the kidneys, or does it remain in the gut lumen, thereby lessening the water load on the kidneys?
REFERENCES
Adams, N.J., Pinshow, B. & Gannes, L.Z. 1997. Water influx and efflux in free-flying pigeons. J. Comp. Physiol. 167:444-450.
Barnes, K., Nicolson, S.W. & van Wyk. B.-E. 1995. Nectar sugar composition in Erica. Biochem. Syst. Ecol. 23:419-423.
Beuchat, C.A., Calder, W.A.III & Braun, E.J. 1990. The integration of osmoregulation and energy balance in hummingbirds. Physiol. Zool. 63:1059-1081.
Calder, W.A. III. 1979. On the temperature dependency of optimal nectar concentrations for birds. J. Theor. Biol. 78:185-196.
Collins, B.G. 1983. A first approximation of the energetics of Cape sugarbirds (Promerops cafer) and orange-breasted sunbirds (Nectarinia violacea). South African Journal of Zoology 18:363-369.
Collins, B.G. & Cary. G. 1980. Short-term regulation of food intake by the brown honeyeater Lichmera indistincta. Comp. Biochem. Physiol. 68A:635-640.
Collins, B.G., Cary, G. & Payne, S. 1980a. Metabolism, thermoregulation and evaporative water loss in two species of Australian nectar-feeding birds (family Meliphagidae). Comp. Biochem. Physiol. 67A:629-635.
Collins, B.G., Cary, G. & Packard, G. 1980b. Energy assimilation, expenditure and storage by the Brown Honeyeater, Lichmera indisincta. J. Comp. Physiol. 137:157-163.
Downs, C.T. 1997. Sugar digestion efficiencies of Gurney's Sugarbirds, Malachite Sunbirds and Black Sunbirds. Physiol. Zool. 70:93-99.
Dykstra, C.R. & Karasov, W.H. 1992. Changes in gut structure and function of House Wrens (Troglodytes aedon) in response to increased energy demands. Physiol. Zool. 65:422-442.
Goldstein, D.L. & Bradshaw, S.D. 1998a. Renal function in red wattlebirds in response to varying fluid intake. J. Comp. Physiol. 168:265-272.
Goldstein, D.L. & Bradshaw, S.D. 1998b. Regulation of water and sodium balance in the field by Australian honeyeaters (Aves: Meliphagidae). Physiol. Zool. 71:214-225.
Hammond, K.A. & Diamond, J. 1997. Maximal sustained energy budgets in humans and animals. Nature 386:457-462.
Hiebert, S.M. & Calder, W.A. III. 1986. The osmoregulatory consequences of nectarivory and frugivory in hummingbirds and other species. Proc. XIX Int. Ornithol. Congr., University of Ottawa Press 2:1498-1505.
Jackson, S., Nicolson, S.W. & van Wyk, B.-E. 1998. Apparent absorption efficiencies of nectar sugars in the Cape sugarbird, with a comparison of methods. Physiol. Zool. 71:106-115.
Karasov, W.H., Phan, D., Diamond, J.M. & Carpenter, F.L. 1986. Food passage and intestinal nutrient transport in hummingbirds. Auk 103:453-464.
Karasov, W.H. & Cork, S.J. 1994. Glucose absorption by a nectarivorous bird: the passive pathway is paramount. Am. J. Physiol. 267:G18-G26.
Kirkwood, J.K. 1983. A limit to metabolisable energy intake in mammals and birds. Comp. Biochem. Physiol. 75A:1-3.
Leon, B. & Nicolson, S.W. 1997. Metabolic rate and body temperature of an African sunbird, Nectarinia chalybea: daily rhythm and the effect of ambient temperature. S.Afr. J. Zool. 32:31-36.
Lotz, C.N. & Nicolson, S.W. 1996. Sugar preferences of a nectarivorous passerine bird, the Lesser Double-collared Sunbird (Nectarinia chalybea). Funct. Ecol. 10:360-365.
MacMillen, R.E. & Carpenter, F.L. 1977. Daily energy costs and body weight in nectarivorous birds. Comp. Biochem. Physiol. 56A:439-441.
Mitchell, R.J. & Paton, D.C. 1990. Effects of nectar volume and concentration on sugar intake rates of Australian honeyeaters. Oecologia 83:238-246.
Mostert, D.P., Siegfried, W.R. & Louw, G.N. 1980. Protea nectar and satellite fauna in relation to the food requirements and pollinating role of the Cape sugarbird. S. Afr. J. Sci. 76:409-412.
Peterson, C.C., Nagy, K.A. & Diamond, J. 1990. Sustained metabolic scope. Proc. Nat. Acad. Sci. USA. 87:2324-2328.
Powers, D.R. 1992. Effect of temperature and humidity on evaporative water loss in Anna's hummingbird (Calypte anna). J. Comp. Physiol. 162B:74-84.
Powers, D.R. & Conley, T.M. 1994. Field metabolic rate and food consumption of two sympatric hummingbird species in southeastern Arizona. Condor 96:141-150.
Prinzinger, R., Lubben, I., & Schuchman, K.-L. 1989. Energy metabolism and body temperature in 13 sunbird species (Nectariniidae). Comp. Biochem. Physiol. 92A:393-402.
Pyke, G. 1980. The foraging behaviour of some Australian honeyeaters: a review and some comparisons with hummingbirds. Austr. J. Ecol. 5:343-369.
Ricklefs, R.E., Konarzewski, M. & Daan, S. 1996. The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am. Nat. 147:1047-1071.
Roberts, W.M. 1995. Hummingbird licking behaviour and the energetics of nectar feeding. Auk 112:456-463.
Weathers, W.A., Paton, D.A. & Seymour, R.S. 1996. Field metabolic rate and water flux of nectarivorous honeyeaters. Austr. J. Zool. 44:445-460.
Williams, J.B. 1991. On the importance of energy considerations to small birds with gynelateral intermittent incubation. Proc. XX Int. Ornithol. Congr., Christchurch, New Zealand 1:1964-1975.
Williams, J.B. 1993. Energetics of incubation in free-living Orange-breasted Sunbirds in South Africa. Condor 95:115-126.
Williams, J.B. 1996. A phylogenetic perspective of evaporative water loss in birds. Auk 113:457-472.
Williams, J.B., Siegfried, W.R., Milton, S.J., Adams, N.J., Dean, W.R.J., Du Plessis, M.A., Jackson, S. & Nagy, K.A. 1993. Field metabolism, water requirements, and foraging behavior of wild Ostriches in the Namib. Ecology 74:390-404.
Table 1. Energy expenditure in four species of winter-breeding nectarivores.

Table 2. Water balance in four species of winter-breeding nectarivores.