S25.1: Patterns of variation in avian osmoregulatory physiology and their application to questions in ecology
David L. Goldstein
Department of Biological Sciences, Wright State University, Dayton, Ohio 45435, USA, e-mail david.goldstein@wright.edu
Goldstein, D.L. 1999. Patterns of variation in avian osmoregulatory physiology and their application to questions in ecology. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1417-1426. Johannesburg: BirdLife South Africa.Homeostatic regulation involves the interaction of a number of regulating physiological systems which respond to environmental change so as to protect the values of regulated variables. The capacities of these regulating systems, and potentially the values of the regulated variables, can vary within individuals over time, and also between individuals, populations, and species. In the present overview, I use examples from the osmoregulatory systems of birds to illustrate these patterns of variation, including aspects of inter- and intra-specific variation in laboratory and field settings. Data from such physiological studies can be used to explore questions at several levels in ecology. How does physiological demand influence individual behaviour? How do physiological constraints influence geographic ranges? To what extent is plasticity in physiological systems itself an evolved trait? Currently available data provide only glimpses into these connections between physiology and ecology.
INTRODUCTION
Physiologists study the ways in which organisms maintain the integrity of their ‘internal milieu’, and how the whole animal and its subordinate components (organs, tissues, cells) respond to the constantly changing cues about the internal and external environment. For comparative and ecological physiologists, many of the questions of interest relate to patterns of variation surrounding this central objective, and to understanding physiological responses to naturally occurring environmental demands. How does the physiology of an individual species respond to some controlled environmental change in the laboratory? How do these responses differ between species, and how is this related to the demands which the animals might face in their natural habitats? How are these physiological capacities utilised in the field? Thus, physiology naturally addresses questions about the interaction between an animal and its environment, and as such has obvious links to ecology. Physiology per se entails the study of individuals, and so might address most naturally those questions in ecology that relate to the biology of individual organisms. What are the energy costs, the implications for water balance, the pH sensitivities of various processes? How does this influence the pattern of habitat use, or of food choice? Are animals physiologically stressed in the field, how do they prevent or alleviate this, how does this relate to their ability to survive and reproduce? Additionally, though, the patterns of physiological variation between and within species can also address broader issues in ecology. What determines the geographic ranges of species? Are widespread species more generalised, or more broadly tolerant of physiological variation, than more localised species (Brown 1995)? What are the implications of such relations for responses of species to global climate change (Clarke 1996)? In the present overview, I will describe the patterns that emerge from studies of avian osmoregulatory physiology. In so doing, I hope to emphasise the paucity of data available to address these ecological questions.
OVERVIEW: CATEGORIES OF VARIABILITY
Interspecific vs. intraspecific variation
The focus of studies on ‘adaptive physiology’ has typically been comparisons between species. Such research might ask, for example, whether desert birds differ in some consistent way from non-desert species in their physiological capacities for desiccation resistance. A variety of comparative approaches, including allometric (e.g. Nagy & Peterson 1988, MacMillen & Baudinette 1993) and phylogenetic (e.g. Garland et al. 1997, Williams 1996) analyses can then be applied toward elucidating patterns of physiological differentiation from these studies. It is often the case that differentiation in physiology can be most clearly documented in species occupying relatively extreme environments, and the tendency has often been to study such species (Schmidt-Nielsen 1967). Moreover, it may be difficult to find a single species that occupies more than one such environment. Because of this, examinations of environmental adaptation typically derive from interspecific analyses, with subsequent comparisons between species or groups of species.
On the other hand, studies of intraspecific variation in avian osmoregulation are few, as is the case for many other areas of vertebrate physiology (Garland & Adolph 1992). Intraspecific studies can avoid the potential analytical pitfalls of multi-species studies related to confounding influences of phylogenetic relatedness, and could offer robust insights into physiological plasticity. Such studies are also necessary to address questions relating physiology to ecology and evolution. For example, understanding the role of physiological specialisation and constraint in establishing species boundaries may require elucidating patterns of variation between populations within a species (Brown 1995; see below).
