
S21.5: Brain aromatase in laboratory and free-living songbirds: Relationships with reproductive behaviour
Jacques Balthazart1, Agnčs Foidart1, Michelle Baillien1 & Bengt Silverin2
1Laboratory of Biochemistry, Research Group in Behavioural Neuroendocrinology, University of Ličge, 17 place Delcour, B-4020 Ličge, Belgium, fax 32 4 366 59 71, e-mail
jbalthazart@ulg.ac.be; 2Institute of Zoology, University of Göteborg, Medicinargatan 18, 413 90, Sweden, e-mail zmbs@liszt.zool.gu.se Balthazart, J., Foidart, A., Baillien, M. & Silverin, B. 1999. Brain aromatase in laboratory and free-living songbirds: Relationships with reproductive behaviour. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1257-1289. Johannesburg: BirdLife South Africa.In many avian species, reproductive behaviour is activated in the spring by the increase in plasma testosterone (T) levels in response to increasing daylength. Many behavioural effects of T are mediated by its action on hypothalamic and limbic brain areas and produced, at the cellular level, by oestrogens derived from local T aromatisation. This is demonstrated by pharmacological studies showing that effects of T on behaviour are blocked by the concurrent administration of aromatase inhibitors (that suppress oestrogen synthesis) or antioestrogens (that prevent access of oestrogens to their receptors). Aromatase activity, and cells containing the aromatase protein and mRNA have accordingly been identified in the areas involved in the control of reproductive behaviour. Most of these studies have however been performed on laboratory/domesticated species (e.g., Japanese Quail Coturnix japonica, Ring Dove Streptopelia risoria, Zebra Finch Taeniopygia guttata) and their relevance for free-living species is therefore difficult to establish. The distribution of aromatase activity in these ‘domestic’ species was compared with the distribution in free-living Chaffinches Fringilla coelebs, (Fringillidae), Willow Warblers Phylloscopus trochilus (Sylviidae), Great Tits Parus major (Paridae), and Pied Flycatchers Ficedula hypoleuca (Muscicapidae). High levels of aromatase activity were observed in the diencephalon of all species. The high levels of aromatase activity that had been observed in the Zebra Finch telencephalon, and were thought to be typical of songbirds, were also present in the 4 wild oscine species contrary to quail or doves. The distribution of aromatase activity was also described by immunocytochemistry in Pied Flycatchers. In this last species, aromatase activity was significantly higher in the diencephalon of birds engaged in territorial defence than in birds feeding nestlings, suggesting that the drop in plasma T caused by the testicular regression observed at that time controls limbic aromatase as previously described in quail and doves. These changes did not affect telencephalic aromatase.
INTRODUCTION
In most, if not all, avian species, the male-typical behaviours that are associated with reproduction are androgen-dependent. They are readily exhibited by males with developed testes that secrete significant amounts of testosterone (T) but not by males that have regressed testes or birds that have been surgically castrated. The androgen-dependence of avian reproductive behaviours was in fact demonstrated long ago when Adolph Berthold showed that castration abolishes the behavioural manifestations of sexual maturity in domestic cocks and that effects of castration could be reversed by a testicular graft (Berthold 1849). This experiment demonstrating that sexual behaviour in cocks is controlled by a compound secreted by the testes in the blood stream is classically considered as the first experiment in behavioural endocrinology. More sophisticated experiments have since confirmed and extended this finding to a variety of experimental models (e.g. Beach 1948; Nelson 1994; Balthazart 1983) and with the advent of sensitive radioimmunoassays, it has become possible to correlate circulating levels of testosterone with the behaviour exhibited by birds in the laboratory as well as in the field (Wingfield et al. 1994; Wingfield 1990; Wingfield et al. 1990; Wingfield et al. 1997). These studies identified a great diversity of patterns in the annual/seasonal changes in plasma levels of T in different species of birds as a function of their reproductive strategies but have also confirmed the key role played by this steroid in the control of reproduction.
TESTOSTERONE IS EXTENSIVELY METABOLISED IN THE BRAIN
Studies carried out during the past 20-30 years in a variety of mammalian and avian species have shown that steroids, and in particular T, can be extensively metabolised when they enter their target cells. As will be illustrated below, this metabolism is highly relevant to the control of reproductive behaviour (Balthazart 1989). The metabolites that are formed from the parent steroid either have their own receptors in the brain and regulate the behaviour (they mimic and actually cause at the molecular level some or all effects of T) or have no receptor and therefore represent inactivation products for the parent steroid. The ratio of active versus inactive metabolites that are produced can be affected by factors such as the sex, age, season or hormonal condition of the subjects and this provides a mechanism for the fine adjustment of behaviour. In addition, there is an anatomical specialisation of these steroid-metabolising enzymes: their concentration and/or activity varies from one brain area to the other. A same steroid can therefore act through different neuroendocrine and neurochemical mechanisms in different brain regions.
These general principles hold true for all major classes of sexual steroids: androgens (e.g. T), oestrogens (e.g. estradiol-17b,E2), progestagens (e.g. progesterone, P) and corticoids (e.g. aldosterone, corticosterone). It must also be stressed that this metabolism takes place not only in the brain but in all steroid target organs. Steroid action throughout the body is therefore controlled by the intracellular metabolism in target structures (see Feder 1981; Balthazart 1989 for more information on this topic). In mammals and in a small number of avian models (mainly poultry), the pathways of T, E2 and P metabolism in the brain have been described and their significance for the control of reproductive behaviour has been to some extent experimentally studied. In other species of birds, the available knowledge is more restricted and with a few exceptions, only the metabolism of T and its role in the control of sexual and/or vocal behaviour has been analysed.
T is the major androgen secreted by the testes in birds. In the brain and pituitary gland, as well as in a number of peripheral target structures, T is extensively metabolised into other steroids that are either androgenic, or oestrogenic, or have no hormonal properties. A number of in vivo and in vitro studies have described the metabolism of T in the avian brain and a summary of the major pathways identified in the Quail Coturnix japonica, Ring Dove Streptopelia risoria and Zebra Finch Taeniopygia guttata brain is presented in Fig. 1 (see Balthazart 1989 for a more extensive discussion of this problem in a broader context). (Fig.1).
In the avian brain, three enzymes catalyse the transformation of T into behaviourally relevant metabolites: aromatase leads to the production of oestrogens, 5a-reductase produces 5a-dihydrotestosterone (5a-DHT) and 5b-reductase leads to the formation of the behaviourally inactive 5b-dihydrotestosterone (5b-DHT). These transformations are thermodynamically irreversible, at least in physiological conditions. In addition, a 17b-hydroxysteroid dehydrogenase (17b-HSDH) permits the reversible interconversion of T with androstenedione (D4). D4 is itself aromatizable into estrone. The two dihydrotestosterones can be further metabolised into the corresponding 3a- and 3b-diols under the catalytic action of the 3a- or 3b-hydroxysteroid dehydrogenase (3a- or 3b-HSDH). These transformations are also reversible.
The formation of all these steroids has been confirmed in the Zebra Finch brain during in vitro experiments (Vockel et al. 1988; Vockel et al. 1990a; Schlinger & Arnold 1991; Schlinger & Arnold 1992b). The presence of an active aromatase and 5
b-reductase has also been confirmed in vivo (Schlinger & Arnold 1992a). With only a few exceptions (Schlinger & Arnold 1992a; Hutchison et al. 1986), all available data concerning T metabolism in the avian brain are however derived from in vitro studies carried out on brain homogenates. They certainly demonstrate the presence of the enzymes in the central nervous system but the measures of activity obtained in these studies may be ignoring some regulatory mechanisms that could play a key role in the live animal. Some caution should therefore be exercised when using these assay data to interpret physiological phenomena.By incubating equal amounts of brain homogenate in the presence of increasing amounts of radioactive substrate (i. e. T), it has been possible to obtain estimates of the maximum velocity (Vmax) and of the affinity (Km) of the T metabolising enzymes in the avian brain. In adult birds, an inverse relationship was found between the estimated Km and Vmax of the three enzymes that irreversibly metabolise T (see Table 1 for data in Zebra Finches). Enzymes that produce biologically active metabolites such as aromatase and 5
a-reductase have a high affinity for and therefore a privileged access to the substrate (T) but they have a low maximum velocity and cannot produce huge amounts of these biologically active substances (E2 and 5a-DHT). Conversely, the enzyme that produces biologically inactive steroids such as the 5b-reduced androgens does not have a priority of access to the substrate but it can metabolise huge amounts of it. (Table 1).These data therefore indicate that in conditions where a relatively low concentration of T is available (which is presumably the case most of the time in vivo), the steroid will be preferentially transformed into oestrogens by the aromatase that has a high affinity (low Km) for this substrate. By contrast, if higher amounts of substrate become present, as could for example happen in a bird that has been injected with exogenous T, then a larger proportion could be inactivated into 5
b-reduced steroids that have no hormonal activity by themselves. The capacity of the 5b-reductase is extremely high compared to the other two enzymes. This enzyme should be able to catalyse the transformation of huge amounts of substrate when they become available.TESTOSTERONE ACTION ON MALE REPRODUCTIVE BEHAVIOUR IS MODULATED BY THE INTRACELLULAR METABOLITES OF THE STEROID
As already mentioned above, a large part of the behavioural effects of T are mediated at the cellular level by the interaction of T metabolites, in particular E2 and 5
a-DHT with specific receptors, respectively the oestrogen receptors (ER) and androgen receptors (AR). The receptors when occupied by their specific ligand act at the nuclear level as transcription factors and modulate gene expression which in turn results in a host of neurochemical changes (yet to be identified for the most part) that lead to behaviour activation. These general principles apply broadly in vertebrates and data supporting these ideas have been previously reviewed (for references reviewing the avian literature by priority, see: Adkins-Regan 1981b; Adkins-Regan 1983; Balthazart 1989; Hutchison 1987; Balthazart et al. 1996b).In birds, the most extensive analysis of the implication of T metabolism in the control of reproductive behaviour has been performed in the Japanese Quail and Ring Dove. The activation of copulatory behaviour in castrated male quail can be obtained by treatment with high doses of oestrogens or by a combined treatment with physiological doses of E2 and 5
a-DHT (Adkins & Pniewski 1978; Adkins et al. 1980; Schumacher & Balthazart 1983; Balthazart et al. 1985; Balthazart & Foidart 1993; Balthazart et al. 1996b). There is a clear synergism between oestrogens and androgens: a marked activation of the behaviour can be obtained by the combined administration of doses of E2 and 5a-DHT that have little or no activity on their own. This suggests that, in physiological conditions, it is indeed these two metabolites that are responsible for the behavioural activation caused by T secreted from the testes or administered by injections. This conclusion has been supported by a large number of experiments showing that the blockade of androgen or oestrogen receptors inhibits T action on copulatory behaviour (Adkins & Nock 1976; Alexandre & Balthazart 1986). A stronger inhibition of the behavioural effects of T was in fact observed after the injection of antioestrogens as compared to antiandrogens (Alexandre & Balthazart 1986; Balthazart & Surlemont 1990) suggesting that oestrogenic metabolites of T are more important for the behavioural activation than T itself or its androgenic metabolites such as 5a-DHT. This conclusion is also supported by the observation that aromatase inhibitors can completely suppress the effects of T on copulatory behaviour while 5a-reductase inhibitors have little or no effect at this level (Adkins et al. 1980; Alexandre & Balthazart 1986; Watson & Adkins-Regan 1989; Balthazart et al. 1990b; Balthazart & Surlemont 1990; Balthazart et al. 1990a). It should be noted that this comparison may be the result of a technical artifact due to the higher efficiency of aromatase inhibitors in general (independent of the response considered). Nevertheless, it is clear that oestrogens alone can activate male copulatory behaviour in quail and that the inhibition of aromatase completely blocks the effects of T on this behaviour. This firmly demonstrates that the aromatisation of T is a limiting step for the activation of sexual behaviour by T (Balthazart & Foidart 1993). Interestingly, other reproductive behaviours in quail such as the courtship display referred to as strut and male-specific vocalisations such as crows are activated in quail by androgens only (T or 5a-DHT). Oestrogen have no effect on these behaviours or even inhibit their activation by 5a-DHT (Adkins 1977; Adkins & Pniewski 1978; Schumacher & Balthazart 1983; Balthazart & Schumacher 1984; Balthazart 1989). These behaviours can therefore be conveniently used to monitor the endocrine specificity of steroid treatments applied to experimental subjects. Similar conclusions have been obtained in Ring Doves (e.g. Adkins-Regan 1981a; Hutchison 1971; Hutchison et al. 1989).The 5
b-reduced androgens such as 5b-DHT appear by contrast to be largely inactive in a reproductive context. Treatment of castrated male birds with this compound or the derived diols failed for example to activate copulatory behaviour in Japanese Quail (Adkins 1977; Deviche et al 1982) and also did not activate courtship behaviour in Ring Doves (Hutchison & Steimer 1981; Cohen & Cheng 1982). 5b-DHT also does not depress the circulating levels of gonadotropins (Davies et al. 1980) and does not stimulate the growth of androgen-dependent secondary sexual characteristics such as the cloacal gland in quail or the comb and wattles in chicken (Adkins 1977; Balthazart and Hirschberg 1979). These compounds appear therefore to be almost entirely devoid of any androgenic action with the exception that they were found to stimulate copulatory reactions in newly hatched domestic chicks (Balthazart and Hirschberg 1979; Balthazart et al. 1981) in a hand-test situation originally developed by Andrews (1975). The origins of this discrepancy are unknown at present (see Balthazart 1989 for further discussion).One song bird species, the Zebra Finch has also been used in detailed experiments analysing steroid-specificity in the activation of male reproductive behaviours. In a first set of studies (Harding et al. 1983), castrated males were treated with Silastic implants providing one of six hormone treatments with either two aromatisable androgens, T , and androstenedione or two nonaromatisable androgens, androsterone and dihydrotestosterone (DHT), or an oestrogen (E2) or the combined treatment E2+DHT. Half the males treated with DHT received the alpha isomer (5
a-DHT) while the other half received the non-androgenic beta isomer (5b-DHT). Reproductive behaviours were then quantified during three weekly tests with different females. All aspects of the reproductive behaviour that were considered were markedly decreased or totally suppressed in castrated birds. All treatments that provided a combination of androgenic and oestrogenic steroids (T, androstenedione, E2+5a-DHT) restored to normal levels reproductive behaviours such as courtship displays (including female-directed songs), beak wiping and copulation attempts. Other treatments providing pure androgenic stimulation were ineffective as was also the case for the low dose of E2 that had been selected for study. These data therefore indicated that in Zebra Finches like in quail or doves, reproductive behaviours are activated by a synergistic action of oestrogens and androgens. The implication of oestrogens at this level was subsequently confirmed in another study demonstrating that the behavioural effects of an aromatisable androgen such as androstenedione can be markedly reduced by a simultaneous treatment with the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD). These inhibitory effects of ATD were reversed when exogenous E2 was concurrently administered indicating that the behavioural inhibition resulted from the lack of oestrogenic metabolites rather than from a non specific adverse effect (Walters & Harding 1988).This synergistic action of oestrogens and androgens in the activation of reproductive behaviours in songbirds may be a general feature as indicated by the fact that in Red-winged Blackbirds Agelaius phoeniceus the frequencies of singing and of three common vocalisations (chucks, checks and ips) that had been markedly decreased by castration were restored by treatments that provided both androgenic and oestrogenic metabolites (e.g. T, androstenedione, E2+ 5
a-DHT) but not by nonaromatisable androgens or oestrogens alone (Harding et al. 1988). Additional tests with a broader variety of species should however be carried out before this conclusion can be accepted as being applicable to songbirds in general.NEUROANATOMICAL DISTRIBUTION OF AROMATASE IN SONGBIRDS
The studies described above establish beyond any doubt that many behavioural effects of T actually result from the action at the cellular level of endogenously produced metabolites, and in particular of oestrogens derived from T aromatisation. Several questions are raised by this observation: where does the T metabolism take place (brain vs periphery)? Is the metabolism a constant feature of the species or is it modulated by the age, sex and/or hormonal status of the subject? If T metabolism takes place in the brain, what is the anatomical specificity of the enzyme distribution at the macroscopic and at the cellular level?
