S24.1: Post fledging survival of Great Tits Parus major in relation to sex
Jeremy K. Blakey & Christopher M. Perrins
Edward Grey Institute of Field Ornithology, Department of Zoology, South Parks Road, Oxford OX1 3XJ, UK, fax 01865 271168, e-mail jeremy.blakey@zoology.oxford.ac.uk
Blakey, J.K. & Perrins, C.M. 1999. Post fledging survival of Great Tits Parus major in relation to sex. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1364-1380. Johannesburg: BirdLife South Africa.Previous studies in Wytham Woods, Oxfordshire U.K., have shown that breeding male Great Tits have a higher lifetime reproductive output than females. Males live longer than females, so fewer males than females breed. Possible explanations for this difference are a male biased fledgling sex-ratio, higher male post-fledging mortality or some surviving males do not breed. Fledgling survival is positively correlated with fledging weight, and the size of the beech crop Fagus sylvatica in the autumn after fledging. In this study, we used a molecular technique to sex cohorts of nestlings to examine sex-related post-fledging survival. Cohorts contained significantly more males than females; this is partly due to female-biased nestling mortality. In a good beech year overall post-fledging survival was higher and the relationship between the probability of survival and fledging weight is not significantly different between the sexes. In the non-beech year, overall survival was lower, but females survived significantly better than males. Similar significant year and sex differences in the pattern of post-fledging survival with fledging weight are a feature of other Great Tit populations, although the nature of the phenotype/environmental interactions that are responsible are largely unknown. We found that the presence of Beech mast in the autumn after fledging is a significant predictor of mean cohort fledging weight; since post-fledging mortality occurs before beech mast is produced, we suggest that the between-year differences are at least partially linked to fledglings being lighter in non-beech years.
INTRODUCTION
The post fledging period is critical for many avian species. For a small passerine, such as a Great Tit Parus major, there are four possible outcomes of the immediate post-fledging period: predation, mortality (other than by predation), dispersal from the natal population or recruitment into the local population. All of these outcomes could be controlled by stochastic, phenotype dependent or environmentally determined factors, and interactions between them. If fledgling phenotype (e.g. sex, weight, condition) is an important determinant in these processes, then it should be reflected in reproductive decisions by parent birds to maximise their fitness by manipulating offspring phenotype in a varying environment (e.g. Verboven & Visser 1998). In addition, the post-fledging period may be a key determinant in population dynamics.
The Great Tit is a hole-nesting species which lays large clutches and, in prime habitats at least, normally raises all or most of its young (Perrins 1979); in our study population in Wytham Woods near Oxford, England, the annual mean number of fledglings per pair lies in the range of three to eight (Perrins in press). Since populations are fairly stable and since annual adult mortality in Great Tits is about 50%, it follows that only approximately one young per pair survives to enter the breeding population. Hence, many young die before they reach breeding age and a large part of this loss seems to occur within a few weeks of leaving the nest (Perrins 1965). In Wytham the percentage of fledglings which survived to recruit into the breeding population has varied between 3% and 21% in different years (Perrins in press), though an unknown number of other fledglings will have survived and emigrated from the study area to breed elsewhere (Verhulst et al. 1997). The year effect is important because the number of young surviving to breed is the "key factor" in population change (Perrins 1980), and accounts for much of the variation in individual lifetime reproductive success (McCleery & Perrins 1988).
There are two major factors which affect the survival of the individual young (Perrins 1965, Tinbergen & Boerjlist 1990). First, survival is closely correlated with fledging mass, heavier chicks surviving better than lighter ones in most years; factors affecting fledging mass include brood-size and date in the season at which the brood is raised. Hence, chick phenotype is partly dependent on parental reproductive decisions. Second, there are large year effects, whose cause is not understood but which lead to the large variations in annual survival of the young mentioned above. However, we do know that post-fledging survival to the following November is positively correlated with the presence of Beech Fagus sylvatica mast (Perrins 1965), an important winter food source for Parus species.
Until recently we were unable to reliably determine the sex of the young while they were in the nest, so it was not possible for us to examine the pattern of post fledging survival of the two sexes. However, in adult Great Tits the two sexes differ in mass by about 1g, the males being about 5% heavier than the females. The same is true for those fledglings which survive and moult into adult plumage (and so can be sexed); at the age of 15 days, the males were heavier than the females by about 1 g. If we assume that the same is true for all nestlings, then the males must grow faster while in the nest.