Analysing osmoregulation: regulated vs. regulating variables
Successful osmoregulation involves the application of a set of negative feedback systems. Such systems can be described in terms of two classes of variables: those that are homeostatically regulated and those which are involved in achieving that regulation. For example, an environmental perturbation (e.g., lack of water) might result in an initial change in one of the regulated variables (e.g., a rise in plasma osmolality). This would initiate a homeostatic response involving one or more of the regulating effector organs (e.g. a drive to drink, a renal conservation of water). This response would prevent the regulated variable from fluctuating too far from its set point, and over some time course would return the regulated variable back toward its original set point value.
Regulated variables
For osmoregulation, the regulated variables relate to the volume and composition of the body fluids. The two principal regulated variables are the osmolality and the volume of the extracellular fluid, though birds appear generally more sensitive to perturbation of the former than the latter (Stallone & Braun 1986). Concentrations of specific solutes, such as sodium and potassium, are also regulated (see Skadhauge 1981). Other variables might be indicators of these regulated parameters, though not direct targets of osmoregulation themselves. For example, constancy of the total body water may be a consequence of regulating cellular and extracellular volumes and osmolalties, without there being any actual mechanism (e.g. a sensor) related to the body water volume per se. Likewise, the haematocrit (proportion of blood volume accounted for by red blood cells) reflects the interplay between systems regulating extracellular fluid volume and those regulating oxygen delivery (blood cell count). In practice, some of these variables, including extracellular fluid osmotic and ionic concentrations, total body water, and haematocrit, are easier to measure than others, such as extracellular fluid volume.
One expects values of regulated variables to be determined by the set points of the negative feedback systems. Thus, they should vary little over time. For such variables, differences among species, populations, or time points could indicate any of several possibilities. First, it is possible that one is observing episodes of homeostatic imbalance that is, individuals or populations are unable to regulate normally under some set of environmental circumstances (Bradshaw 1986). Second, it is possible that the set points have acclimated to the prevailing environmental circumstances. Third, it is possible that the populations or species actually differ in the genetically determined set points for the variable under investigation. Distinguishing between these possibilities may require a substantial research program.
Regulating variables
The regulating variables for osmoregulation relate to all of those systems which influence the balance of water and electrolytes. On the input side, these include ingestion and drinking. On the output side, they include evaporation and excretion. In addition, several neuroendocrine factors are involved in adjusting the function of these regulatory organs (see Skadhauge 1981). In principle, a number of indices reflecting the function of these organ systems could be measured. For the excretory losses, for example, one could measure aspects of renal function (rates of glomerular filtration and urine flow, concentration of the urine), of intestinal function (ion transport capacities, tissue transporting surface area), and of hormonal control (circulating levels of antidiuretic hormone or aldosterone). The overall water turnover rate also provides insight into the integrated function of these organ systems. In both laboratory and field studies, some of these variables (e.g. water turnover rate, plasma osmolality, haematocrit) have been reported more often than others (e.g. urine composition, circulating hormone concentrations). Regulating variables are used by animals to respond to fluctuating environmental conditions. Thus, the capacities of the regulating systems and the range over which they can operate might vary between species or populations. However, in contrast to the regulated variables, the function of the regulating systems is likely to vary over time as environmental circumstances change.
Evidence for adaptation or response to environmental demand?
Some studies of physiological systems can provide insight into what might be called adaptations, differences in functional design that enhance survivorship under some set of environmental circumstances. For the regulated variables, differences in design could be reflected in variation in set points. For example, one species or populations might regulate its plasma osmotic concentrations at 320 mosmol/kg, another at 350 mosmol/kg. Alternatively, adaptation might entail enhanced tolerance of deviation from the ‘normal’ value, and thus be reflected in variability. For example, it has been suggested that birds are more tolerant of change in plasma osmolality than are mammals (Braun 1985).