These questions have been approached by two types of techniques. In vitro radioenzyme assays combined with a variety of micro-dissection techniques have been used to identify the presence of specific enzymes in a series of brain nuclei and to analyse the potential regulations of these enzymatic activities. More recently, immunocytochemical and in situ hybridisation techniques that permit the visualisation of the aromatase protein or of its messenger RNA have additionally become available and studies have been carried out to analyse the cellular distribution of this enzyme in the brain of a few avian species. The most extensive data have been collected in Japanese Quail and Ring Doves (Balthazart & Foidart 1993; Panzica et al. 1996; Balthazart 1997; Balthazart et al. 1996c; Hutchison 1991; Hutchison 1993; Hutchison et al. 1995) but a substantial amount of work has also been performed in one songbird species that breeds easily in laboratory conditions, the Zebra Finch.
In a first series of studies, the activity of the aromatase, 5
a-, and 5b-reductase were measured by radioenzyme assays in brain nuclei that had been dissected by the Palkovits punch technique (Vockel et al. 1990a; Vockel et al. 1990b). The 5a-reductase activity was more or less evenly distributed in all brain nuclei that were considered with the noticeable exception of the nucleus robustus archistriatalis (RA) where enzyme activity was twice higher than in all other regions (Fig. 2). High levels of 5b-reductase activity were found throughout the brain (note the very different scale of the graph) but especially in the nucleus magnocellularis of the anterior neostriatum (MAN), in the high vocal centre (HVC; formerly referred to as the hyperstriatum ventrale, pars caudalis) and in the nucleus striae terminalis (nST). These two reductases were even present in two steroid-insensitive regions that had been included in the study as controls, the ectostriatum (E) and the nucleus rotundus. (Fig. 2).High levels of aromatase were observed in the diencephalic and limbic nuclei that were classically known to express this enzyme in mammals and other avian species, namely the nucleus preopticus anterioris (POA), the nucleus periventricularis magnocellularis (PVM), the nucleus medialis hypothalami posterioris (PMH), the nucleus stria terminalis (nST) and the nucleus taeniae (Tn; homologue to parts of the mammalian amygdala).
Interestingly, these studies also reported the presence of significant levels of aromatase activity in telencephalic song control nuclei such as the area X (X), MAN, HVC and RA. They also indicated that the area parahippocampalis (APH), that had originally been selected as a control region, was characterised by an extremely high enzymatic activity larger than in any of the diencephalic or limbic nuclei that had been studied (Vockel et al. 1990a). Additional studies confirmed the presence of a very high aromatase activity in the dorsal telencephalon and showed that the enzyme is actually present in the entire roof of the forebrain in the neostriatum, hippocampal and parahippocampal region. This high level of telencephalic aromatase activity has been independently confirmed (Schlinger & Arnold 1991; Schlinger & Arnold 1992b) and a series of elegant studies based on the injection of radioactive androgens in the brain or periphery combined with blood collection in the jugulars or carotids has provided experimental evidence indicating that brain aromatase substantially contributes to the circulating levels of oestrogens in the Zebra Finch (Schlinger & Arnold 1992a; Schlinger & Arnold 1993).
These data suggested that the song control nuclei have the capacity to produce the T metabolites (E2 and 5
a-DHT) which had been shown, during behavioural experiments, to mimic the effects of T on song production and courtship behaviour. More recently, the distribution of aromatase protein and mRNA has been described at a cellular level of resolution in the Zebra Finch brain by immunocytochemistry (ICC) and in situ hybridisation (ISH) and this new information has led to a partial revision of these conclusions (Shen et al. 1994; Shen et al. 1995; Balthazart et al. 1996a). Both ICC and ISH studies confirmed the presence of aromatase and its mRNA in the diencephalic and limbic regions such as the POA, PVM, PMH, nST and Tn that are implicated in the control of reproduction and where a high level of enzyme activity had been measured. Unexpectedly however, all telencephalic song control nuclei were found to contain only minimal, often not detectable, amounts of the protein or its mRNA. The presence of large amounts of aromatase (protein and mRNA) could however be confirmed in the neostriatum and its distribution characterized. At the level of HVC or in slightly most rostral parts of the telencephalon, aromatase and its mRNA are present in broad areas of the medial neostriatum adjacent to the lateral ventricles. At more caudal levels, the cluster of aromatase-expressing cells extends more medially as a band coursing along the dorsal edge of the lamina archistriatalis dorsalis; they are also present in the ventrolateral aspects of the neostriatum. Based on these detailed anatomical data, it is likely that the aromatase activity that had been detected in HVC and RA by radioenzyme assays performed on nuclei microdissected by the Palkovits punch technique originated in a contamination by neostriatal tissue immediately surrounding these nuclei.ANATOMICAL RELATIONSHIPS BETWEEN AROMATASE AND OTHER STEROID-METABOLISING ENZYME WITH STEROID RECEPTORS
As described above, the precise mapping of the T-metabolising enzymes in the Zebra Finch brain is still in progress. The only published data for the two reductases are based on assays of the enzyme activity in nuclei dissected by the Palkovits punch technique and these do not provide a cellular level of anatomical resolution as has been obtained for aromatase by ICC and ISH. It is therefore difficult to draw precise correlations between the distribution of these enzymes and the distribution of the steroid receptors that are supposed to bind the locally produced metabolites (E2 and 5
a-DHT).Most nuclei of the song system are steroid-sensitive and contain, at least, androgen receptors as demonstrated by in vivo autoradiography (for review: Ball 1990; Brenowitz 1991), by binding assays (Harding et al. 1984) and, more recently, by immunocytochemistry (Balthazart et al. 1992) and in situ hybridisation (Nastiuk & Clayton 1994; Nastiuk & Clayton 1995; Gahr & Metzdorf 1997). One noticeable exception to this rule is the area X that appears to be devoid of androgen binding sites. A significant 5
a-reductase was found in all these nuclei which suggests that the androgen receptors located in the song control nuclei could be occupied in physiological conditions by T as well as by its metabolite, 5a-DHT. This enzymatic activity was particularly low in area X which fits in well with the absence of androgen binding sites in this nucleus.As already mentioned before, it is usually accepted that the 5
b-reduced androgens are biologically inactive compounds as far as activation of reproductive behaviour is concerned and that the 5b-reduction is an inactivation pathway for testosterone (Steimer & Hutchison 1981b; Balthazart 1989). It could therefore be expected that the production of 5b-reduced compounds should be lower in steroid target areas than in non-target tissue as previously shown in a variety of avian species (reviewed in Balthazart 1983; Balthazart 1989). The distribution pattern of the 5b-reductase observed in the Zebra Finch brain to some extent contradicts this generalisation. As expected, the enzyme activity was low in all hypothalamic nuclei as demonstrated previously in other species and this fits in well with the fact that the hypothalamus is a major site of androgen action in the control of reproduction. Unexpectedly however, the 5b-reductase activity was very high in several nuclei of the song system which are also well-known targets for androgens (MAN, HVC, and to a lower extent RA). Conversely, the lowest production of 5b-DHT was observed in the two nuclei of the visual system, E and Rt which are not steroid-sensitive and where a high rate of T inactivation would have been expected. The mechanism underlying this unexpected distribution of the 5b-reductase activity and its biological meaning are totally unclear at present.The relationships between aromatase activity and oestrogen receptors can be evaluated in more detail and this comparison has revealed some striking discrepancies. Some brain areas such as the preoptic-hypothalamic nuclei (POA, PVM and PMH) and limbic regions (Tn and nSt) contain oestrogen receptors as demonstrated by both autoradiography (Nordeen et al. 1987) and immunocytochemistry (Gahr et al. 1987; Gahr & Konishi 1988; Gahr et al. 1993) as well as a high aromatase activity and a high density of aromatase-expressing cells. Some mismatches can however be observed. In the nucleus intercollicularis (ICo), identified in all studies as a site with dense oestrogen receptors (autoradiography: Nordeen et al. 1987, ICC: Gahr et al. 1987; Gahr et al. 1993, binding assays on punched nuclei: Walters et al. 1988) enzyme assays reveal no or barely detectable levels of enzyme activity and no aromatase-expressing cells can be detected by ICC or ISH. In contrast, high levels of aromatase activity are observed in a large portion of the dorsal telencephalon (hippocampus, area parahippocampalis, dorsal neostriatum) while these brain regions are not known as a classical target for steroids. Anatomical studies that have documented the distribution of aromatase and oestrogen receptors in these brain regions also confirm the presence of broad discrepancies between areas expressing the enzyme and the receptor. Oestrogen receptors have been demonstrated in a large neostriatal area located under the lateral ventricle at the level of HVC in the Zebra Finch brain (Gahr et al. 1987) where a high aromatase activity and numerous aromatase-expressing cells are detected. However aromatase-immunoreactive cells and cells expressing aromatase mRNA are found beyond this specific region of the neostriatum namely in the area dorsal to the lamina archistriatalis dorsalis and in the ventrolateral aspects of the neostriatum where no oestrogen receptors have been detected so far (Gahr et al. 1987). These facts therefore raise questions concerning how oestrogens locally produced in the dorsal telencephalon may affect directly or indirectly the control of reproduction and of singing behaviour.
These studies were however completed when a single nuclear receptor for oestrogens was known. Recently, a novel oestrogen receptor, named oestrogen receptor beta (ER
b) has been cloned in the mammalian brain (Kuiper et al. 1996) and its neuroanatomical distribution was shown to be quite different from the distribution of the classical oestrogen receptor, now re-named oestrogen receptor alpha (ERa; see Shughrue et al. 1997). This new form of receptor is now known to be present in the avian (quail) brain and it has been partly sequenced (Lakaye et al 1998). We showed that, in the Japanese Quail brain, ERb expression is present in classical steroid targets such at the preoptic area, bed nucleus striae terminalis and nucleus taeniae but no information to this date is available for song birds, and for the song control nuclei in particular. When these data become available, they may help explain the discrepancies that have been noted between the neuroanatomical distribution of oestrogen receptors (ERa) and aromatase.During enzyme assays performed on cell fractions separated by differential centrifugation of the quail preoptic area (Schlinger & Callard 1989) or the Zebra Finch telencephalon (Schlinger & Arnold 1992b), it has been observed, that aromatase activity is found not only in the microsomal but also in synaptosomal (pinched-out nerve terminals) subfractions. The presence of aromatase-immunoreactive material has furthermore been confirmed in cell processes of the quail brain that have an axonal morphology (Foidart et al. 1994b) and, at the electron microscope level, it was confirmed that aromatase-immunoreactive material is present in high density at the level of presynaptic boutons (Naftolin et al. 1996). This local production of oestrogens in the close vicinity of synapses may provide steroid signals that could affect brain activity independent of the binding to nuclear receptors acting as transcription factors (i.e. by non-genomic mechanisms). It is known that oestrogens are able to modify the electrical activity of the neuronal membranes within seconds after their application which essentially rules out the possibility of a classical steroid receptor-mediated effect on protein synthesis (for additional discussion see: Blaustein & Olster 1989; Schlinger & Callard 1989; Schumacher 1990; McEwen 1994; Ramirez et al. 1996). Other effects of oestrogens on brain functioning that appear to be independent of their binding to intracellular receptors have also been described (e.g. Pasqualini et al. 1995; Thompson & Moss 1994; Mermelstein et al. 1996). Whether the presence of aromatase activity in brain areas such as part of the neostriatum that are apparently devoid of intracellular oestrogen receptors relates to these membrane effects of oestrogens is unknown at present.