Such differences in growth rates and body size might be expected to affect the survival rates of the two sexes differently. In Wytham, breeding males have a higher lifetime reproductive output than females, despite being socially monogamous (McCleery & Perrins 1988). This imbalance stems from the fact that recruited males tend to live longer than females, but means that fewer males than females enter the breeding population (McCleery & Perrins 1988). We do not know why this is, but possible explanations include males having a higher mortality than females between fledging and breeding, or more males failing to obtain mates and hence not breeding (McCleery & Perrins 1988).
With the advent of DNA technology, it is now possible to determine the sex of the young birds from a small sample of blood such as can be obtained by clipping a toe-nail or removing a growing feather. In this paper we examine sex and fledging weight as determinants of post-fledging survival and their interaction with strong year effects, particularly the presence of beech mast.
METHODS
Study population
Data were collected from the long term Great Tit population study at Wytham, Oxford, U.K. (Perrins 1979). Wytham is a 320 ha mixed, predominantly deciduous woodland, provided with nesting boxes for Parus species at an average density of 3.1 boxes per hectare; it is thought that virtually all the Great Tits breeding in the wood breed in the nest boxes (East & Perrins 1988). The boxes were visited weekly during the breeding season to determine lay date, clutch size and hatching success. Hatch date was determined by weighing the 2 or 3 heaviest chicks and using a standard growth curve (Gibb 1950). At Day 15 (date of hatch = Day 1), chicks were ringed and weighed to the nearest 0.1 g. This was taken as the fledging weight (fledging occurs from about Day 18), because nestling mass reaches an asymptote by Day 13 (Gibb 1950). A growing secondary wing feather was removed, under licence, from each nestling for subsequent molecular sexing (see below). Parent birds were trapped and weighed whilst feeding nestlings. A very high proportion (<90%) of successful breeders were identified in this way, but few birds from nests that failed before the chicks reached an age of about one week are captured. Thus a small proportion of recruited fledglings are not identified. In Wytham, unlike most of continental Europe, Great Tits are almost exclusively single brooded.
Sexing nestlings
Nestling sex was determined by using the polymerase chain reaction (PCR) to amplify a portion of the W-linked avian CHD gene (CHD-W) in females, and its non-W-linked homologue (CHD-NW) present in both sexes (Griffiths et al. 1996). Genomic DNA was extracted from 2mm of the proximal tip of a growing secondary feather with Guanidine Isothiocyanate and isolated by binding onto silica (Boom et al. 1990). PCR reactions were performed as described by Griffiths et al. (1998), using an annealing temperature of 52°C. PCR products were separated on 3% agarose gels and visualised with Ethidium Bromide. Birds were sexed on the presence of the PCR products of CHD-Z (350 bases; present in both sexes) and CHD-W (385 bases; present in females only). Hence, males give a single band genotype and females two bands (Fig. 1).
Analysis
Differences in survival patterns were examined between three cohorts of fledglings in years that differed in the abundance of beech mast in the autumn following fledging. As beech trees are highly synchronous in their cropping, autumn beech mast crop is scored as a 3-level category for the whole wood each year (1 = no beech crop, 2 = moderate crop; either a small proportion of trees producing mast or a larger proportion of trees producing infertile mast; 3 = high proportion of trees producing fertile mast). The three cohorts used were from three years of different beech score (1988=1, 1994=2, 1995=3). However, the sample of sexed chicks for 1994 was very incomplete with respect to the size of the complete cohort for the wood for that year. The initial model relating survival to weight and sex incorporated this dataset; subsequent analyses comparing beechmast and non-beechmast years used 1988 and 1995 cohorts only. The analysis is based on 159 recruits from 1988 and 307 from 1995.
Since we were interested in individual-specific phenotypic traits, we used the fledgling as the unit of analysis, although this suffered from some pseudoreplication in the data (see discussion). Survival was determined from recapture of recruits into the breeding population at fledging year +1 or, uncommonly, +2. Local recruitment was used as as a binary response variable (0 = not recruited, 1 = recruited) in logistic regression using the GLIM (Generalised Linear Interactive Modelling) statistical package. This allows the inclusion of both categorical and continuous predictor variables into a single linear model. Significance tests are on the basis of the change in deviance (D D) on fitting model terms; this is distributed approximately as c2 with degrees of freedom equal to the change in degrees of freedom (D df) on fitting the new model. Minimum adequate models were constructed by forward inclusion of significant variables, starting from the null model. As well as investigating the effects of year, nestling sex and fledging weight on fledgling survival, we also controlled for hatching date.