For the regulating variables, inferring differences in functional design must relate to the capacity of the regulating system, not to its value under any particular condition. For example, the identification that two species or populations differ in their water turnover rates or in their urine osmolalities in their respective habitats (e.g., Goldstein & Bradshaw 1998) does not by itself imply that the two species differ in their physiologies. The values of these variables might be quite different at some other time or locale, under other circumstances. To evaluate the capacities of the regulating physiological systems, one must bring the animals into the laboratory and evaluate the extent of physiological response to changing demand (e.g. McNabb 1969): what is the bird’s maximum urine osmolality, minimum water flux that maintains mass balance, or hormonal response to water deprivation?
The second type of inference one may make from measures of physiological condition relates not to the capacities or design of the organism, but to the coupling between the animal and its environment. Two birds with similar physiological capacities might be subjected to different regulatory demands, relating, for example, to the availability of sodium, or to the gradient for evaporative water loss. These demands might be reflected in different values of regulating variables such as intestinal transport or of water turnover rate.
Integrating these two types of interpretations may provide an approach to understanding the ability of species to tolerate various and varying environments. What are the limits to this tolerance, and at what point do physiological demands impact survivorship or fitness? One approach to operationally defining periods of physiological stress is to evaluate the regulated along with the regulating variables. A stressful period could then be defined as one in which the environmental demand induces a deviation of a regulated variable from its set point, even while the regulating systems are invoked in an effort to prevent that deviation (Bradshaw 1986). Thus, a rise in plasma osmolality, even while antidiuretic hormone level and urine concentration are elevated, implies a physiological demand beyond the homeostatic capacity.
PATTERNS IN THE DATA
With this framework as background, we can examine the patterns of variability in avian osmoregulatory physiology. Much of our understanding of fundamental mechanisms of avian osmoregulation derives from studies of domestic birds (primarily chickens & ducks), and studies of osmoregulation in the field are particularly few. The present overview is not meant to be comprehensive, but to illustrate the types of data that are available, and to suggest how these apply to answering questions in ecology.
Interspecific studies in the laboratory
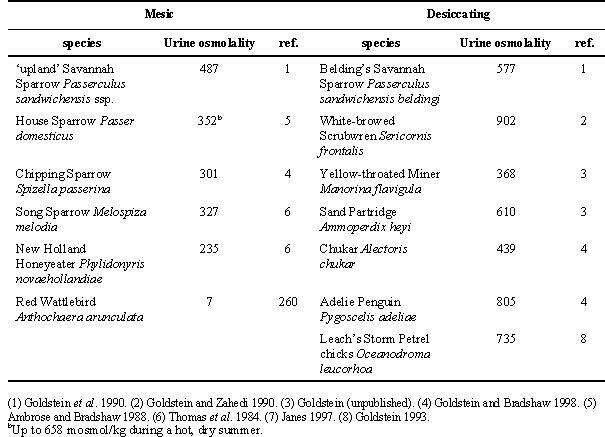
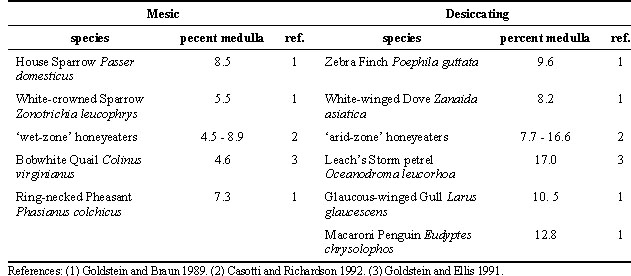
Interspecific comparisons of physiological capacities in the laboratory (see, for example, McNabb 1969a,b, Thomas et al. 1984) provide the opportunity to evaluate possible adaptations in avian osmoregulation. Among the regulated variables, we see no consistent patterns of variation between species or habitats. Plasma osmolality, sodium pool size (meq Na/kg body mass), body water and extracellular fluid volumes (as percent of body mass), and haematocrit are size independent and, so far as we know, invariant with respect to habitat. In contrast, values of regulating systems, including characteristics of both the regulatory organs themselves and of the physiological variables deriving from those organs, may differ markedly among species. Much of this variation may be related to body size, and allometric analyses have been applied to such characteristics as drinking rate (Bartholomew & Cade 1963), evaporative water loss rate (Bartholomew & Cade 1963), kidney mass (Hughes 1970), and renal structure and function (Yokota et al. 1985, Goldstein & Braun 1989). Interestingly, though, patterns of regulatory capacities related to varying habitats are few and just recently emerged. A recent phylogenetic analysis suggests that birds from desiccating environments do have lower rates of evaporative water loss than those from mesic habitats (Williams 1996). Studies of kidney morphology indicate that birds from desiccating environments generally have a higher proportion of medullary renal tissue (Table 1), but there are no data to suggest that urine concentrating abilities are enhanced as a consequence. Similarly, we have yet to elucidate consistent differences in intestinal transport capacity, or in evaporative water loss. Perhaps the absence of physiological differentiation reflects the importance of behavioural adjustment in maintaining hydromineral balance in the field.