In general, the partial mismatch between the T-metabolising enzymes and the steroid receptors raises the question of how T metabolites exert their action on the brain. It was classically assumed that all steroids act in an autocrine fashion and exert their biological effects through the binding with intracellular receptors located in the same cell as the enzymes. The presence of aromatase at the presynaptic level certainly suggests that a paracrine mode of action is also possible. The demonstration that infusion of radioactive androstenedione in the Zebra Finch telencephalon results in increased levels of radioactive oestrogen in the jugular blood (Schlinger & Arnold 1992a; Schlinger & Arnold 1993) even suggests an endocrine action for brain aromatase. This represents without a doubt one of the most exciting recent discovery in the field and its biological significance should be experimentally evaluated.
The specific role of oestrogens in the control of the sexual differentiation of the song control nuclei and in the activation of singing behaviour remains at this point unclear. Anatomical studies have revealed that the song control nuclei MAN, HVC and RA whose size and cell typology can be affected by oestrogens acting during ontogeny (Gurney 1981; Gurney & Konishi 1980; Konishi & Akutagawa 1988) appear to be devoid of aromatase-expressing cells, even if such cells are found in the close vicinity in the neostriatum. In addition, these three nuclei apparently contain no or very few oestrogen receptors (Gahr et al. 1987; Nordeen et al. 1987; Walters et al. 1988) at least in the adult bird. Immunoreactive oestrogen receptors could be present transiently at least in HVC during ontogeny (Gahr & Konishi 1988). It is therefore difficult to understand precisely how oestrogens affect the differentiation of these song control nuclei as well as the vocal behaviour in adulthood (Balthazart & Ball 1995; Arnold 1997; Schlinger 1998). It must be pointed also that the neostriatal production of oestrogen may relate to other functions such as the control of memory capabilities, in particular the spatial memory and encouraging results along these lines have been recently obtained (Schlinger 1997). The medial neostriatum adjacent to the lateral ventricle is also known as an area of active neurogenesis in the adult songbird brain (Alvarez-Buylla et al. 1990). A role of oestrogens in the survival, migration or differentiation of these newly formed neurons should therefore also be considered and experimentally tested. The biology of oestrogen action in the songbird brain is clearly far from being completely uncovered.
DIFFERENTIAL REGULATION OF AROMATASE ACTIVITY IN THE TELENCEPHALON AND IN HYPOTHALAMIC NUCLEI
Biochemical studies carried out on microdissected brain regions or nuclei demonstrated that the activity of the T-metabolising enzymes in Zebra Finches is markedly affected by the age and sex of the birds (Balthazart et al. 1986; Vockel et al. 1988; Vockel et al. 1990a; Vockel et al. 1990b). As an example, the aromatase activity measured in limbic nuclei and in telencephalic areas centred on the song control nuclei (they included the nuclei but were probably contaminated by surrounding tissue; see above) of young (20 day old) and adult (>100 day old) males is shown in Fig. 3A.
As can be immediately seen, the enzymatic activity observed in our assay conditions was usually higher in the adult than in the juvenile birds. This was true for both the limbic and the song control nuclei. The interpretation of such a difference is, however, complicated by the fact that the apparent affinity (Km) of the aromatase for its substrate seems to vary with age in Zebra Finches (Vockel et al. 1988), contrary to what had been observed previously in quail (Hutchison & Schumacher 1986). The double reciprocal plots of a saturation analysis shown in Fig. 3B illustrates this fact. In very young birds (below 35 days post-hatch), a high hypothalamic aromatase activity was detected (Vmax = 350 fmol/mg protein/h) but it was associated with a low affinity of the enzyme for its substrate (Km =70 nM). As the birds became sexually mature (40-45 days post hatch), a shift in the enzyme affinity occurred and the Km of the aromatase for T reached its adult values that are typically below 30 nM. An increase in the enzyme maximal velocity was subsequently observed as birds grew older (80-85 day old group).
Because important changes in enzyme affinity are observed with the increasing age, it is likely that the changes in maximum velocity reflect more than a simple change in enzyme concentration. Changes in the apparent enzyme affinity (Km) of aromatase can reflect modifications of the enzyme (its maturation) or the presence of a new form of enzymatic protein or the modulation of the enzymatic activity by new regulatory molecules (co-factors, inhibitors). Discriminating between these alternative explanations would require detailed biochemical and enzymatic studies. Extrapolating the in vitro assays to the physiology in the intact bird is also difficult and, in particular, it is hard to determine, based on these data, the actual activity level of the brain aromatase in the differentiating embryo or neonate. It must also be stressed that these kinetic data are based on studies of hypothalamic homogenates and it is not known whether similar changes would be detected in the telencephalic areas.
The fact that a major shift in the kinetic characteristics of the Zebra Finch aromatase occurred at the age of 40-45 days when the birds begin their sexual maturation as evidenced by molting into adult plumage and change in beak colour from black to red (Pröve 1983) suggests that these changes are induced by steroids, a conclusion that was indirectly supported by the observation that aromatase activity was higher in the hypothalamus of adult males compared to adult females (Vockel et al. 1988).
Such a sex difference in aromatase activity can, in theory, be caused by two distinct actions of steroids. The difference can reflect a differential activation by the endocrine milieu in the adult birds or it could result from some irreversible organising effects of steroids acting earlier in life. To study the endocrine control of these enzymatic differences, the activity of the three main T-metabolising enzymes (aromatase, but also the 5
a- and 5b-reductases) was quantified in the song control and limbic nuclei of adult male and female Zebra Finches that were either gonadectomised, or gonadectomised and submitted to a steroid replacement therapy (T Silastic implants for two weeks) or sham-operated (sexually mature birds serving as control groups). We shall only discuss here the effect of these treatments on aromatase activity that are the most relevant to the present review. Changes in the activity of the reductases have been published in detail (Vockel et al. 1990b). In the hypothalamic and preoptic nuclei of Zebra Finches, aromatase activity is usually higher in males than in females but this sex difference appears to result mainly if not exclusively from the presence of higher T levels in males. Gonadectomy indeed reduces the enzyme activity to basal levels and treatment with T usually induces high levels of enzymatic activity in both sexes (see example Fig. 4A). This pattern is very similar to what had been previously described for many hypothalamic structures in other avian species such as the quail and dove (Balthazart 1989 for review; see however Schumacher & Balthazart 1986 for an exception to this rule). (Fig. 4).A different pattern emerged however in the telencephalic samples. In areas such as the dorsal neostriatum or the APH, no clear sex difference in enzyme activity could be detected in sexually mature, gonadally intact birds. Furthermore, gonadectomy associated or not with a chronic treatment with exogenous T did not seem to affect much enzyme activity (no change at all in APH, small numerical increase in the neostriatum) but the changes failed to reach statistical significance; (Fig. 4B). The data therefore suggested that telencephalic aromatase is not sexually differentiated and not regulated by T. A lack of a sex difference in the telencephalic aromatase activity has also been reported in young Zebra Finches (Schlinger & Arnold 1992b).
It must also be mentioned here that in the original papers reporting on these data, a ‘reverse’ sex difference in aromatase activity had been indicated for the song control nuclei HVC and RA (higher enzyme activity in females than in males; Vockel et al. 1990a) and this difference did not appear to be caused by a differential activation by steroids because it was not affected by gonadectomy and T treatment (Vockel et al. 1990b). These studies were however carried out when it was technically impossible to localise aromatase-containing cells at the cellular level so that microdissections could only be guided by the tissue appearance in Nissl stain and not by the actual distribution of the enzyme. As noted above, when anatomical methods for the visualisation of aromatase became available, it became clear that HVC and RA actually contain little or no aromatase so that the enzyme activity that had been measured in these nuclei presumably resulted from a contamination by surrounding positive tissue. Because HVC and RA are significantly larger in males than in females and punches of the same size had been used in both sexes to measure aromatase activity, it now appears likely that the sex difference that had been noted resulted from a more important contamination by surrounding tissue in females than in males.
It remains true, however, that there appears to be a differential regulation of aromatase in the telencephalic area and in the limbic structures. This suggests that different forms of enzyme may be present in these two parts of the brain. Recent studies utilising the tools of molecular biology actually bring some support to this idea (Ramachandran et al. 1997) but it is beyond the scope of the present study to analyse the molecular biology of aromatase regulation (see: Simpson et al. 1991; Bulun & Simpson 1994; Simpson et al. 1994; Lephart 1996; Lephart 1997 for recent reviews).
AROMATASE ACTIVITY IN FREE-LIVING SONGBIRDS
The data summarized above demonstrated that aromatization of T is a key factor in the regulation of reproductive behaviour in one oscine species, the Zebra Finch, like it is in mammals and several previously investigated avian species. They also showed that in hypothalamic and limbic areas the Zebra Finch aromatase is distributed and regulated by steroids like in quail and doves. Interestingly, however, an extremely active broadly distributed aromatase was found in the Zebra Finch telencephalon that seems to distinguish this species from other avian species that had been previously considered. This telencephalic aromatase also does not seem to be controlled by steroids like the hypothalamic enzyme.
It is quite tempting to generalize these features of the Zebra Finch aromatase to the entire songbirds order (passeriformes) but until quite recently data supporting this generalization were critically missing with the exception of limited studies devoted to a few species such as the Brown-headed Cowbird Molothrus ater, White-crowned Sparrow Zonotrichia leucophrys, House Sparrow Passer domesticus, or Island Canary Serinus canaria (Saldanha & Schlinger 1997; Schlinger et al. 1992; Schlinger 1996). Such generalisations may appear quite hazardous for two reasons. On the one hand, most Zebra Finches studied in the laboratory are derived from semi-domesticated stocks and they may consequently exhibit significant differences by comparison with the wild species but these are difficult to appreciate. On the other hand, the Zebra Finch is a non-photoperiodic species that inhabits the australian deserts and its use as a model species for the songbird group that contains many photoperiodic species originating from the temperate zone appears questionable. For these reasons, it appeared desirable to investigate aromatase distribution, regulation and function in a broader variety of songbird species. Additionally, it was also of interest to research whether the dramatic changes in hypothalamic aromatase activity that had been observed following castration and/or treatment with exogenous T would also be detected after more physiological changes in plasma T such as those that are normally observed during a breeding cycle .
Therefore, in a first set of studies, we researched whether the high level of telencephalic aromatase that had been detected in Zebra Finches would also be found in a variety of wild European songbird species caught in the wild during the breeding season. Males from four different species of songbirds belonging to four different families were for this purpose caught by mist nests in southwest Sweden at a field site close to Göteborg. These included 7 chaffinches (Fringilla coelebs, Fringillidae; caught between May 29 and June 6 with fully developed testes), 7 Pied Flycatchers (Ficedula hypoleuca, Muscicapidae; caught between May 28 and June 7 while defending secondary territories), 7 great tits (Parus major, Paridae; caught between June 5 and 26 while feeding nestlings [n=6] or when the female was incubating [n=1]) and 6 willow warblers (Phylloscopus trochilus, Sylviidae; caught on May 30). Aromatase activity was measured in the brain of these birds and compared to the activity found in previously studied species, namely the Zebra Finch (Taeniopygia guttata, Estrildidae, 6 two-year old males raised in the laboratory), Japanese Quail (Coturnix japonica, Phasianidae, 5 adult sexually mature males raised in the laboratory) and ring dove (Streptopelia roseogrisea [= risoria], Columbidae, 2 males raised and kept in an outdoor aviary and killed in December).
All subjects were sacrificed by decapitation as soon as possible after capture and their brain was immediately dissected out of the skull and frozen on powdered dry ice. Brains were then stored at -70°C until assayed for enzyme activity. Frozen brains were dissected using anatomical landmarks to isolate 5 different brain regions: the telencephalon (Tel.), optic lobes (O.L.), diencephalon (Di.), cerebellum (Cereb.), and brain stem (B.S.). These brain parts were weighed to the nearest tenth of a milligram and then homogenized in buffer (150 mM KCl, 1mM EDTA, 10 mM Tris-HCl, pH 7.2). Aromatase activity was measured in triplicate in each of these brain fractions by measuring the release of tritiated water during aromatization of [1
b-3H]-androstenedione, as described by Roselli and Resko (Roselli & Resko 1991) with minor modifications. The assay and its validation for use in the quail (Baillien & Balthazart 1997) and for the songbird (Foidart et al. 1998) brain have been previously described. The protein content of the homogenates was determined by a micromodification of the Bradford method (Bradford 1976) using commercial Coomassie plus protein assay reagent (Pierce, Rockford, IL 61105, USA). Enzyme activity was expressed either as fmol per brain fraction (total activity present in the area) or in fmol. h-1. mg protein-1. We shall only discuss here the data concerning the relative levels of aromatase activity in the diencephalon and telencephalon.As can be observed in Fig. 5A, this study confirmed that in Zebra Finches, contrary to quail and doves, the aromatase activity, expressed per mg protein is larger in the telencephalon than in any other brain area including the diencephalon. The same situation was observed in the flycatcher but not in the other songbird species that were considered (chaffinch, tit, warbler), therefore questioning the generalization of this pattern to the passeriformes order. One must however consider that the telencephalon is at least one order of magnitude larger than the diencephalon. When aromatase activity is therefore recalculated for the entire brain area, the activity difference between the telencephalon and diencephalon becomes more prominent in all songbird species and a 5 to 20 fold excess is found in the former by comparison with the latter area (see Fig. 5B). With this expression of activity, the aromatase activity is however still lower in the telencephalon than in the diencephalon of quail but not of doves. It must be noted that the Ring Doves used here for comparison were killed outside breeding season when diencephalic aromatase was presumably minimal. Assays performed on reproductively active birds would probably yield a pattern of activity that would favor the diencephalic aromatase in this species also.