An alternative method of examining survival with respect to phenotype is to calculate selection differentials. This has the advantage of summarising the magnitude and shape of the relationship between weight and survival into single statistics, which can be compared between samples. Analysis of selection differentials were based on the methods described by Price & Boag (1987) and Merila et al. (1998). Univariate standardised selection differentials were calculated by regressing relative fitness (w’; calculated as the individual survivorship score divided by the population mean survivorship) on standardised (z; mean = 0, variance = 1) mass values. The presence of stabilising selection was tested by calculating the non-linear selection gradient (
g)=2b2, where w’=c+bz+b2z2RESULTS
Fledgling sex ratios and size dimorphism
The three cohort groups all showed a slight, but non significant, male biased sex ratio at fledging (proportion of males: 0.52-0.53, G1-tests, all not significant). Amongst broods with no nestling mortality (either all eggs produced a fledgling, or all eggs hatched produced a fledgling), or where chicks were sampled before mortality took place, we found no evidence for systematic sex-ratio bias (Table 1). Broods with nestling mortality were slightly male-biased (Table 1); however, this does not necessarily imply a higher probability of mortality for females, since the probability of mortality may co-vary with the starting sex ratio in the nest at hatching (Fiala 1980). We do not know the sexes of the majority of the dead nestlings because most of them die early and are almost invariably removed from the nest by the parents. However, late nestling mortality (where nestlings died after day 15), although relatively rare, was strongly female-biased (G
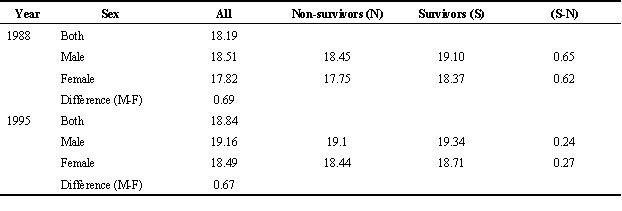
1=4.28, n = 46 nestlings, P<0.05).Male fledglings were significantly heavier than females in both beech and non-beechmast years (Table 2). Considering a group of 50 nests where nestlings were weighed at regular intervals, this size dimorphism is evident even at day 4 (Fig. 2) despite hatching from similarly sized eggs. Although siblings within a brood are not independent data since they share common nest environment and parental effects, even after controlling for this females are significantly smaller at Day 4 (F58, 378=15.50, P<0.05, controlling for brood effect and brood size) right through to Day 15 (F58,374=15.50, P<0.001).
Patterns of post-fledging survival
Analysis of the probability of local recruitment with a logistic model for the three years showed that, as with other studies (e.g. Lindén et al. 1992, Verboven & Visser 1998) this was negatively related to fledging date and positively to fledging mass (Table 3). In addition, the significant sex, sex*weight and sex*year interaction terms in the model suggest that the shape of the curve relating fledgling weight to recruitment is different for the two sexes, and that this differs between years. When between-year differences in recruitment rate are controlled for, inclusion of a quadratic term (weight2) improves the fit of the weight/recruitment relationship. This suggests that the relationship between weight and survival is non-linear, at least for some sex/year classes, as suggested by Perrins (1965).
The probability of recruitment of the two sexes was investigated by constructing separate models for the beech and non-beech years (Table 4). The quadratic term (weight2) is significant for females in the non-beech year only. Because the significance of this quadratic term may be based on the rather small number of heavy females that were not recruited, the weight/recruitment curves are presented with fitted models incorporating weight only, and only over the range that recruitment occurred (Fig. 3). In the beech year, overall survival was higher, the relationship between the probability of survival and fledging weight is approximately linear and not significantly different between the sexes. In the non-beech year, overall survival was lower, and inclusion of fledgling sex as a factor significantly increased the fit of the model. Females survived significantly better than males in all except the highest weight classes, where the sample size was very small. However, given that the mean weight of male fledglings is higher than females, the proportion of recruits produced within each weight class is different between the sexes, at least in the non-beech year. The modal weight class for producing male recruits is higher than in females (Fig. 4); heavier males survive less well than females, but there are more of them. This suggests that the process of selection is not for absolute weight, but some interaction between sex and weight.