Intraspecific studies in the laboratory
Intraspecific studies in the laboratory illuminate several patterns of variation in avian osmoregulation. First, even under controlled laboratory conditions there is a range of values observed for all measured variables; this is a basic premise of physiology, that homeostasis establishes a ‘relative constancy.’ Physiologists have typically focused on the mean values of these traits. However, the variation around the mean may also be of interest, and this variation is greater for some variables than for others. For example, in House Sparrows Passer domesticus plasma osmolality of birds provided with unlimited access to food and water had a coefficient of variation (c.v.) of 1.7 %, whereas the c.v. for haematocrit under the same circumstances was 6.9% (Goldstein & Zahedi 1990).
Second, a single population of birds can undergo physiological adjustment (acclimation) in response to sustained environmental demand. This phenomenon has been examined in captive-bred Bobwhite Quail, Colinus virginanus, which have an extended growth phase (typical of the precocial galliforms) during which they might be subjected to varying availability of water. When raised with limited access to water, these quail developed an enhanced capacity for renal tubular reabsorption of water, resulting in reduced urine flow, compared with birds raised with unrestricted water (Goldstein 1995).
Third, different populations of a species may have different physiological capacities. This phenomenon is well exemplified by two species of North American emberizids (the Song Sparrow, Melospiza melodia, and the Savannah Sparrow, Passerculus sandwichensis), in which some populations (subspecies) are salt marsh residents and others inhabit mesic habitats away from the salt marshes. In both species, the salt marsh birds are more tolerant of saline drinking waters, and for the Savannah Sparrows these differences are accompanied by substantial differences in the size and concentrating ability of the kidneys (Cade & Bartholomew 1959, Basham & Mewaldt 1987, Goldstein et al. 1990). Similar differences have been documented for a small Australian passerine, the White-browed Scrubwren Sericornis frontalis, in which several subspecies occupy habitats with a range of aridity and salt availability (Ambrose & Bradshaw 1988). In other cases, less differentiated wild populations may also diverge in physiological responses. For example, House Sparrows from the arid southwest of the U.S.A. lost body mass at a slower rate in response to water deprivation than did birds from the more mesic northeastern part of the country (Goldstein & Zahedi 1990). Application of experimental designs in which differentiated populations, for example the subspecies of Savannah Sparrow described above, are raised in common environments (‘common garden’ designs) could address the relative contributions of acclimation vs. genetics to these patterns. Such studies have not been conducted for any aspect of osmoregulation in wild birds.
Interspecific studies in the field
Studies of avian osmoregulatory function in the field are for the most part limited to a few variables (e.g. water turnover rate) at selected times of year. These studies do permit some broad generalisations about coupling between the animal and its environment. One of the most striking is the consistency with which desert species have lower water turnover rates than non-desert species. This first became apparent in two widely separated studies of desert phasianids (Goldstein & Nagy 1985, Kam et al. 1987), and has since received support from a number of other studies (see Williams et al. 1993), including a recent study of three species within the same family (Goldstein & Bradshaw 1998). In addition to lower water fluxes, desert birds also have higher urine osmolalities in the field (Table 2). These two regulating variables (water flux and urine concentration) differ in the arid-zone species without any detectable differences in regulated variables such as body water content or plasma osmolality. Analogous findings have been reported with respect to sodium balance (higher fluxes in birds from saline environments, but without any effect on the body sodium pool; see Goldstein and Bradshaw 1998). Thus, in the face of differences in environmental demand, birds are indeed responding with physiological adjustment, not only with behaviour.