In conclusion, these data support the notion that songbirds are characterized by a high level of telencephalic aromatase activity by comparison with other avian groups. The difference is however not as dramatic as previously thought and some overlap can be observed especially if enzyme activities are normalized for the weight of brain areas and expressed per mg of protein. It should also be pointed out that aromatase activity is present in the telencephalon of all species studied so far including non songbirds. The evolutionary significance of the high or low ratio between telencephalic and diencephalic aromatase activity remains unclear at present and will probably be impossible to specify until the enzyme has been studied in a larger number of species originating from a broad range of families and displaying a variety of ecological life styles.
SEASONAL CHANGES OF AROMATASE IN THE PIED FLYCATCHER BRAIN: RELATIONSHIP WITH REPRODUCTIVE STAGE
As mentioned above, most studies on brain aromatase in birds have so far been concerned with domestic or semi-domestic species such as the ring dove, quail, chicken or Zebra Finch and little information is available on the anatomical distribution and on possible changes in brain aromatase activity during the normal reproductive cycle in free-living birds. In these semi-domestic species, the regulation and neuroanatomical distribution of aromatase has to this date been studied by product formation assays, immunocytochemistry and molecular biology (assays of mRNA concentration or their visualisation by in situ hybridisation). As reviewed above, these studies have revealed that the aromatase activity is high in the preoptic area and hypothalamus (Callard et al. 1978; Steimer & Hutchison 1981a; Schumacher & Balthazart 1986) that are implicated in the activation of male reproductive behaviour (Panzica et al. 1996). Moreover, the preoptic aromatase activity is strongly increased by T in several bird species including the quail, dove and Zebra Finch (Steimer & Hutchison 1981a; Schumacher & Balthazart 1986; Vockel et al. 1990b) which supports the notion that this enzyme plays a critical role in the control of male sexual behaviour (see: Balthazart 1989; Balthazart et al. 1996b for review). Additional studies indicate that, in the few species studied so far the T-induced changes in preoptic aromatase activity result from changes in the concentration of the enzyme caused by an increased transcription of the corresponding mRNA (see: Balthazart & Foidart 1993; Foidart et al. 1995b for additional discussion). High levels of aromatase activity are also present in the telencephalon of songbirds (Vockel et al. 1990a; Vockel et al. 1990b; Schlinger & Arnold 1991) but the control of aromatase by T that is present in the preoptic area and anterior hypothalamus is surprisingly not observed in the telencephalon of the only songbirds species that has been studied in this respect, the Zebra Finch (Vockel et al. 1990b).
Seasonally breeding species that live in the temperate zone show marked seasonal changes in testis weight and in testosterone (T) plasma levels. These variations are paralleled by pronounced behavioural modifications. In many species, males defend a territory and vigorously attack any intruder, they court a female, and eventually help feeding the young. The high level of aggressiveness that characterizes the establishment of the territory is generally lost during the period of incubation and feeding of nestlings (Silverin & Deviche 1991; Silverin 1993). The endocrine mechanisms that control these behaviour changes have been investigated in a variety of species and it is now widely accepted that both aggressive and reproductive male behaviours are activated by T in many vertebrate species (Silver et al. 1979; Balthazart 1983; Wingfield 1985).
The central conversion of T into estradiol (E2), or aromatization, appears to be a critical step in the activation of male copulatory behaviour and also possibly of parts of the aggressive repertoire (Adkins & Pniewski 1978; Balthazart 1989; Balthazart & Foidart 1993; Schlinger & Callard 1990) but to this date, little or no data are available concerning the neuroanatomical distribution and functional regulation of the aromatase enzyme in the brain of free-living species. We therefore decided to initiate studies on this topic in male Pied Flycatchers Ficedula hypoleuca. The Pied Flycatcher is a migratory species common in northern Europe during the spring breeding season. It is a poly-territorial, hole-nesting bird that breeds preferentially in deciduous forests. The Pied Flycatcher is a polygamous species in which the male leaves his home territory usually on the day his female lays her first egg and establishes a secondary territory some 100-400 m away where he tries to attract a second female. He will return to the home territory during the later part of the incubation period of the first female and participate in the feeding of the nestlings. The second female will incubate and feed her nestlings alone and these young will therefore have a lower survival success (Silverin 1983b; Silverin 1983a).
Male Pied Flycatchers arrive in the breeding area and occupy territories before the females arrive. When a female settles in a territory, she starts nest-building more or less immediately. Within just a day or two, the male is found to have drastically increased luteinizing hormone (LH) and T titers that remain elevated during the early part of the nest-building period (Silverin & Wingfield 1982). The Pied Flycatcher is also known for its early and extremely rapid gonadal regression starting during the later part of the incubation period. All males have basal T levels at the time of egg hatching and, just a few days later, the gonads are completely regressed (Silverin 1975; Silverin 1983b; Silverin & Wingfield 1982). Immunocytochemical and biochemical studies were initiated in this species to describe the distribution of aromatase-containing cells in the brain and research whether changes in enzyme activity are associated with the natural changes in plasma T that physiologically occur during the reproductive cycle (see Foidart et al. 1998 for a full description of these results).
Changes in brain aromatase activity Male Pied Flycatchers were captured by automatic traps inside the nest-boxes or by mist nests in the territories they had established around nest boxes in a mixed forest just outside Göteborg (Sweden). Males were captured at two different stages of their breeding cycle: in late May/early June when they were defending a secondary territory and in late June, when they had returned to their primary territory and were helping females in feeding the nestlings (n = 5-7 in each case). A blood sample was taken from the jugular vein (a procedure not taking more than 15-30 seconds) within 1-3 min after capture and kept on ice until centrifuged later on the same day. Testosterone was measured by a single antibody assay in all plasma samples after extraction and chromatographic separation on celite:glycol microcolumns (see: Wingfield & Farner 1975; Ball & Wingfield 1987 for description and validation of these procedures). The assays confirmed the existence of a statistically significant drop in plasma T levels when birds switch from territory defence to feeding nestlings (from 557.2±72.9 to 138.2±40.7 pg/ml; mean±SE). This decrease in plasma T levels was associated with the seasonal collapse of the testes (from 91.8±6.1 mg to 11.9±1.2 mg; means of paired testes weight ± SE).One set of birds (seven males defending a secondary territory and six males feeding nestlings) were sacrificed by decapitation and brains were immediately dissected out of the skull and frozen on powdered dry ice. Aromatase activity was then measured by the radioenzyme assay quantifying the release of tritiated water described above in 6 brain regions: the telencephalon (Tel.), the anterior diencephalon (ant.Di., corresponding to the preoptic area and rostral hypothalamus including the POM), the posterior diencephalon (post.Di., including the ventromedial nucleus of the hypothalamus [VMN] and the tuberal region), the optic lobes (O.L.), the cerebellum (Cereb.), and the brain stem (B.S.). Results of these assays expressed as fmol of water (or oestrogen) produced per hour and per mg protein are summarised in Fig. 6.
As already reported above, the highest level of aromatase activity was observed in the telencephalon (Fig. 6A) where enzyme activity clearly exceeded the activity detected in any other brain area including the anterior or posterior parts of the diencephalon. Low to non detectable levels of enzyme activity were observed in the other brain regions. The aromatase activity per mg protein was approximately twice higher in the telencephalon than in the diencephalon, a difference that, given the large size of the telencephalon, translates into a 20 fold excess when total activities are computed for the entire area (see also Fig. 5).
Analysis of these data by a mixed two-way ANOVA with the two periods of the reproductive cycle as an independent variable and the 6 brain regions as a repeated measure clearly indicated the heterogeneous distribution of aromatase activity between the 6 brain regions (P < 0.001) as well as the overall significant difference between the two experimental groups (P = 0.002) . Interestingly, a strong interaction between these two variables was also detected (P = 0.002) and additional post hoc t-tests using the relevant mean square of the ANOVA as the basis for comparison showed that aromatase activity is significantly higher in the secondary territory males than in males feeding nestlings in the anterior (P < 0.05) and posterior (P < 0.01) diencephalon but this difference is not present in the other brain regions including the telencephalon (P > 0.05 in each case).
These data showed that territorial males who have higher plasma T levels also have a higher aromatase activity in the diencephalon as compared with males feeding nestlings. Because previous studies in other avian species have shown that T increase aromatase activity (e.g. Steimer & Hutchison 1981a; Schumacher & Balthazart 1986), we further analysed the relationship between the individual variations in these two variables in the different brain regions for the two groups of birds considered together or separately. In the pooled data from all subjects studied at the two points in the reproductive cycle a linear regression analysis demonstrated the existence of a significant correlation between plasma T level and aromatase activity per mg protein in the anterior and posterior diencephalon (ant.Di.: r = 0.887, P > 0.001; post.Di.: r = 0.910, P > 0.001) but not in the telencephalon (P > 0.05; see Fig. 6B). These significant correlations obviously originated in part in the seasonal variation and in part in the individual differences at one given stage of the reproductive cycle. To separate these two sources of variance, correlations between the same variables were computed again for the territorial birds and the birds feeding nestlings separately. All these correlation coefficients were then found to be non significant but in birds defending a territory, correlations fell short of significance (statistical tendencies) for the relationship between plasma T levels and aromatase activity in the anterior and posterior diencephalon (r = 0.754, P = 0.083 and r = 0.773, P = 0.071 respectively) while no relationship was seen between plasma T levels and aromatase activity in the telencephalon (P > 0.10). Similarly no relationship could be detected between plasma T levels and aromatase activity in all brain areas of the birds feeding nestlings (P > 0.10 in each case).
Neuroanatomical distribution of aromatase-immunoreactive (ARO-ir) cells Another set of birds were anesthetised within 30-60 min after capture and then perfused through the heart with fixative (4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer, pH 7.2). Brains were dissected out of the skull, cryoprotected overnight in a 20% sucrose solution and frozen on powdered dry ice. These brains were then cut with a cryostat in 25 µm-thick coronal sections from the caudal to the rostral edge and sections were stained by immunocytochemistry for the aromatase protein. The method based on a primary polyclonal antibody raised against quail recombinant aromatase has been fully described previously (Foidart et al. 1995a; Balthazart et al. 1996a).The overall pattern of distribution of ARO-ir cells was similar in males defending secondary territories and in males feeding nestlings and strongly resembled the distribution previously reported for ARO-ir cells and cells expressing aromatase mRNA in the Zebra Finch brain (Balthazart et al. 1990c; Balthazart et al. 1996a; Shen et al. 1995). In the anterior preoptic area, a medial cluster of immunoreactive cells adjacent to the third ventricle was observed in a ventral position under the tractus septomesencephalicus and extended through the rostro-caudal extent of the preoptic area to end up in a position just ventral to the anterior commissure (CA). A similar group of ARO-ir cells has been described in Zebra Finches (Balthazart et al. 1996a) and identified as the homologueue of the medial preoptic nucleus (POM) of the quail (Panzica et al. 1991; Panzica et al. 1996). The same terminology will be used here (Fig. 7A).
At the same level, a small group of ARO-ir neurons is observed between the ventral edge of the lateral ventricles and the dorsal edge of the CA (Fig. 4B). This cluster has been identified as the rostral part of the nucleus striae terminalis (nST) in our work on Zebra Finches (Balthazart et al. 1996a) but is located in a slightly more ventral position than the nST as indicated in the stereotaxic atlas of the canary brain (Stokes et al. 1974). A small number of weakly labelled cells was in addition observed at the lateral tip of the lateral ventricles (Fig. 7A) in a position identified as the nST in the canary brain atlas (Stokes et al. 1974).
In sections immediately caudal to the CA, the groups of ARO-ir neurons located in the nST and in the caudal POM appear to merge and form a V-shaped group of immunopositive cells identified as the caudal part of the nST in the Zebra Finch and quail (Balthazart et al. 1996a; Foidart et al. 1995a). At these rostro-caudal levels, another more ventral group of ARO-ir cells is observed at the level of the nucleus ventromedialis hypothalami (Fig. 7A; VMN; = posteromedial hypothalamus, PMH in the canary brain atlas). This group of immunoreactive cells is present without discontinuity throughout the mediobasal hypothalamus but the number of positive cells progressively decreases in the most caudal part of the hypothalamus. Finally, a small number of weakly labelled cells is also found ventral to the VMN in the nucleus tuberis (Tu).