Examination of the selection coefficients on fledgling weight confirm these patterns (Table 5). Directional selection in the non beech year for both sexes, and significantly larger for males. In addition, the non-linear selection coefficient (
g) is negative and approaches significance for females (g=-0.266±0.14, P=0.058); this implies weak stabilising selection, suggesting that the heaviest females do survive significantly less well. In the beech year, there is weak (non-significant) directional selection which is not different between the sexes. Hence the shape of the fitness function relating weight to survival appears to vary both between years and between the sexes in some years.Beech mast and fledging weight
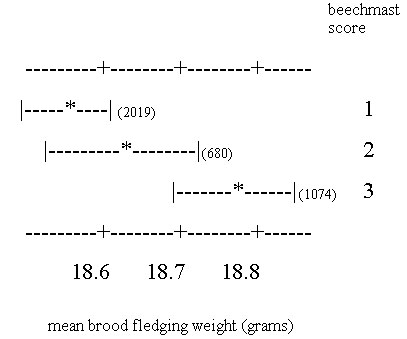
The presence of beech mast is a significant determinant of post fledging survival, both in terms of the proportion of fledglings surviving, and the shape of the fledgling weight /survivorship curve. Although beechmast is a major food source for tits in the winter, the majority of post-fledging mortality is believed to occur before beech mast is produced (Perrins 1980) so the relationship cannot be a simple one. Because the mean fledgling weight of the whole cohort can vary considerably between years, we investigated the relationship between fledging weight and the size of the beech crop in the autumn after fledging. For the years 1978-1996, the mean brood fledgling weight differed significantly between the three categories of differing beech mast abundance (F2, 3770=7.20, P<0.001); fledgling weights were lower in non-beech years than in beech years (Fig. 5). However, the magnitude of the difference was only about one sixth of the extreme between-year difference in mean fledging weight. Because a number of different factors are known to affect fledgling weight, it is perhaps not surprising that the presence of beech mast only partially explains the between-year variation. To investigate this further, beech score was used as categorical variable in a linear model incorporating other predictors of fledging weight (Table 6). When other variables that are known to affect fledging weight (hatch date, brood size, habitat) are controlled for, the abundance of beech mast in the following autumn is still a significant correlate of fledging weight.
Potential biases in the analyses
We have used recruitment into the breeding population as the measure of local survival on the basis that the major component of post-fledging mortality occurs soon after fledging and has occurred by the beginning of autumn (Dhondt 1979). This is an indirect estimate of survival over the post-fledging period because, although recruits have a high probability of capture, some over-winter mortality also occurs. However, it overcomes the potential bias of weight-dependent recapture if, for example, heavier birds of one sex are more likely to be captured at winter feeders. Although mortality rates differ between age, sex and immigrant status classes of Great Tits within Wytham (Clobert et al. 1988), we do not expect mortality between autumn and recruitment the following spring to be dependent on fledging weight (Tinbergen & Boerlijst 1990). Thus using recruitment as a measure of post fledging survival may reduce the amount of data available, but we do not expect the pattern of survival to recruitment with respect to weight to be different from that to winter. The winter trapping programme in Wytham has been patchy, varying in intensity between years and different areas of the wood. Even so, using a subset of the 1988 cohort from the areas of Wytham where winter trapping was regularly carried out confirms that the pattern of post fledging survival to the winter (
c21= 4.15, P<0.05 on inclusion of sex in a logistic model having controlled for date and weight) was similar to that found using survival to recruitment.A major potential problem in analysing local survival probability with respect to phenotype is an inability to distinguish between local and global survival, since quantitative studies of emigration are rather difficult to undertake in most avian populations. If the probability of dispersal is non-random with respect to body mass or sex or both, then the relationships described between fledging mass and local survival do not accurately reflect the selection episodes. Within Wytham, female natal dispersal is on average greater than males (Delestrade et al. 1996); similarly, among birds dispersing to the nearby surrounding area, females move further than males (Verhulst et al. 1997). However, among birds dispersing in either direction the proportion of the two sexes is similar, hence the overall exchange of birds between Wytham and the surrounding area is not sex-biased. In addition, the probability that a Wytham fledgling dispersed to the surrounding area was independent of its mass (Verhulst et al. 1997). It is still possible that dispersal over larger distances is weight dependent, but we have no evidence to confirm or refute this. In other Great Tit populations, the probability of dispersal seems to be independent of fledging mass (Verboven & Visser 1998, Lindén et al. 1992, Smith et al. 1989), although this may not be true in all cases (Drent 1984). It is also possible that predation of fledglings may not be random with respect to their mass. The main predator of fledgling tits in Wytham is the Sparrowhawk Accipiter nisus. Whilst predation on tits in winter is mass-dependent, with heavier birds more likely to be taken (Gosler et al., 1995), we have no evidence of any relationship between fledging weight and the probability of predation of fledglings by Sparrowhawks in Wytham (Geer 1978).
All of our analyses have used the individual (fledgling) as the unit of analysis; this causes pseudoreplication which inflates the degrees of freedom in the model. Conversely, using the brood as the unit of analysis is too conservative and results in a loss of information (due to using brood-mean values for weight sub-divided by sex). We used individuals in our analyses on the basis that pseudoreplication may inflate the degrees of freedom in the model, but that this was unlikely to be important in our studies since the degrees of freedom due to broods is already large so extra degrees of freedom are unlikely to affect the conclusions of probability tests.