Intraspecific studies in the field
Intraspecific studies of osmoregulatory function or condition in the field are few. However, the studies that have been conducted indicate both temporal and spatial (geographic) patterns of variation. Studies indicating temporal variation in osmoregulatory function have taken place in habitats ranging from desert to mesic (Alkon et al. 1982, Ambrose & Bradshaw 1988, Rooke et al. 1986, Goldstein & Bradshaw 1998, Goldstein & Zahedi 1990) and have evaluated both regulated (plasma osmolality, body water volume, haematocrit, body exchangeable sodium pool) and regulating physiological systems (water turnover rate, urine osmolality, circulating hormone concentrations). The general patterns that emerge from these studies are quite consistent.
Within a species there is little evidence for variation of most regulated variables over time. An exception to this generality is that haematocrit is often higher during the colder times of year than during the warmer (Swanson 1988, Goldstein & Zahedi 1990). However, this most likely relates to changes in the circulating number of red blood cells (erythropoiesis), related to elevated metabolic rates during the cold times, rather than to changes in the plasma (extracellular fluid) volume. Body water content also sometimes varies (Alkon et al. 1982), but it is not clear to what extent this represents changes in the body water compartments or in body solids. It is also noteworthy that the range and variance in the field of even closely regulated variables like plasma osmolality may exceed that occurring under controlled conditions in the laboratory (Goldstein & Zahedi 1990). Together with the observation that plasma osmolality was significantly correlated with ambient temperature, these findings are suggestive of environmental demands impinging on the precision of osmoregulation.
In contrast to the regulated variables, a number of regulating systems do change seasonally. Urine osmolality may be elevated during warm or dry times of year, water turnover may be decreased, and circulating concentrations of hormones may change (see above references). As in the interspecific comparisons, these changes indicate physiological responses to changing environmental conditions; behaviour alone is not used, or is not adequate, to offset changing availability of water and electrolytes.
There are fewer data to address questions of geographic variation in osmoregulatory physiology. Different water turnover rates have been measured in two populations of the New Holland Honeyeater Phylidonyris novaehollandiae (Weathers et al. 1996, Goldstein & Bradshaw 1998). However, many factors (diet, moult stage, ambient temperature, etc.) could account for this difference. Perhaps the most intensive study of osmoregulatory differentiation among bird populations in the field is that of Ambrose & Bradshaw (1988), who examined water and electrolyte metabolism in White-browed Scrubwrens (Sericornis frontalis) from three sites ranging over a gradient of aridity. Populations differed in a number of variables, including regulated variables such as plasma osmolality, exchangeable sodium pool size, and percent body water content, and also in regulating variables such as water turnover rate and urine osmolality. In Savannah Sparrows, too, there is evidence that different subspecies (salt-marsh resident vs. non-salt-marsh migrant) differ in their osmoregulatory function in the field. The flow rate, osmolality, and sodium concentration of urine in salt-marsh birds all exceeded those of upland individuals (Goldstein et al. 1990), and this was accompanied by a slightly, but significantly, higher plasma osmolality in the salt marsh birds (349 vs. 339 mosmol/kg). For both of these species (Scrub-wren and Savannah Sparrow), there are accompanying laboratory studies of physiological differentiation between sub-species (see section above). However, it is still not possible to determine the extent to which the differences in regulated variables in the field represent acclimation, selection, or physiological duress. The laboratory and field studies together do present a picture of species divided into subspecies with differing physiological capacities, though, and these capacities are exerted in the field under natural circumstances.
APPLICATIONS: ANSWERING ECOLOGICAL QUESTIONS WITH PHYSIOLOGICAL DATA
Successful osmoregulation is a fundamental requirement of homeostasis for birds. Thus, appropriate availability of water and electrolytes (i.e., availability within the birds’ regulatory abilities) is necessary for survival, along with other requirements such as adequate food and a tolerable thermal environment. In the discussion above, I have outlined the patterns that exist in available data on osmoregulation by wild birds. How can these data be used to address questions in ecology?