In addition to the ARO-ir cells observed in the diencephalic and limbic areas, large numbers of immunoreactive cells were seen in the flycatcher telencephalon with a pattern of distribution very reminiscent of the distribution reported for in the Zebra Finch telencephalon (Balthazart et al. 1996a; Shen et al. 1995). These telencephalic ARO-ir cells appeared in general to be less numerous in flycatchers than in Zebra Finches but it is impossible at this stage to know whether the difference in abundance reflects a true species difference or is related to technical differences (different cross-reactivity with antibodies, different levels of optimization for the immunocytochemical technique). In the flycatcher, telencephalic ARO-ir cells were mainly found in the neostriatum (Fig. 7B). At the most rostral levels, the positive cells were exclusively located in the medial neostriatum and in the ventral hyperstriatum. In more caudal sections, this cell cluster progressively extended ventrally along the lateral ventricle until the point where this ventricle crosses the lamina medullaris dorsalis. In even more more caudal levels, the immunopositive cells became more numerous and were spread over a broader area that covered at its maximal extent a large area dorsal to the lamina archistriatalis dorsalis and a large part of the caudal neostriatum. A small number of immunopositive neurons were also observed in dorsomedial position throughout the hippocampus and the area parahippocampalis. No ARO-ir cells could be detected in the nucleus taeniae (Tn), nor in the telencephalic nuclei of the song control system: the high vocal centre (HVC) and nucleus robustus archistriatalis (RA).
Changes in aromatase-immunoreactive cells associated with testes regression In the last part of these studies, we also researched whether the changes in aromatase activity that had been observed in the flycatcher diencephalon as a function of the stage in the reproductive cycle were reflected in the enzyme concentration as indicated by the number of detectable ARO-ir cells. In the preoptic area, immunopositive cells were counted in five equally distant brain sections throughout the rostral to caudal axis of the POM and the mean numbers of positive cells per section for the two experimental groups were calculated (Fig. 7C). The number of ARO-ir cells progressively increased as one moves from the most rostral to caudal sections (level CA-8 to CA-2) and this increase was more pronounced in territorial birds than in birds feeding nestlings. A larger number of ARO-ir cells was therefore detected in the caudal (but not in the rostral) part of the POM in territorial birds by comparison with birds feeding nestlings. The analysis of these data by a two way ANOVA with the two experimental groups as the independent factor and the successive sections as the repeated factor failed however to detect an overall difference between the experimental groups or an interaction between the groups and the anatomical position. There was simply a significant effect of the position in the rostro-caudal axis but this effect was statistically similar in the two groups of birds collected at different points of the breeding cycle. The numerical difference in cell numbers observed in the caudal part of the POM was however in agreement with the difference in enzyme activity that had been detected by biochemical assays.Surprisingly however, the analysis by the same technique of the numbers of ARO-ir cells counted at five successive rostro-caudal levels in the VMN identified an effect of the position in the rostro-caudal axis but also an overall difference between the experimental groups and an interaction between these two variables. The number of ARO-ir cells was unexpectedly higher in the posterior diencephalon of the males feeding nestlings than in territorial birds, whereas aromatase activity was higher in territorial birds (see: Foidart et al. 1998 for the detail of these results).
Previous studies in Japanese Quail showed that changes in aromatase activity as a function of the sex of the subjects, gonadal state (castrated vs gonadally intact) or endocrine treatment (replacement therapy with androgens and/or oestrogens) are almost always paralleled by differences in the number of ARO-ir cells, suggesting that the changes in enzyme activity are caused largely if not entirely by a change in enzyme concentration (Balthazart & Foidart 1993; Panzica et al. 1996). Why such correlations between enzymatic activity and numbers of ARO-ir cells were not clearly observed in flycatchers remains partly unknown. Territorial males appeared to have more ARO-ir cells in the POM than birds feeding nestlings, especially in the caudal part of the nucleus. This result fits in well with previous data in quail showing that a treatment with exogenous T increases the number of ARO-ir cells specifically in the caudal part of the POM (Foidart et al. 1994a; Balthazart et al. 1996d) and with the enzyme assay data in flycatchers showing a decrease in aromatase activity in birds feeding nestlings by comparison with territorial birds. The seasonal changes in number of ARO-ir cells in POM did not reach statistical significance presumably because a relatively small sample size was available (6 -7 subjects further decreased by technical problems so that 4 pairs of birds only could be used in the general ANOVA comparing all rostro-caudal levels in all subjects).
The discrepancy between the changes in aromatase activity (decrease between May/June and late June) and in ARO-ir cell numbers (increase during the same period) observed in the VMN is however more surprising. As mentioned above, previous studies have often identified a parallel between the enzymatic activity and the number of immunoreactive cells. It must however be pointed out that these studies exclusively concern a semi-domesticated species, the Japanese Quail and that the changes observed were due to surgical castration or treatment with exogenous hormones and never concerned the physiological variations as a function of the season. The present data suggests that the testicular collapse that occurs at the beginning of the summer in flycatcher is associated at least for a period of time with a drop in aromatase activity but with an increase in ARO-ir cell numbers and therefore presumably in aromatase concentration. The mechanism underlying this dissociation is not clear at present. The discrepancy is however reminiscent of a situation observed in quail treated with non steroidal aromatase inhibitors such as vorozole and fadrozole: these inhibitors completely block aromatase activity but they unexpectedly increase the number and optical density of ARO-ir cells (Foidart et al. 1994b; Foidart et al. 1995b). It has been suggested that these compounds extend, in some unidentified way, the half-life of the enzyme so that the inactive protein accumulates in the neurons and leads to a better immunocytochemical detection (Foidart et al. 1995b; Balthazart 1997). The same mechanisms could be operating during the breeding cycle of the flycatcher: the transition from photosensitivity to photorefractoriness would inhibit the aromatase activity but not the enzyme synthesis (at least for some time) so that inactive enzyme could build up in neurons of the VMN. It is in this respect interesting to note that the transition from photosensitivity to photorefractoriness is associated in another songbird species, the starling (Sturnus vulgaris) with neuroanatomically differentiated changes in 5
b-reduction of T (Bottoni & Massa 1981) and that 5b-reduced metabolites of T appear to inhibit aromatase activity (Schumacher et al. 1991).CONCLUSIONS
Anatomical results
A host of studies have shown in Zebra Finches, quail and doves that T aromatisation is a critical step in the induction of most behavioural effects of the androgen and in particular, it has been shown that , in two species of song birds (Zebra Finch and red-winged blackbird), song is activated by a synergistic action of oestrogenic and androgenic metabolites of T. The role of the central T metabolism in the control of vocal behaviour remains, however, partly unclear. If it has been possible to demonstrate clear effects on song production of systemic treatments that affect the availability of T metabolites (Harding et al. 1983; Walters & Harding 1988), the specific role played by the enzymes in each brain area is completely unknown. In particular, the specific implication of the high telencephalic aromatase activity identified in songbirds in the control of vocalisations remains unknown given that ARO-ir cells do not appear to overlap with song control nuclei (see: Balthazart et al. 1996a for further discussion on this topic).
The anatomical mismatch between aromatase, oestrogen receptor and song control nuclei in the Zebra Finch central nervous system questions the way in which steroids affect behaviour. An autocrine mode of action has always been assumed. Oestrogens derived from intracellular aromatisation would act in the cell where they are produced and these should therefore contain both oestrogen receptors and aromatase. The recent anatomical (immunocytochemistry and in situ hybridisation) and physiological evidence suggest the possibility of paracrine or even endocrine effects for the oestrogens produced in the brain.
The data presented in this paper indicate that, as could be suspected based on the limited amount of available data (Saldanha & Schlinger 1997; Schlinger et al. 1992; Schlinger 1996), the existence of high levels of aromatase activity in the telencephalon can be considered as a typical feature of songbirds, even if the difference with other avian orders is of a smaller magnitude than could have been expected based only on the study of the zebra finch. The anatomical distribution of this telencephalic aromatase or of its messenger RNA has now been mapped with some detail in three songbird species, the zebra finch, brown-headed cowbird and Pied Flycatcher. A great degree of similarity was observed in the hypothalamic and limbic distribution of the enzyme in these species and all studies agree to localize the majority of telencephalic aromatase-containing cells in the neostriatum, ventral hyperstriatum, hippocampus and area hippocampalis. In all studies also, little or no aromatase was detected in the song control nuclei HVC and RA.
In Pied Flycatchers like in Zebra Finches (Vockel et al. 1990a; Schlinger & Arnold 1991; Balthazart et al. 1996a; Shen et al. 1995), a high level of aromatase activity was observed in the telencephalon and this enzymatic activity corresponded to the presence of broad populations of ARO-ir cells in the neostriatum, hyperstriatum, hippocampus and area parahippocampalis. These ARO-ir cells had remained undetected in our original study of the Zebra Finch brain (Balthazart et al. 1990c) but they have been more recently identified by immunocytochemistry with the help of a new more sensitive antibody or by pretreating the subjects with a non aromatizable inhibitor (Balthazart et al. 1996a). Their distribution has been confirmed by in situ hybridisation (Shen et al. 1995). It was thought originally that the presence of aromatase in the telencephalon was a characteristic of songbirds but this conclusion was only based on studies in a few species (see above). The present study brings further support to this idea with a broader set of data.
It must also be noted that a significant level of aromatase activity was recently detected in the telencephalon of the Japanese Quail, in parallel with the existence of large numbers of weakly immunoreactive aromatase cells (Balthazart et al. 1998). Therefore, the presence of aromatase in the telencephalon is probably not a unique characteristic of songbirds as opposed to other avian species but it should rather be considered that songbirds differ from other avian species by the higher level of telencephalic expression of the enzyme. When expressed per mg of protein, aromatase activity appears to be twice higher in the telencephalon than in the hypothalamus in Zebra Finches or Pied Flycatchers but this ratio is more than 10 times lower in quail (Balthazart et al. 1998). Because the telencephalon weight is about one order of magnitude larger than the diencephalon weight, the total amount of enzyme activity in the telencephalon remains important by comparison with the hypothalamic aromatase activity even in species where activity per mg protein is relatively modest (e.g. quail). Based on the anatomical studies described above, it appears that the aromatase activity in the telencephalon of songbirds may not be directly related to the control of song expression because ARO-ir cells and cells expressing the ARO mRNA do not appear to be present in the song control nuclei HVC and RA (Shen et al. 1995; Balthazart et al. 1996a). Indirect functional relationships cannot be ruled out at present but the possibility that telencephalic aromatase could be related to completely different biological functions is certainly supported by the presence of a significant level of aromatase activity in avian species that do not possess a song control system such as quail and doves.
Seasonal and endocrine control of aromatase activity
During the last few years, the activity of T-metabolising enzymes has been measured in the brain of a few avian species including one songbird, the Zebra Finch. These studies have revealed the presence of large enzymatic differences related to the sex, age or endocrine status of the birds. In some cases, the aromatase activity appears to be controlled by the adult endocrine environment but in others cases it is not, suggesting the existence of differential regulation mechanisms in different species and neuroanatomical loci. The nature of these regulatory mechanisms is currently being uncovered largely with the help of molecular biology techniques.
Studies carried out almost exclusively in laboratory species had shown that experimentally-induced changes in plasma T are rapidly reflected (within a few hours or days) in changes preoptic-hypothalamic aromatase activity. We have now demonstrated that the testicular collapse and dramatic fall in plasma T that take place during the natural breeding cycle of Pied Flycatchers when birds cease to defend a territory and start feeding young nestlings are also paralleled by a similar drop in the preoptic and hypothalamic aromatase activity. In contrast, the telencephalic aromatase appears to be insensitive to this control by T in flycatcher, just like it was not affected by castration in Zebra Finches (Vockel et al. 1990b).
These data strongly argue for the presence of different regulatory mechanisms for aromatase activity and presumably aromatase synthesis in these two brain areas. In all species that have been studied so far in some detail, a good parallel has been observed between T-induced changes in the aromatase activity, in the number of ARO-ir cells and in the aromatase mRNA concentration in the preoptic area and hypothalamus (Steimer & Hutchison 1981a; Schumacher & Balthazart 1986; Vockel et al. 1990b; Balthazart & Foidart 1993; Panzica et al. 1996; Balthazart et al. 1996b). These data strongly suggest that T enhances the transcription of aromatase in the diencephalon, a fact that is consistent with the recent discovery of an androgen responsive element at the 5’-end of the first untranslated brain-typical exon of the aromatase gene in rat (Kato et al. 1997). Why this control mechanism does not appear to be present in the telencephalon has remained mysterious so far but the analysis of the aromatase gene by molecular biology techniques is beginning to provide a detailed answer to this question (Simpson et al. 1991; Bulun & Simpson 1994; Simpson et al. 1994; Lephart 1996; Lephart 1997). The control of aromatase activity by different hormones or growth factors in different tissues appears to be related to the fact that aromatase transcription is regulated by different promoters located in or at the 5’-end of different tissue-specific untranslated exons 1 (Simpson et al. 1997). A specific exon 1 (called exon 1f) has been isolated and sequenced in the rat brain and presumably explains the peculiar regulation of the enzyme synthesis in the brain compared to peripheral tissues in this species (Yamada-Mouri et al. 1997; Kato et al. 1997; Lephart 1996). Interestingly, a recent report in abstract form indicates that different types of exon 1 called exons 1a and 1b are also present in the Zebra Finch brain (Ramachandran et al. 1997) and these could be differentially expressed in the diencephalon and telencephalon. These anatomically specific first exons associated with different regulatory elements could explain why aromatase transcription is regulated in a different fashion within specific brain areas. Detailed studies of the telencephalic and diencephalic exons 1 and of their 5’-end should help explaining why aromatase activity is controlled by T (Zebra Finches) or changes with season (Pied Flycatcher) in one brain area but not in the other. Although we did not formally test here the idea that the seasonal change in diencephalic aromatase activity is related to the changes in plasma T levels in Pied Flycatchers, the fact that levels of aromatase activity are closely correlated with plasma T in the two diencephalic regions but not in the telencephalon strongly supports this idea.