DISCUSSION
The fate of birds in the immediate post-fledging period depends on a variety of factors. Here we show that the interaction between an environmental determinant (the presence of beech mast in autumn) and phenotypic traits (sex, fledging mass) can produce different patterns of survival for different individuals. Year effects on post-fledging survival have been well documented for Great Tits (Perrins 1965, Verboven & Visser 1998, Lindén et al. 1992) and can be remarkably different between years. In the Swedish study, Lindén et al. (1992) found evidence for stabilising selection, directional selection and no statistical relationship between mass and survival at all, in different years. In general, years can broadly be classified into those where survival is positively correlated with fledging mass, and those (particularly beech mast years) where mass is relatively unimportant for survival above a threshold value (Perrins 1965). In years where survival increases with fledging weight, reduced survival of the heaviest weight class seems to be a common feature (Tinbergen & Boerlijst 1990, Verboven & Visser 1998, Lindén et al. 1992); we have found this to be true only for females in the non-beech year.
In general the post-fledging survival pattern of the two sexes is remarkably similar to that for the two sexes combined (e.g. Perrins 1965). The surprising feature of this is that females survive better than males of the same weight, at least for the weight range where recruitment probability increases with mass. This is also the case for the Vlieland population over a large number of years (Verboven & Visser 1998), but apparently not in Hoge Veluwe (ibid; Tinbergen & Boerlijst 1990), pointing to population differences. In one sense this is not surprising inasmuch as for any given mass, a female is relatively heavier, compared to the female distribution, than is a male of the same mass, compared with the male distribution. Hence if probability of survival was simply related to mass in each sex, then this is what might be expected. However, what is more surprising is that, for any given mass, a female is more likely to survive than a male of the same mass. This suggests that survival is mediated by mass within, but not between, the sexes.
We do not know how the survival of the young birds is related to mass. Garnett (1981) suggested that since the fat stores on the larger birds were barely sufficient to enable the bird to survive for a day, it was unlikely that the extra mass simply functions as a food store as suggested by Perrins (1965). However, Smith et al. (1989) pointed out that it was unlikely that such a fat reserve would be used in this way and small amounts of extra reserves, used each day during the early post-fledging period might well have an important effect on survival. When the young birds leave the nest, many of them quickly join into small flocks with other juveniles and there is some evidence that dominance hierarchies are rapidly formed within these flocks and that dominance is related to mass (Garnett 1981). However, since females survive better than males of the same mass, it seems unlikely that the dominance pattern or the mass-mediated survival pattern is quite as simple as this.
It is known that the mass of the male may effect its future success, but we do not know whether female future success is affected in the same way. For example, Sandell & Smith (1991) showed that in males, prior residence and age were important components of dominance in Great Tits and, other things being equal, older birds tended to win; however first year males were able to defeat older ones if they were heavier. Perrins (1988) showed that, for young male recruits it was particularly important to be heavy in years when few young males recruited into the population. He inferred that the large size gave them a competitive advantage over their smaller rivals; however, there was no evidence that body mass had a similar effect on a female's chance of recruiting into the population. One reason why mass may not always be so important for females is that they often obtain their social rank from their mate. This is particularly clear in some of the other parids such as the Black-capped Chickadee P. atricapillus where the birds live in small social groups through the winter (Smith 1995). Here, pairs that bred together tend to stay together in the following winter. Females paired to alpha males gain advantage by having little aggression from their mate and less frequent aggression from other birds and so can maintain a high feeding rate. A wintering female's pair bond status may be more important than her own dominance rank (Lemmon et al. 1997). However, for much of the year female Great Tits do not consort with a mate and so are less likely to benefit in this way.
The magnitude of the selection differential for fledging weight (the difference between mean cohort fledging weight and the mean weight of survivors) is dependent on mean fledging weights and the post-fledging selection episode itself. Both of these seem to be related to the production of beech mast. Because much post-fledging mortality occurs before beech mast is produced, we suggest that population decrease (which follows strong selection episodes) is at least partially linked to fledglings being lighter in non-beech years. One possible mechanism as to how this could occur is as follows. Trees produce phenolic compounds, including tannins, in their leaves as a defence against caterpillar predation (Levin 1971) and these adversely affect caterpillar development (e.g. Feeny 1970). However, the levels of tannins in leaves varies between years, being lower in mast years when more phenolics are diverted into fruiting bodies; this gives rise to better larval growth and survival in those years (Selas 1997). The implication is that caterpillar quality and abundance will be lower in non-mast years because they have ingested higher levels of tannins. This in turn may result in lighter tit fledglings, since increased tannin levels in caterpillars seem to retard the growth rates of nestling tits (Perrins 1976). Whilst it seems that some of the between-year variation in fledging weight is explained by differences in beech mast abundance, we do not know how this occurs. Future research will investigate the relationship between diet quality and nestling development in fruiting and non-fruiting years.