Balancing the individual water budget: what are the roles of physiology and behaviour?
How does a bird balance its water budget? Most studies of avian osmoregulatory ecology address this level of analysis, and we have a substantial data for particular types of measures related to this question. For example, rates of water turnover have now been measured for several dozen bird species, ranging from desert to mesic to marine, and clear allometric and habitat-related patterns emerge (Nagy & Peterson 1988). These data suggest that water fluxes do vary among habitats, and both inter- and intraspecific variation in values of regulating variables (e.g. urine concentration) in the field support the notion that birds respond physiologically to changing osmoregulatory demand. Examples also exist where osmoregulatory concerns drive behavioural patterns. Regular visitation to water holes is an obvious example (Fisher et al. 1972). In the southeastern United States, parent White Ibis Eudocimus albus fly long distances (up to 25 km) to procure fresh-water prey for their nestlings, despite themselves being able to survive on prey from the nearby saline environments (Johnston & Bildstein 1990). Nevertheless, our understanding of the interplay between physiology and behavior in balancing osmoregulatory demands is quite limited. For example, evaporative water loss rates are apparently lower in arid-zone birds (Williams 1996), and this route of water efflux may be a substantial and component of overall water balance. Yet we are essentially ignorant of the partitioning of water loss between evaporative and excretory routes in the field, and we have just a rudimentary understanding of the way in which evaporative water loss varies with changes in microclimate or osmoregulatory condition of the animal. Thus, we are unable to assess the impact of behavioural adjustment (e.g. movement between microclimates) on water balance or, conversely, to evaluate the extent to which osmregulatory demand influences habitat use. This contrasts with our understanding of thermoregulation, for which both empirical and theoretical foundations allow analysis of the interplay between physiology and behaviour (see, e.g., Bakken 1980, Buttemer et al. 1985).
The evolution of osmoregulation: which traits are critical?
An understanding of the mechanistic bases of osmoregulation, and of the exploitation of these mechanisms in the field, would better allow us to ask questions about selective pressures on these physiological systems. Avian osmoregulation represents a complex interaction between several organ systems, and it is still unclear how birds prioritise these systems. To what extent are cutaneous evaporation, respiratory evaporation, or excretory water loss varied to defend against desiccation? How does this vary among species, or in species faced with different sorts of environmental challenges (e.g. hot vs. cold dehydration)? A general question that persists in evolutionary biology is why some species or species groups can adapt to changing environments, or can expand their ranges into new environments, whereas others apparently cannot (e.g. see discussion in Clarke 1996). Before we can answer such questions, we must understand the limits of flexibility in the physiological systems. We still have scant data on acclimation of avian osmoregulation, and most of the regulating variables themselves (e.g. hormonal systems, intestinal function, regulation of evaporation) remain largely unstudied in non-domestic species, either in the laboratory or in the field.
The geographic ranges of species: what role of physiological constraints?
Answers to questions about physiological flexibility can also address questions concerning patterns in biogeography. Among these are patterns related to the number of species per geographic region and those concerning the sizes of geographic regions occupied by species. Explanations for such patterns range from stochastic models to mechanistic explanations based on physiological tolerances. Evaluation of these models requires an understanding of the ‘physiological geography’ of species. A few studies (e.g. Degen et al. 1983, MacMillen & Baudinette 1993) have attempted to analyse the role of osmoregulatory differentiation in determining spatial or temporal patterns of range segregation between species. A few also suggest physiological differentiation among well defined subspecies (see discussion above of Savannah and Song sparrows). However, we have little understanding of broader patterns. Are widespread species more physiologically generalised (more tolerant of varying external environments, or perhaps of more varying internal environments) than species with narrower distributions? Or are widespread species instead composed of populations expressing a gradation of physiological characteristics, so that each is adapted to its regional environment?