Many studies in birds and other vertebrates demonstrate that T is a key endocrine factor responsible for the activation of sexual and aggressive behaviour. The endocrine changes seen in the Pied Flycatcher when males begin to feed nestlings are therefore presumably responsible for the dramatic behavioural metamorphosis seen at that time: males stop defending a territory, they no longer show aggressive reactions towards territory intruders, they no longer try to attract additional females and they start helping the primary female in feeding her young nestlings (Silverin 1993). In flycatchers, the diencephalic aromatase is located in nuclei that are obviously related to the control of various aspects of reproduction (POM, VMN, nST) and the observation that this enzymatic activity decreases when birds switch from territorial defence to caring for nestlings certainly reflects the underlying control of these behavioural activities. It is known for example that oestrogens contribute to the activation of singing and possibly of aspects of aggressive behaviours in songbirds (Harding et al. 1988; Harding et al. 1983; Walters & Harding 1988; Walters et al. 1991). The diencephalic drop in aromatase activity may therefore be implicated in the seasonal control of these behavioural changes. More studies should however be carried out in a variety of avian species to investigate whether control mechanisms for steroid-metabolising enzymes that have originally been discovered in (semi-) domestic species also apply to free-living birds. Additional regulatory mechanisms may be uncovered as suggested here by the discrepancy between seasonal changes in aromatase activity and in the number of aromatase-immunoreactive cells in the posterior hypothalamus of flycatchers.
ACKNOWLEDGMENTS
The work of the authors described in this review was supported in part by grants of the NIMH (MH50388), the NINDS (NS35467), the Belgian FRFC (9.4565.96F), and the Government of the French Community of Belgium (Action Concertée #93/98-171) to JB and by grants from the Swedish National Research Council (Dnr B-AA/BU 04342-321), from the ‘Magn. Bergvalls Stiftelse’ and the’Kungl. Vetenskaps- och Vitterhets Samhallet i Goteborg’ foundations to BS. AF is Research Associate of the Belgian FNRS
REFERENCES
Adkins, E.K. & Nock, B.L. 1976. The effects of the antioestrogen CI-628 on sexual behaviour activated by androgen and oestrogen in quail. Hormones and Behaviour 7: 417-429.
Adkins, E.K. 1977. Effects of diverse androgens on the sexual behaviour and morphology of castrated male quail. Hormones and Behaviour 8: 201-207.
Adkins, E.K. & Pniewski, E.E. 1978. Control of reproductive behaviour by sex steroids in male quail. Journal of Comparative and Physiological Psychology 92: 1169-1178.
Adkins, E.K., Boop, J.J., Koutnik, D.L., Morris, J.B. & Pniewski, E.E. 1980. Further evidence that androgen aromatization is essential for the activation of copulation in male quail. Physiology and Behaviour 24: 441-446.
Adkins-Regan, E. 1981a. Effect of sex steroids on the reproductive behaviour of castrated male ring doves (Streptopelia sp.). Physiology and Behaviour 26: 561-565.
Adkins-Regan, E. 1981b. Hormone specificity, androgen metabolism, and social behaviour. American Zoologist 21: 257-271.
Adkins-Regan, E. 1983. Sex steroids and the differentiation and activation of avian reproductive behaviour. In Balthazart, J. & Gilles, R. (eds) Hormones and behaviour in higher vertebrates. Springer-Verlag, Berlin: 219-228
Alexandre, C. & Balthazart, J. 1986. Effects of metabolism inhibitors, antioestrogens and antiandrogens on the androgen and oestrogen induced sexual behaviour in Japanese Quail. Physiology and Behaviour 38: 581-591.
Alvarez-Buylla, A., Theelen, M. & Nottebohm, F. 1990. Proliferation ‘hot spots’ in adult avian ventricular zone reveal radial cell division. Neuron 5: 101-109.
Andrew, R. 1975. Effects of testosterone on the behaviour of the domestic chick. I. Effects present in males but not in females. Animal Behaviour 23: 139-155.
Arnold, A.P. 1997. Sexual differentiation of the zebra pinch song system: Positive evidence, negative evidence, null hypotheses, and a paradigm shift. Journal of Neurobiology 33: 572-584.
Baillien, M. & Balthazart, J. 1997. A direct dopaminergic control of aromatase activity in the quail preoptic area. Journal of Steroid Biochemistry and Molecular Biology 63: 99-113.
Ball, G.F. & Wingfield, J.C. 1987. Changes in plasma levels of luteinizing hormone and sex steroid hormones in relation to multiple-broodedness and nest-site density in male starlings. Physiological Zoology 60: 191-199.
Ball, G.F. 1990. Chemical neuroanatomical studies of the steroid-sensitive songbird vocal control system: a comparative approach. In: Balthazart, J. (ed.) Hormones, Brain and Behaviour in Vertebrates. 1. Sexual Differentiation, Neuroanatomical Aspects, Neurotransmitters and Neuropeptides. Comp. Physiol vol 8, Karger, Basel: 148-167.
Balthazart, J. & Hirschberg, D. 1979. Testosterone metabolism and sexual behaviour in the chick. Hormones and Behaviour 12: 253-263.
Balthazart, J., Malacarne, G. & Deviche, P. 1981. Stimulatory effects of 5
b-dihydrotestosterone on the sexual behaviour in the domestic chick. Hormones and Behaviour 15: 246-258.Balthazart, J. 1983. Hormonal correlates of behaviour. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology. Academic Press, New York: 221-365.
Balthazart, J. & Schumacher, M. 1984. Organization and activation of behaviour in quail: role of testosterone metabolism. Journal of Experimental Zoology 232: 595-604.
Balthazart, J., Schumacher, M. & Malacarne, G. 1985. Interaction of androgens and oestrogens in the control of sexual behaviour in male Japanese Quail. Physiology and Behaviour 35: 157-166.
Balthazart, J., Schumacher, M. & Pröve, E. 1986. Brain testosterone metabolism during ontogeny in the Zebra finch. Brain Research 378: 240-256.
Balthazart, J. 1989. Steroid metabolism and the activation of social behaviour. In: Balthazart, J. (ed.) Advances in Comparative and Environmental Physiology. Vol 3; Springer Verlag, Berlin: 105-159.
Balthazart, J., Evrard, L. & Surlemont, C. 1990a. Effects of the non-steroidal aromatase inhibitor, R76713 on testosterone-induced sexual behaviour in the Japanese Quail (Coturnix coturnix japonica). Hormones and Behaviour 24: 510-531.
Balthazart, J., Foidart, A. & Hendrick, J.C. 1990b. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behaviour. Physiology and Behaviour 47: 83-94.
Balthazart, J., Foidart, A., Surlemont, C., Vockel, A. & Harada, N. 1990c. Distribution of aromatase in the brain of the Japanese Quail, ring dove, and zebra finch: An immunocytochemical study. Journal of Comparative Neurology 301: 276-288.
Balthazart, J. & Surlemont, C. 1990. Androgen and oestrogen action in the preoptic area and activation of copulatory behaviour in quail. Physiology and Behaviour 48: 599-609.
Balthazart, J., Foidart, A., Wilson, E.M. & Ball, G.F. 1992. Immunocytochemical localization of androgen receptors in the male songbird and quail brain. Journal of Comparative Neurology 317: 407-420.
Balthazart, J. & Foidart, A. 1993. Brain aromatase and the control of male sexual behaviour. Journal of Steroid Biochemistry and Molecular Biology 44: 521-540.
Balthazart, J. & Ball, G.F. 1995. Sexual differentiation of brain and behaviour in birds. Trends in Endocrinology and Metabolism 6: 21-29.
Balthazart, J., Absil, P., Foidart, A., Houbart, M., Harada, N. & Ball, G.F. 1996a. Distribution of aromatase-immunoreactive cells in the forebrain of Zebra Finches (Taeniopygia guttata): Implications for the neural action of steroids and nuclear definition in the avian hypothalamus. Journal of Neurobiology 31: 129-148.
Balthazart, J., Foidart, A., Absil, P. & Harada, N. 1996b. Effects of testosterone and its metabolites on aromatase-immunoreactive cells in the quail brain: Relationship with the activation of male reproductive behaviour. Journal of Steroid Biochemistry and Molecular Biology 56: 185-200.
Balthazart, J., Tlemçani, O. & Ball, G. F. 1996c. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behaviour? What 25 years of research on the Japanese Quail tells us. Hormones and Behaviour 30: 627-661.
Balthazart, J., Tlemçani, O. & Harada, N. 1996d. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. Journal of Chemical Neuroanatomy 11: 147-171.
Balthazart, J. 1997. Steroid control and sexual differentiation of brain aromatase. Journal of Steroid Biochemistry and Molecular Biology 61: 323-339.
Balthazart, J., Foidart, A., Baillien, M., Harada, N. & Ball, G.F. 1998. Anatomical relationships between aromatase and tyrosine hydroxylase in the quail brain: double label immunocytochemical studies. Journal of Comparative Neurology 391: 214-226.
Beach, F.A. 1948. Hormones and behaviour. Paul B. Hoeber, Inc., New York.
Berthold, A.A. 1849. Transplantation der Hoden. Archiv Für Anatomie Und Physiologie 16: 42-46.
Blaustein, J.D. & Olster, D.H. (1989). Gonadal steroid hormone receptors and social behaviours. In: Balthazart, J. (ed.) Advances in Comparative and Environmental Physiology. Vol 3; Springer Verlag, Berlin: 31-104.
Bottoni, L. & Massa, R. 1981. Seasonal changes in testosterone metabolism in the pituitary gland and central nervous system of the European starling (Sturnus vulgaris). General and Comparative Endocrinology 43: 532-536.
Bradford, M.M. 1976. A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248-254.
Brenowitz, E.A. 1991. Evolution of the vocal control system in the avian brain. Seminars in the Neurosciences 3: 399-407.
Bulun, S.E. & Simpson, E.R. 1994. Regulation of aromatase expression in human tissues. Breast Cancer Research and Treatment 30: 19-29.
Callard, G.V., Petro, Z. & Ryan, K.J. 1978. Conversion of androgen to oestrogen and other steroids in the vertebrate brain. American Zoologist 18: 511-523.
Cohen, J. & Cheng, M.F. 1982. Effects of testosterone metabolites and oestrogen in the midbrain control of courtship behaviour in the male ring dove (Streptopelia risoria). Neuroendocrinology 34: 64-74.
Davies, D.T., Massa, R. & James, R. 1980. Role of testosterone and of its metabolites in regulating gonadotrophin secretion in the Japanese Quail. Journal of Endocrinology 84: 211-222.
Deviche, P., Bottoni, L. & Balthazart, J. 1982. 5b-dihydrotestosterone is weakly androgenic in the adult Japanese Quail (Coturnix coturnix japonica). General and Comparative Endocrinology 48: 421-424.
Foidart, A., De Clerck, A., Harada, N. & Balthazart, J. 1994a. Aromatase-immunoreactive cells in the quail brain: Effects of testosterone and sex dimorphism. Physiology and Behaviour 55: 453-464.
Feder, H.H. (1981). Essentials of steroid structure, nomenclature, reactions, biosynthesis, and measurements. In: Adler, N.T. (ed.) Neuroendocrinology of Reproduction. Physiology and behaviour. Plenum Press, New York and London: 19-63.
Foidart, A., Harada, N. & Balthazart, J. 1994b. Effects of steroidal and non steroidal aromatase inhibitors on sexual behaviour and aromatase-immunoreactive cells and fibers in the quail brain. Brain Research 657: 105-123.
Foidart, A., Reid, J., Absil, P., Yoshimura, N., Harada, N. & Balthazart, J. 1995a. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. Journal of Chemical Neuroanatomy 8: 267-282.
Foidart, A., Tlemçani, O., Harada, N., Abe-Dohmae, S. & Balthazart, J. 1995b. Pre- and post-translational regulation of aromatase by steroidal and non-steroidal aromatase inhibitors. Brain Research 701: 267-278.
Foidart, A., Silverin, B., Baillien, M., Harada, N. & Balthazart, J. 1998. Neuroanatomical distribution and variations across the reproductive cycle of aromatase activity and aromatase-immunoreactive cells in the Pied Flycatcher (Ficedula hypoleuca). Hormones and Behaviour 33: 180-196.
Gahr, M., Flügge, G. & Güttinger, H.R. 1987. Immunocytochemical localization of oestrogen binding neurons in the songbird brain. Brain Research 402: 173-177.
Gahr, M. & Konishi, M. 1988. Developmental changes in oestrogen sensitive cells in the forebrain of the zebra finch. Proceedings of the National Academy of Science 85: 7380-7383.
Gahr, M., Güttinger, H. -R. & Kroodsma, D.E. 1993. Oestrogen receptors in the avian brain: Survey reveals general distribution and forebrain areas unique to songbirds. Journal of Comparative Neurology 327: 112-122.