ACKNOWLEDGEMENTS
We thank A. Becher and F. Sarrazin for sexing some of the samples in the laboratory. JKB was supported by a BBSRC research grant.
REFERENCES
Boom, R., Sol, C.J.A., Salimans, M.M.M., Jansen, C.L., Wertheim-van Dillen, P.M.E. & van der Noordaa, J. 1990. Rapid and simple methods for purification of nucleic acids. J. Clin. Microbiol. 28: 495-503.
Clobert, J., Perrins, C.M., McCleery, R.H. & A.G. Gosler. 1988. Survival rate in the great tit Parus major in relation to sex, age and immigration status. J. Anim. Ecol. 57: 287-306.
Delestrade, A., McCleery, R.H. & Perrins, C.M. 1996. Natal dispersal in a heterogeneous environment: the case of the Great Tit in Wytham. Acta Oecologica 17: 519-529.
Dhondt, A.A. 1979. Summer dispersal and survival of juvenile great tits in southern Sweden. Oecologia 42: 139-157.
Drent, P.J. 1984. Mortality and dispersal in summer and its consequences for the density of Great Tits Parus major at the onset of autumn. Ardea 72: 127-162.
East, M.L. & Perrins, C.M. 1988. The effect of nestboxes on breeding populations of birds in broadleaved temperate woodlands. Ibis 130: 393-401.
Feeny, P.P. 1970. Oak tannins and caterpillars. Ecology 51: 565-581.
Fiala, K.L. 1980. On estimating the primary sex ratio from incomplete data. Amer. Nat. 115: 442-444.
Garnett, M.C. 1981. Body size, its heritability and influence on juvenile survival among great tits Parus major. Ibis 123: 31-41.
Geer, T.A. 1978. Effects of nesting Sparrowhawks on nesting tits. Condor 80: 419-422.
Gibb, J.A. 1950. The breeding biology of Great and Blue Titmice. Ibis 92: 507-539.
Gosler, A.G., Greenwood, J.J.D. & Perrins, C. M. 1995. Predation risk and the cost of being fat. Nature 377: 621-623.
Griffiths, R., Daan, S. & Dijkstra, C. 1996. Sex identification in birds using two CHD genes. Proc. Roy. Soc. Lond. 263: 1251-1256.
Griffiths, R., Double, M.C., Orr, K., & Dawson, R.J.G. A DNA test to sex most birds. Mol. Ecol. 7: 1071-1075.
Lemmon, D., Withiam, M.L. & Barkan, C.P.L. 1997. Mate-protection and winter pair bonds in Black-capped Chickadees. Condor 99: 424-433.
Levin, D.A. 1971. Phenolic compounds in plants. Am. Nat. 105: 157-181.
Lindén, M., Gustafsson, L. & Pärt, T. 1992. Selection on fledging mass in the Collared Flycatcher and the Great Tit. Ecology 73: 336-343.
McCleery, R.H. & Perrins, C.M. 1988. Lifetime reproductive success of the Great Tit, Parus major. In Clutton-Brock, T.H. (ed.) Reproductive Success. Chicago; Chicago University Press: 136-153.
Merila, J., Sheldon, B.C. & Ellegren, H. 1998. Antagonistic natural selection revealed by molecular sex identification of nestling collared flycatchers. Mol. Ecol. 6: 1167-1175.
Perrins, C.M. 1965. Population fluctuations and clutch size in the Great Tit Parus major. J. Anim. Ecol. 34: 601-647.
Perrins, C.M. 1976. Possible effects of qualitative changes in the insect diet of avian predators. Ibis 118: 580-584.
Perrins, C.M. 1979. British Tits. London; Collins.
Perrins, C.M. 1980. Survival of young Great Tits, Parus major. Proc. XVIIth Int. Orn. Cong. Berlin 1978: 159-173.
Perrins, C.M. 1998. A fifty-year study of Great Tits, Parus major. Biol. Cons. Fauna 102: in press.
Price, T.D. & Boag, P.T. 1987. Selection in natural populations of birds. In: Cooke, F. & Buckley, P.A. (eds). Avian Genetics: A Population and Ecological Approach. London; Academic Press: 257-287.
Selas, V. 1997. Cyclic population fluctuations of herbivores as an effect of cyclic seed cropping of plants: the mast depression hypothesis. Oikos 80: 257-268.
Smith, H.G., Källinder, H. & Nilsson, J-Å. 1989. The trade-off between offspring number and quality in the great tit Parus major. J. Anim. Ecol. 58: 383-402.