Thus, questions in physiology naturally grade from those relating to individual function on to broader application in ecology and evolution. Evaluation of these questions will require some shifts and expansion from the data base that currently exists. We will need more studies of integrated physiological systems, more studies of intraspecific variation and of acclimational flexibility, and more combination of laboratory with field studies. By attempting these studies, physiologists and ecologists will have much to discuss.
REFERENCES
Alkon, P.U., Pinshow, B. & Degen, A.A. 1982. Seasonal water turnover rates and body water volumes in desert Chukars. Condor 84: 332-337.
Ambrose, S. J. & Bradshaw, S.D. 1988. The water and electrolyte metabolism of free-ranging and captive White-browed Scrubwrens, Sericornis frontalis (Acanthizidae), from arid, semi-arid, and mesic environments. Aust. J. Zool. 36: 29-51.
Bakken, G.S. 1980. The use of standard operative temperature in the study of the thermal energetics of birds. Physiol. Zool. 53: 108-119.
Bartholomew, G.A. & Cade, T.J. 1963. The water economy of land birds. Auk 80: 504-539.
Basham, M.P. & Mewaldt, L.R. 1987. Salt water tolerance and the distribution of South San Francisco Bay song sparrows. Condor 89: 697-709.
Braun, E.J. 1985. Comparative aspects of the urinary concentrating process. [Review]. Renal Physiol. 8: 249-60.
Bradshaw, S.D. 1986. Ecophysiology of desert reptiles. Academic Press, New York.
Brown, J. 1995. Macroecology. Chicago University Press, Chicago.
Buttemer, W.A. 1985. Energy relations of winter roost-site utilization by American Goldfinches (Carduelis tristis). Oecologia 68: 126-132.
Cade, T.J. & Bartholomew, G.A. 1959. Sea-water utilization by Savannah Sparrows. Physiol. Zool. 32: 230-238.
Casotti, G. & Richardson, K.C. 1992. A stereological analysis of kidney structure of honeyeater birds (Meliphagidae) inhabiting either arid or wet environments. J. Anat. 180: 281-288.
Clarke, A. 1996. The influence of climate change on the distribution and evolution of organisms. Pp. 377-407 in I. A. Johnston and A. F. Bennett, eds. Animals and Temperature. Cambridge University Press, Cambridge.
Degen, A.A., Pinshow, B. & Alkon, P.U. 1983. Summer water turnover rates in free-living Chukars (Alectoris chukar) and Sand Partridges (Ammoperdix heyi) in the Negev desert. Condor 85: 333-337.
Fisher, C.D., Lindgren, E. & Dawson, W.R. 1972. Drinking patterns and behavior of Australian desert birds in relation to their ecology and abundance. Condor 74: 111-136.
Garland, T. Jr. & Adolph, S.C. 1991. Physiological differentiation of vertebrate populations. Ann. Rev. Ecol. System. 22: 193-228.
Garland, T. Jr., Martin, K.L.M. & Díaz-Uriarte, R. 1997. Reconstructing ancestral trait values using squared-change parsimony: plasma osmolarity at the origin of vertebrates. In: Sumida, S.S. & Martin, K.L.M. (eds) Amniote Origins. Academic Press, New York: Pp. 425-5014._
Goldstein, D.L. 1995. Effects of water restriction during growth and adulthood on renal function of Bobwhite Quail, Colinus virginianus. J. Comp. Physiol. B. 164: 663-670.
Goldstein, D.L. & Bradshaw, S.D. 1998. Regulation of water and sodium balance in the field by Australian honeyeaters (Aves: Meliphagidae). Physiol. Zool. 71: 214-225.
Goldstein, D.L. & Braun, E.J. 1989. Structure and concentrating ability in the avian kidney. Am J. Physiol. 256: R501-9.
Goldstein, D.L. & Ellis, C.C. 1991. Effects of chronic water restriction on whole body and kidney growth in Bobwhite Quail. Am J. Physiol. 261: R117-R125.
Goldstein, D.L. & Nagy, K.A. 1985. Resource utilization by desert quail: time and energy, food and water. Ecology 66: 378-387.