Gahr, M. & Metzdorf, R. 1997. Distribution and dynamics in the expression of androgen and oestrogen receptors in vocal control systems of songbirds. Brain Research Bulletin 44: 509-517.
Gurney, M.E. & Konishi, M. 1980. Hormone-induced sexual differentiation of brain and behaviour in Zebra Finches. Science 208: 1380-1383.
Gurney, M.E. 1981. Hormonal control of cell form and number in the Zebra Finch song system. Journal of Neuroscience 1: 658-673.
Harding, C.F., Sheridan, K. & Walters, M.J. 1983. Hormonal specificity and activation of sexual behaviour in male Zebra Finches. Hormones and Behaviour 17: 111-133.
Harding, C.F., Walters, M.J. & Parsons, B. 1984. Androgen receptor levels in hypothalamic and vocal control nuclei in the male zebra finch. Brain Research 306: 333-339.
Harding, C.F., Walters, M.J., Collado, D. & Sheridan, K. 1988. Hormonal specificity and activation of social behaviour in male red-winged blackbirds. Hormones and Behaviour 22: 402-418.
Hutchison, J. B. 1971. Effects of hypothalamic implants of gonadal steroids on courtship behaviour in Barbary doves (Streptopelia risoria). Journal of Endocrinology 50: 97-113.
Hutchison, J.B. & Steimer, Th. 1981. Brain 5b-reductase. A correlate of behavioural sensitivity to androgen.
Hutchison, J.B. & Schumacher, M. 1986. Development of testosterone-metabolising pathways in the avian brain: enzyme localization and characteristics. Developmental Brain Research 25: 33-42.
Hutchison, J.B., Steimer, Th. & Hutchison, R.E. 1986. Formation of behaviourally active oestrogen in the dove brain: induction of preoptic aromatase by intracranial testosterone. Neuroendocrinology 43: 416-427.
Hutchison, J.B. 1987. A role for brain metabolism in the behavioural action of androgen. In: Christiansen, C. & Riis, B. J. (eds) Highlights on Endocrinology. Bogtrykkeri, Copenhagen: 429-436.
Hutchison, J.B., Joris, S., Hutchison, R.E. & Steimer, T. 1989. Steroid control of sexual behaviour and brain aromatase in the dove: Effects of nonaromatizable androgens, methyltrienolone (R1881), and 5a-dihydrotestosterone. Hormones and Behaviour 23: 542-555.
Hutchison, J.B. 1991. Hormonal control of behaviour: steroid action in the brain. Current Opinion in Neurobiology 1: 562-571.
Hutchison, J.B. 1993. Aromatase: Neuromodulator in the control of behaviour. Journal of Steroid Biochemistry and Molecular Biology 44: 509-520.
Hutchison, J.B., Beyer, C., Hutchison, R.E. & Wozniak, A. 1995. Sexual dimorphism in the developmental regulation of brain aromatase. Journal of Steroid Biochemistry and Molecular Biology 53: 307-313.
Kato, J., Yamada-Mouri, N. & Hirata, S. 1997. Structure of aromatase mRNA in the rat brain. Journal of Steroid Biochemistry and Molecular Biology 61: 381-385.
Konishi, M. & Akutagawa, E. 1988. A critical period for oestrogen action on neurons of the song control system in the zebra finch. Proceedings of the National Academy of Science 85: 7006-7007.
Kuiper, G.G.J.M., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J.A. 1996. Cloning of a novel oestrogen receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Science 93: 5925-5930.
Lakaye, B., Foidart, A., Grisar, T. & Balthazart, J. 1998. Partial cloning and distribution of oestrogen receptor beta in the avian brain. Neuroreport 9: 2743-2748.
Lephart, E.D. 1996. A review of brain aromatase cytochrome P450. Brain Research Reviews 22: 1-26.
Lephart, E.D. 1997. Molecular aspects of brain aromatase cytochrome P450. Journal of Steroid Biochemistry and Molecular Biology 61: 375-380.
McEwen, B.S. 1994. Steroid hormone actions on the brain: When is the genome involved? Hormones and Behaviour 28: 396-405.
Mermelstein, P.G., Becker, J.B. & Surmeier, D.J. 1996. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. Journal of Neuroscience 16: 595-604.
Naftolin, F., Horvath, T.L., Jakab, R.L., Leranth, C., Harada, N. & Balthazart, J. 1996. Aromatase immunoreactivity in axon terminals of the vertebrate brain - An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63: 149-155.
Nastiuk, K.L. & Clayton, D.F. 1994. Seasonal and tissue-specific regulation of canary androgen receptor messenger ribonucleic acid. Endocrinology 134: 640-649.
Nastiuk, K.L. & Clayton, D.F. 1995. The canary androgen receptor mRNA is localized in the song control nuclei of the brain and is rapidly regulated by testosterone. Journal of Neurobiology 26: 213-224.
Nelson, R.J. 1994. An introduction to behavioural endocrinology. Sinauer Associates, Inc, Sunderland, Mass.
Nordeen, K. W., Nordeen, E. J. & Arnold, A. P. 1987. Oestrogen accumulation in Zebra Finch song control nuclei: implications for sexual differentiation and adult activation of song behaviour. Journal of Neurobiology 18: 569-582.
Panzica, G.C, Viglietti-Panzica, C., Sanchez, F., Sante, P. & Balthazart, J. 1991. Effects of testosterone on a selected neuronal population within the preoptic sexually dimorphic nucleus of the Japanese Quail. Journal of Comparative Neurology 303: 443-456.
Panzica, G.C., Viglietti-Panzica, C. & Balthazart, J. 1996. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behaviour. Frontiers in Neuroendocrinology 17: 51-125.
Pasqualini, C., Olivier, V., Guibert, B., Frain, O. & Leviel, V. 1995. Acute stimulatory effect of estradiol on striatal dopamine synthesis. Journal of Neurochemistry 65: 1651-1657.
Pröve, E. 1983. Hormonal correlates of behavioural development in male Zebra Finches. In: Balthazart, J., Pröve, E. & Gilles, R. (eds) Hormones and behaviour in higher vertebrates. Springer Verlag, Berlin: 368-374.
Ramachandran, B., Schlinger, B.A., Arnold, A.P. & Campagnoni, A.T. 1997. Structure and expression of the Zebra Finch aromatase gene. Society for Neuroscience Abstracts 23: 61.
Ramirez, V.D., Zheng, J.B. & Siddique, K.M. 1996. Membrane receptors for oestrogen, progesterone, and testosterone in the rat brain: Fantasy or reality. Cellular and Molecular Neurobiology 16: 175-198.
Roselli, C.E. & Resko, J.A. 1991. In vitro assay of aromatase activity in the central nervous system. In: Greenstein, B. (ed.) Neuroendocrine research methods. Vol 2; Harwood Academic Publishers, Chur, Switzerland: 937-951.
Saldanha, C.J. & Schlinger, B.A. 1997. Oestrogen synthesis and secretion in the brown-headed cowbird (Molothrus ater). General and Comparative Endocrinology 105: 390-401.
Schlinger, B.A. & Callard, G.V. 1989. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology 49: 434-441.
Schlinger, B.A. & Callard, G.V. 1990. Aromatization mediates aggressive behaviour in quail. General and Comparative Endocrinology 79: 39-53.
Schlinger, B.A. & Arnold, A.P. 1991. Brain is the major site of oestrogen synthesis in a male songbird. Proceedings of the National Academy of Science 88: 4191-4194.
Schlinger, B.A. & Arnold, A.P. 1992a. Circulating oestrogens in a male songbird originate in the brain. Proceedings of the National Academy of Science 89: 7650-7653.
Schlinger, B.A. & Arnold, A.P. 1992b. Plasma sex steroids and tissue aromatization in hatchling Zebra Finches: Implications for the sexual differentiation of singing behaviour. Endocrinology 130: 289-299.
Schlinger, B.A., Slotow, R.H. & Arnold, A.P. 1992. Plasma oestrogens and brain aromatase in winter white-crowned sparrows. Ornis Scandinavica 23: 292-297.
Schlinger, B.A. & Arnold, A.P. 1993. Oestrogen synthesis in vivo in the adult zebra finch: Additional evidence that circulating oestrogens can originate in brain. Endocrinology 133: 2610-2616.
Schlinger, B.A. 1996. Evolution of steroid-metabolising enzyme expression in the avian brain. Italian Journal of Anatomy and Embryology 101 (Suppl 1): 67-68.
Schlinger, B.A. 1997. The activity and expression of aromatase in songbirds. Brain Research Bulletin 44: 359-364.
Schlinger, B.A. 1998. Sexual differentiation of avian brain and behaviour: Current views on gonadal hormone-dependent and independent mechanisms. Annual Review of Physiology 60: 407-429.
Schumacher, M. & Balthazart, J. 1983. The effects of testosterone and its metabolites on sexual behaviour and morphology in male and female Japanese Quail. Physiology and Behaviour 30: 335-339.
Schumacher, M. & Balthazart, J. 1986. Testosterone-induced brain aromatase is sexually dimorphic. Brain Research 370: 285-293.
Schumacher, M. 1990. Rapid membrane effects of steroid hormones: an emerging concept in neuroendocrinology. Trends in Neurosciences 13: 359-362.
Schumacher, M., Hutchison, R. E. & Hutchison, J. B. 1991. Inhibition of hypothalamic aromatase activity by 5beta-dihydrotestosterone. Journal of Neuroendocrinology 3: 221-226.
Shen, P., Campagnoni, C.W., Kampf, K., Schlinger, B.A., Arnold, A.P. & Campagnoni, A.T. 1994. Isolation and characterization of a Zebra Finch aromatase cDNA: In situ hybridization reveals high aromatase expression in brain. Molecular Brain Research 24: 227-237.
Shen, P., Schlinger, B.A., Campagnoni, A.T. & Arnold, A.P. 1995. An atlas of aromatase mRNA expression in the Zebra Finch brain. Journal of Comparative Neurology 360: 172-184.
Shughrue, P.J., Lane, M. V. & Merchenthaler, I. 1997. Comparative distribution of oestrogen receptor-
a and -b mRNA in the rat central nervous system. Journal of Comparative Neurology 388: 507-525.Silver, R., O'Connell, M. & Saad, R. (1979). The effects of androgens on the behaviour of birds. In edited by Beyer, C. Endocrine control of sex behaviour, pp. 223-279. Raven Press, New York.
Silverin, B. 1975. Reproductive organs and breeding behaviour of the male Pied Flycatcher Ficedula hypoleuca (Pallas). Ornis Scandinavica 6: 15-26.
Silverin, B. & Wingfield, J.C. 1982. Patterns of breeding behaviour and plasma levels of hormones in a free-living population of Pied Flycatchers Ficedula hypoleuca. Journal of Zoology 198: 117-129.
Silverin, B. 1983a. Population endocrinology of the female Pied Flycatcher, Ficedula hypoleuca. In edited by Balthazart, J., Pröve, E. & Gilles, R. Hormones and Behaviour in Higher Vertebrates, pp. 388-397. Springer Verlag, Berlin.
Silverin, B. 1983b. Population endocrinology and gonadal activities of the male Pied Flycatcher (Ficedula hypoleuca) . In: Mikami, S., Homma, K. & Wada, M. (eds) Avian endocrinology: environmental and ecological perspectives. Japan Sci. Soc. Press/Springer-Verlag, Tokyo/Berlin: 289-305.
Silverin, B. & Deviche, P. 1991. Biochemical characterization and seasonal changes in the concentration of testosterone-metabolising enzymes in the European great tits (Parus major) brain. General and Comparative Endocrinology 81: 146-159.
Silverin, B. 1993. Territorial aggressiveness and its relation to the endocrine system in the Pied Flycatcher. General and Comparative Endocrinology 89: 206-213.
Simpson, E.R., Means, G.D., Mahendroo, M.S., Kilgore, M.W., Corbin, C.J. & Mendelson, C.R. 1991. Analysis of the gene encoding human aromatase cytochrome P-450. In: Hochberg, R.B. & Naftolin, F. (eds) The new biology of steroid hormones. Raven Press, New York: 1-13.
Simpson, E.R., Mahendroo, M.S., Means, G.D., Kilgore, M.W., Hinshelwood, M.M., Graham-Lorence, S., Amarneh, B., Ito, Y., Fisher, C.R., Michael, M.D., Mendelson, C.R. & Bulun, S.E. 1994. Aromatase cytochrome P450, the enzyme responsible for oestrogen biosynthesis. Endocrine Reviews 15: 342-355.
Simpson, E.R., Michael, M.D., Agarwal, V.R., Hinshelwood, M.M., Bulun, S.E. & Zhao, Y. 1997. Expression of the CYP19 (aromatase) gene: An unusual case of alternative promoter usage. FASEB Journal 11: 29-36.
Steimer, T. & Hutchison, J.B. 1981a. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature 292: 345-347.
Steimer, Th. & Hutchison, J.B. 1981b. Metabolic control of the behavioural action of androgens in the dove brain: testosterone inactivation by 5
b-reduction. Brain Research 209: 189-204.Stokes, T.M., Leonard, C.M. & Nottebohm, F. 1974. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. Journal of Comparative Neurology 156: 337-374.
Thompson, T.L. & Moss, R.L. 1994. Oestrogen regulation of dopamine release in the nucleus accumbens: Genomic- and nongenomic-mediated effects. Journal of Neurochemistry 62: 1750-1756.