Sandell, M. & Smith, H.G. 1991. Dominance, prior occupancy and winter residency in the Great Tit (Parus major). Behav. Ecol. Sociobiol. 29: 147-152.
Smith, S.M. 1995. Age-specific survival in breeding Black-capped Chickadees (Parus atricapillus). Auk 112: 840-846.
Tinbergen, J.M. & Boerjlist, M.C. 1990. Nestling weight and survival in individual great tits (Parus major). J. Anim. Ecol. 59: 1113-1128.
Verboven, N. & Visser, M. 1998. Seasonal variation in local recruitment of great tits: the importance of being early. Oikos 81: 511-524.
Verhulst, S., Perrins, C.M. & Riddington, R. 1997. Natal dispersal of Great Tits in a patchy environment. Ecology 78: 864-872.
Table 1. Fledgling sex ratios (proportion males) in three classes of Great Tit broods for 1988. All G-tests are non-significant.
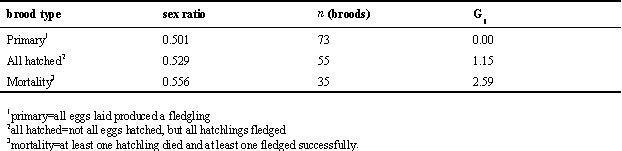
Table 2. Mean cohort fledgling weights (grams±SD) by sex for Wytham Great Tits in two different years.

Table 3. Logistic regression analysis of local recruitment probability of individual fledglings for the three cohorts 1988, 1994 and 1995 combined. The null model represents the variation present when only the equation constant is in the model. All variables except sex were initially fitted in isolation to assess their inclusion in the model, then all significant terms were included in the order shown.
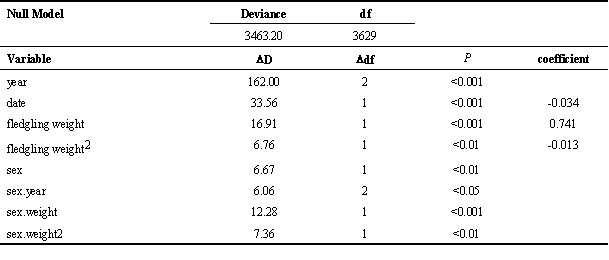
Table 4. Minimum adequate logistic regression models of recruitment probability for the years 1988 and 1995 separately (***= P<0.001). The analysis is based on 223 male and 243 female recruits respectively.
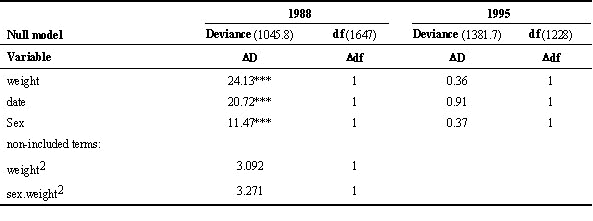
Table 5. Standardised linear selection differentials (S) of viability on fledgling body mass for two Great Tit cohorts, divided by sex.
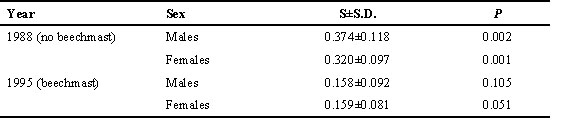
Table 6. Linear model of mean fledgling weight per nest for Great Tit nests in Wytham 1978-1996. The null model is the fitted constant to the raw data only; the minimal adequate model contains all significant predictors and their significant interactions. Significance was determined by the change in deviance (D D) and its associated change in degrees of freedom (D d.f.) on deleting the factor from the model, starting with higher order interactions. Beech is a 3-level factor of the size of the beechmast crop in the autumn after fledging (1 = no crop, 2 = moderate crop, 3 = substantial crop) for each year. Habitat is a 7-level factor incorporating differences in woodland character and nest density (see Delestrade et al. 1996). Brood size (BS) is brood size at fledging.
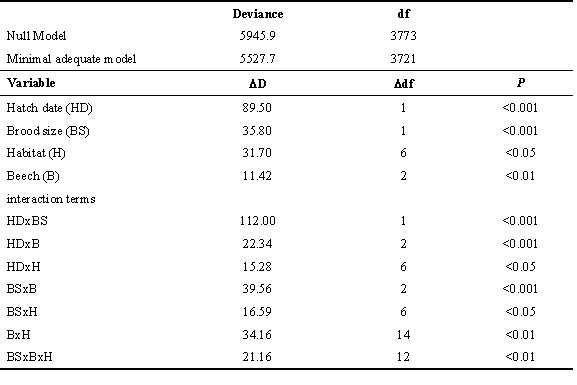
Fig. 1. PCR sex determination in a Great Tit family. Photograph of an ethidium bromide stained agarose gel after electrophoresis of CHD gene products amplified from Great Tit genomic DNA. M = male parent, F = female parent, 1 - 9 = brood of nestlings, S = DNA size marker. Both CHD-W and CHD-NW PCR products are present in the female parent and in female nestlings (2, 5, 6, 7, 9); the CHD-NW product only is present in the male parent and in male nestlings (1, 3, 4, 8).