Goldstein, D.L., Williams, J.B. & E.J. Braun. 1990. Osmoregulation in the field by salt-marsh savannah Savannah Sparrows Passerculus sandwichensis beldingi. Physiol. Zool. 63: 669-682.
Goldstein, D.L. & Zahedi, A. 1990. Variation in osmoregulatory parameters of captive and wild House Sparrows Passer domesticus. Auk 107: 533-538.
Hughes, M.R. 1970. Relative kidney size in nonpasserine birds with functional salt glands. Condor 72: 174-178.
Janes, D.N. 1997. Osmoregulation by Ad?lie Penguin chicks on the Antarctic peninsula. Auk 114: 488-495.
Johnston, J.W. & Bildstein, K.L. 1990. Dietary salt as a physiological constraint in White Ibis breeding in an estuary. Physiol. Zool. 63: 190-207.
Kam, M., Degen, A.A. & Nagy, K.A. 1987. Seasonal energy, water and food consumption of Negev Chukars and Sand Partridges. Ecology 68: 1029-1037.
MacMillen, R.E. & Baudinette, R.V. 1993. Water economy of granivorous birds: Australian parrots. Func. Ecol. 7: 704-712.
McNabb, F.M.A. 1969. A comparative study of water balance in three species of quail-I. Water turnover in the absence of temperature stress. Comp. Biochem. Physiol. 28: 1045-1058.
McNabb, F.M.A. 1969. A comparative study of water balance in three species of quail-II. Utilization of saline drinking solutions. Comp. Biochem. Physiol. 28: 1059-1074.
Nagy, K.A. & Peterson C.C. 1988. Scaling of water flux rate in animals. University of California Publications in Zoology Vol 120, University of California Press, Los Angeles.
Rooke, I.J., Bradshaw, S.D., Langworthy, R.A. & Tom. J.A. 1986. Annual cycle of physiological stress and condition of the Silvereye, Zosterops lateralis (Aves). Aust. J. Zool. 34: 493-501.
Schmidt-Nielsen, K. 1967. The unusual animal, or to expect the unexpected. Fed. Proceed. 26: 981-986.
Skadhauge, E. 1981. Osmoregulation in birds. Springer-Verlag, New York.
Stallone, J.N., & Braun, E.J. 1986. Osmotic and volemic regulation of plasma arginine vasotocin in conscious domestic fowl. Am. J. Physiol. 250: R644-57.
Swanson, D.L. 1988. Seasonal variation in cold hardiness and peak rates of cold-induced thermogenesis in the Dark-eyed Junco (Junco hyemalis). Auk 107: 561-566.
Thomas, D.H., Pinshow, B. & Degen, A.A. 1984. Renal and lower intestinal contributions to the water economy of desert-dwelling phasianid birds: comparison of free-living and captive Chukars and Sand Partridges. Physiol. Zool. 57: 128-136.
Ward D, & Pinshow B. Temperature regulation of the Great Grey Shrike (Lanius excubitor) in the Negev Desert-II. Field measurements of standard operative temperatures and behaviour. J. Thermal Biol. 20(3). 1995. 271-279.
Weathers, W.W., Paton, D.C., & Seymour, R.S. 1996. Field metabolic rate and water flux of nectarivorous honeyeaters. Aust. J. Zool. 44: 445-460.
Williams, J.B. 1996. A phylogenetic perspective of evaporative water loss in birds. Auk 113: 457-472.
Williams, J.B., Siegfreid, W.R., Milton, S.J., Adams, N.J., Dean, W.R.J., Du, P.M.A., Jackson, S. & Nagy, K.A. 1993. Field metabolism water requirements and foraging behavior of wild oOstriches in the Namib. Ecology 74: 390-404.
Yokota, S.D., Benyajati, S. & Dantzler, W.H. 1985. Comparative aspects of glomerular filtration in vertebrates. Renal Physiol. 8: 193-221.
Table 1. Percent of kidney accounted for by medullary tissues in birds from mesic and from desiccating environments

Table 2. Urine osmolality in birds from mesic and desiccating environments.