Vockel, A., Pröve, E. & Balthazart, J. 1988. Changes in the activity of testosterone-metabolising enzymes in the brain of male and female Zebra Finches during the post-hatching period. Brain Research 463: 330-340.
Vockel, A., Pröve, E. & Balthazart, J. 1990a. Sex- and age-related differences in the activity of testosterone-metabolising enzymes in microdissected nuclei of the Zebra Finch brain. Brain Research 511: 291-302.
Vockel, A., Pröve, E. & Balthazart, J. 1990b. Effects of castration and testosterone treatment on the activity of testosterone-metabolising enzymes in the brain of male and female Zebra Finches. Journal of Neurobiology 21: 808-825.
Walters, M.J. & Harding, C.F. 1988. The effects of an aromatization inhibitor on the reproductive behaviour of male Zebra Finches. Hormones and Behaviour 22: 207-218.
Walters, M.J., McEwen, B.S. & Harding, C.F. 1988. Oestrogen receptor levels in hypothalamic and vocal control nuclei in the male zebra finch. Brain Research 459: 37-43.
Walters, M.J., Collado, D. & Harding, C.F. 1991. Oestrogenic modulation of singing in male Zebra Finches: Differential effects on directed and undirected songs. Animal Behaviour 42: 445-452.
Watson, J.T. & Adkins-Regan, E. 1989. Testosterone implanted in the preoptic area of male Japanese Quail must be aromatized to activate copulation. Hormones and Behaviour 23: 432-447.
Wingfield, J.C. & Farner, D.S. 1975. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-bindin. Steroids 26: 311-327.
Wingfield, J.C. 1985. Environmental and endocrine control of territorial behaviour in birds. In: Follett, B.K., Ishii, S. & Chandola, A. (eds) The endocrine system and the environment. Japan Sci. Soc. Press/Springer-Verlag, Tokyo/Berlin: 265-277
Wingfield, J.C. 1990. Interrelationships of androgens, aggression, and mating systems. In: Wada, M., Ishii, S. & Scanes, C.G. (eds) Endocrinology of birds. Molecular to behavioural. Japan Scientific Societies Press and Springer Verlag, Tokyo and Berlin: 185-205.
Wingfield, J.C., Hegner, R.E., Dufty, A.M. & Ball, G.F. 1990. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems and breedings strategies. American Naturalist 136: 829-846.
Wingfield, J.C., Whaling, C.S. & Marler, P. 1994. Communication in vertebrate aggression and reproduction: the role of hormones. In: Knobil, E. & Neill, J.D. (eds) The physiology of reproduction. 2nd Edition, Raven Press, New York: 303-341.
Wingfield, J.C., Jacobs, J. & Hillgarth, N. 1997. Ecological constraints and the evolution of hormone-behaviour interrelationships. Annals of the New York Academy of Sciences 807: 22-41.
Yamada-Mouri, N., Hirata, S., Kato, J. & Hoshi, K. 1997. Expression and distribution of cortical type aromatase mRNA variant in the adult rat brain. Journal of Steroid Biochemistry and Molecular Biology 60: 325-329.
Table 1. Apparent affinity (Km) and maximum velocity (Vmax) of the three major enzymes that catalyze irreversible transformations of T in the Zebra finch brain (data from Vockel et al. 1988).

Fig 1. Schematic representation of the testosterone metabolic pathways identified in the Zebra Finch brain. Single arrows indicate thermodynamically irreversible transformations; double arrows represent reversible interconversions. DHT = dihydrotestosterone; 5b-3a,17b-diol: 5b-androstane-3a, 17b-diol; 3a-HSDH: 3a-hydroxysteroid dehydrogenase; 17b-HSDH: 17b-hydroxysteroid dehydrogenase. Reconstructed from data published in a series of papers presenting in vitro and in vivo studies (Balthazart et al. 1986; Vockel et al. 1988; Vockel et al. 1990a; Vockel et al. 1990b; Schlinger & Arnold 1991; Schlinger & Arnold 1992b; Schlinger & Arnold 1992a)
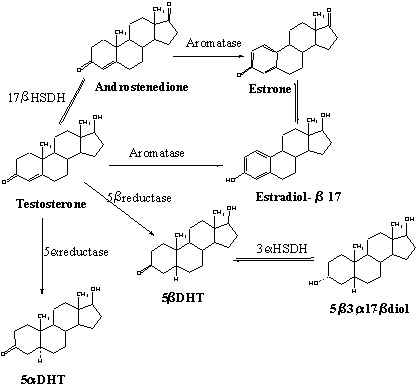
Fig 2. Formation of 5a-DHT (5a-reduction), 5b-DHT (5b-reduction) and estradiol (aromatization) by different brain nuclei of adult male Zebra Finches. Data represent the mean ± SE of 6-11 replicates in each case. Note that different scales have been used to represent the activity of the 3 enzymes. X: Area X of the lobus parolfactorius; MAN: nucleus magnocellularis of the anterior neostriatum; HVC: hyperstriatum ventrale, pars caudalis; RA: nucleus robustus archistriatalis; ICo: nucleus intercollicularis; POA: nucleus preopticus anterioris; PVM: nucleus periventricularis magnocellularis; PMH: nucleus medialis hypothalami posterioris; E: ectostriatum; Rt: nucleus rotundus; nSt: nucleus stria terminalis; Tn: nucleus taeniae; APH: area parahippocampalis. Based on data from Vockel et al. (1990a).
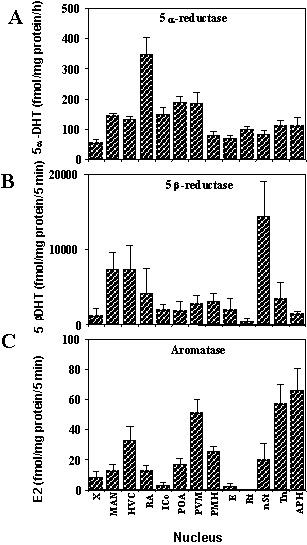
Fig 3. (A) Comparison of the aromatase activity in different brain nuclei of young (20 day old) and adult (>100 days old) male Zebra Finches. (B) Double reciprocal plot of a saturation analysis of aromatase activity in the hypothalamus of male Zebra Finches at different ages (bottom). In the top graph, each column represents the mean ± SE of 4-11 individual data points. See figure 2 for the name of the different brain areas. The bottom graph shows a plot of the inverse of the substrate concentration (1/S) versus the inverse of the observed enzymatic velocity (1/V). The intercept of the experimental lines with the vertical axis provides an estimate of the maximum velocity (1/Vmax) while the intercept with the horizontal axis gives a measure of the enzyme affinity (-1/Km). Based on data published in Vockel et al. (1988).
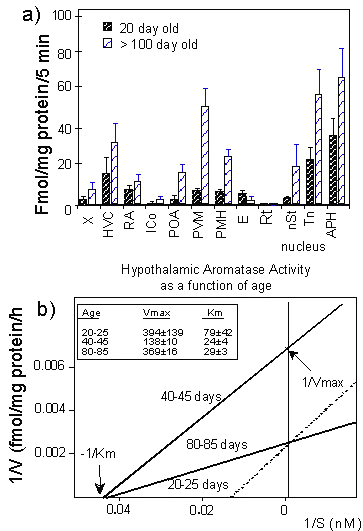
Fig 4. Effect of castration associated or not with a T replacement therapy on the aromatase activity measured in the nucleus periventricularis magnocellularis (PVM) and in the dorsal neostriatum of adult male and female Zebra Finches. Each bar represents the mean ± SE of 5-8 individual data points. Based on data published in Vockel et al. (1990b).
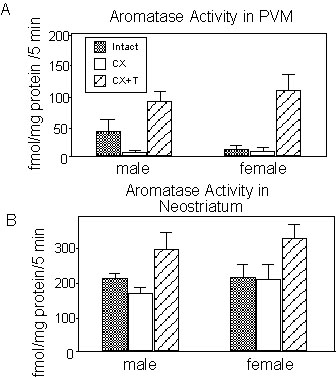
NOTE: Since the publication of this study, the nomenclature of the preoptic area in the Zebra Finch has been reconsidered based largely on the distribution of aromatase-immunoreactive cells. It now appears that the nucleus that had been dissected from Zebra Finch brains for the assay of aromatase activity and labelled PVM based on the atlas of the canary brain (Stokes et al. 1974) should be considered as the homologue of the medial preoptic nucleus (POM) of the quail (Panzica et al. 1996). We retained the original nomenclature for the description of previously published data presented above but we have used the more accurate nomenclature (POM) for the presentation of currently unpublished studies of wild songbird species that is found below.
Fig 5. Aromatase activity in the telencephalon and diencephalon of five species of songbirds by comparison with quail and doves. Each data point represents the mean ± SE of 5-7 determinations except for doves where n = 2. Data are expressed as enzyme activity per hour per mg protein (A) or for the entire brain region considered (B).
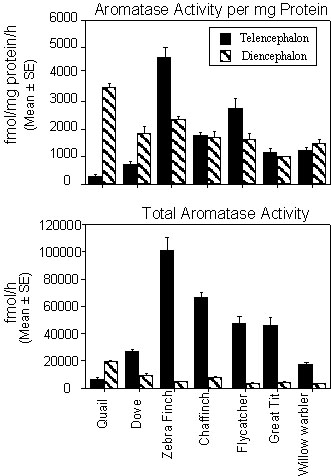
Fig 6. Aromatase activity in male Pied Flycatcher. (A) Mean levels ± SE of aromatase activity observed in six parts of male flycatcher brains that were either defending a secondary territory or feeding nestlings. Enzyme activity is expressed as fmol. per mg protein per hour. (B) Linear regressions between the plasma testosterone levels and the aromatase activity measured in the telencephalon or anterior diencephalon of male Pied Flycatchers. Birds were either defending a territory (filled circles) or feeding nestlings (open circles). The regression equations and correlation coefficients in each graph refer to the analysis of the entire set of data (pool of territory and nestling birds). Redrawn from data in Foidart et al. (1998).
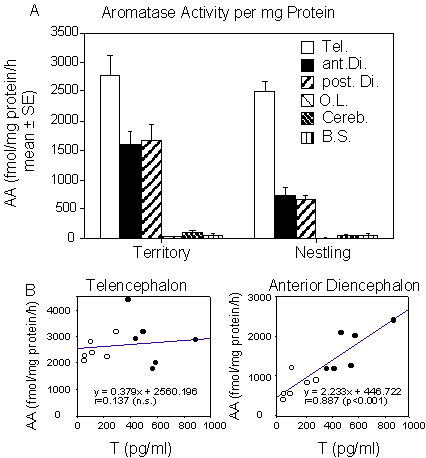
Fig 7. Aromatase immunoreactive cells in male Pied Flycatchers. (A-B) Schematic representation of the distribution of aromatase immunoreactive cells in the male Pied Flycatcher brain at the level of the caudal preoptic area and at the level of the song control nucleus, HVC. Darkly labelled cells are indicated by dots in the diencephalon. In the telencephalon, the distribution of cells showing a weaker immunoreactivity is indicated by the shaded areas. A group of slightly darker cells is indicated by a darker shading in the ventromedial corner of the neostriatum and archistriatum (panel B).(C) Distribution of aromatase-immunoreactive cells along the rostro-caudal axis of the medial preoptic nucleus (POM) and of the ventromedial nucleus of the hypothalamus (VMN) in male Pied Flycatchers that were either defending a secondary territory or feeding nestlings. Sections were aligned in different individuals using fixed anatomical landmarks to permit calculation of group means. The anterior commissure (CA) was used to align data in the POM (cells located in the 8th, 6th, 4th and 2nd sections rostral to CA) while the sections in the VMN were aligned with respect to the most rostral section containing aromatase cells (level 1[Lvl 1]; see text for additional information). Redrawn from data in Foidart et al. (1998).
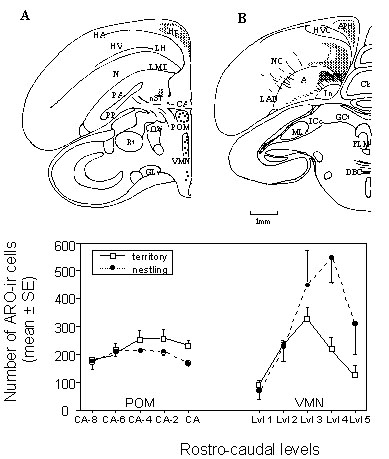
Abbreviations: A: archistriatum; APH: area parahippocampalis; CA: commissura anterior; Cb: cerebellum; DBC: decussatio brachiorum conjunctivorum; FLM: fasciculus longitudinalis medialis; GCt: substantia grisea centralis; GLv: nucleus geniculatus lateralis pars ventralis; HA: hyperstriatum accessorium; Hp: hippocampus; HV: hyperstriatum ventrale; HVC: high vocal centre (formally hyperstriatum ventrale pars caudale); ICo: nucleus intercollicularis; LAD: lamina archistriatalis dorsalis; LH: lamina hyperstriatica; LMD: lamina medullaris dorsalis; MLd: nucleus mesencephalicus lateralis pars dorsalis; N: neostriatum; NC: neostriatum caudale; nST: nucleus striae terminalis; OV: nucleus ovoidalis; PA: paleostriatum augmentatum; POM: nucleus preopticus medialis; PP: paleostriatum primitivum; Rt: nucleus rotundus; Tn: nucleus taeniae; Tu: nucleus tuberis; VMN: nucleus ventromedialis hypothalami.