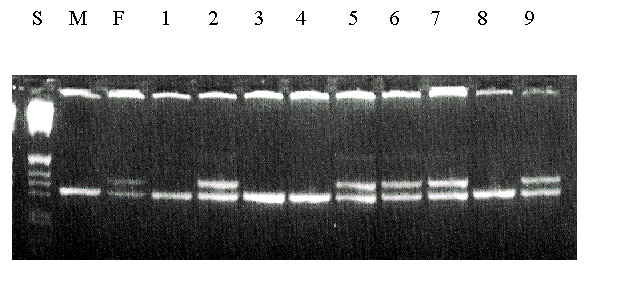
Fig. 2. Mean weight (± S.E.) of sexed nestling Great Tits (n=185 nestlings for each sex) at different stages of the nestling period (Day 1= day of hatch).
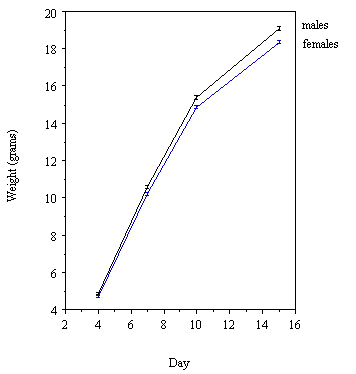
Fig. 3. Local recruitment probability in relation to fledging weight for male and female Great Tits in (A) 1988 and (B) 1995. The points are the proportion recruited from each 1 gram weight class. Fitted logistic regression curves are shown over the weight range where recruitment occurred and are given by recruitment probability = E
a/(1+ea), where a is the linear regression equation including weight.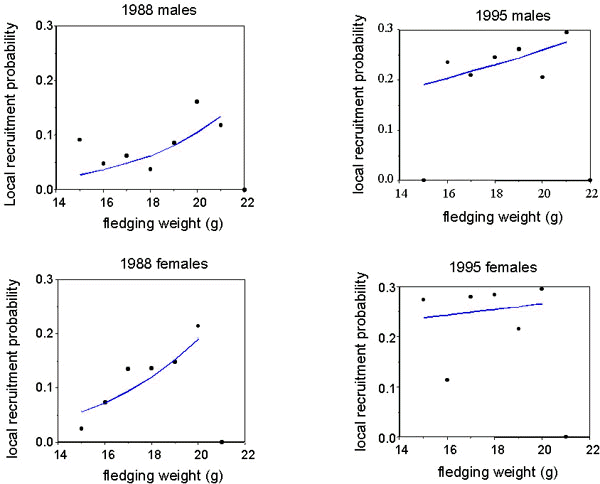
Fig. 4. The proportion of male and female recruits produced expressed by weight class for (A) 1988 (non-beech mast year) and (B) 1995 (beech mast year).
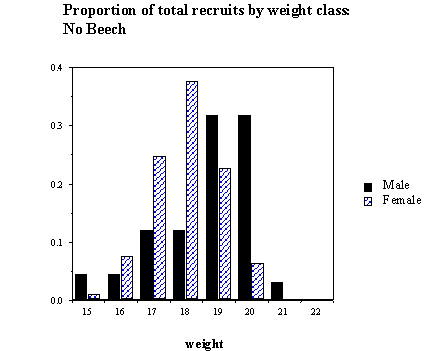
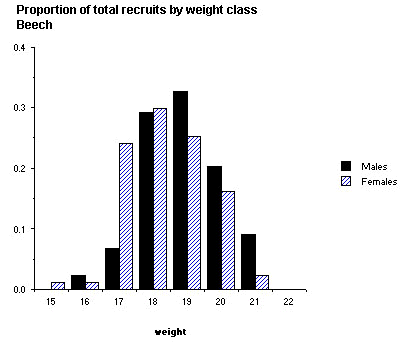
Fig. 5. Mean brood fledging weights ± standard deviation for Wytham Great Tit nests for the years 1978 - 1996, grouped by beechmast score (1=no beech crop, 2=moderate crop, 3=high proportion of trees producing mast). Numbers in brackets are the sample of nests in each beech category.