S21.4: A novel technique for injecting behaviourally-active peptides into the brains of wild, freely-behaving passerines
L. Michael Romero1 & John C. Wingfield2
1Department of Biology, Tufts University, Medford, MA 02155 USA, fax 617-627-3805, e-mail mromero@tufts.edu; 2Department of Zoology, University of Washington, Seattle, WA 98195 USA
Romero, L.M. & Wingfield, J.C. 1999. A novel technique for injecting behaviourally-active peptides into the brains of wild, freely-behaving passerines. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1251-1256. Johannesburg: BirdLife South Africa.A recently-developed novel stereotaxic-like surgical technique allows peptides to be injected directly into the brains of wild passerines. Short surgeries (typically under 10 min) and inexpensive equipment allow experiments to be performed in the field, with subsequent release of birds into their natural habitats within 30-40 min of capture. This allows monitoring a peptide’s effect on natural behaviours. Initial work with this technique focused on how various peptides affect territorial behaviour of White-Crowned Sparrows Zonotrichia leucophrys. We assessed territorial behaviour using simulated territorial intrusions consisting of caged decoy males and tape-recorded songs. After vehicle injection, 65% of birds responded by either approaching the decoy or counter singing. Corticotrophin-releasing factor (CRF) nearly eliminated this response, whereas arginine vasotocin had no effect. This difference did not reflect differential mortality since males usually were still defending their territories 1-2 weeks later. Furthermore, the return rate the following year for these birds was similar to that of unmanipulated birds. These studies demonstrate that we can successfully administer peptides to the brains of passerines for the subsequent monitoring of behavioural changes in free-living and naturally-behaving birds.
INTRODUCTION
Countless laboratory studies demonstrate that peptides can affect behaviour. These studies have traditionally been restricted to the laboratory because the most widely used technique, injecting peptides directly into the brain (Sahgal 1993), is much easier in a laboratory setting. It is often of interest, however, to study peptides’ effects on behaviour in an ethologically relevant context. With this goal, we recently developed a stereotaxic-like technique for rapidly injecting peptides into the lateral ventricles of small passerines (Romero & Wingfield 1997). The apparatus necessary for this technique is simple and inexpensive, making possible peptide injections under field conditions.
The first study to ascertain whether behavioural effects of peptides could indeed be determined in an ethologically relevant context monitored changes in territorial behaviour (Romero et al. 1998). Corticotrophin-releasing hormone (CRF) and arginine vasotocin (AVT) were injected into the lateral ventricles of White-Crowned Sparrows Zonotrichia leucophrys in the Alaskan Arctic (68░ N, 149░ W). Both of these peptide hormones are involved in the vertebrate stress response (Owens & Nemeroff 1991; Antoni 1993), and CRF (Dunn & Berridge 1990; Owens & Nemeroff 1991; Menzaghi et al. 1993) and perhaps AVT (de Kloet et al. 1993) can alter behaviour (stress behaviour for CRF and singing for AVT) when injected into the brain.
In order to assess behavioural changes, we monitored territorial defence by male White-Crowned Sparrows after injecting LRS (Lactated Ringer’s Solution, used as the vehicle to dissolve the peptides), CRF, and AVT (Romero et al. 1998). Territorial behaviour was determined with a simulated territorial intrusion (STI). An STI consists of a live captive male placed in a cage near the centre of a male’s territory while the species-specific song is played through a tape recorder (Wingfield & Ramenofsky 1985; Wingfield et al. 1987). We then monitored the defensive responses of the focal male to the caged intruder. Territorial defence is a very robust behaviour in this species, with males aggressively defending their territories via song and physical attacks (Wingfield 1994). We were able to show that the new stereotaxic-like technique can successfully be used to determine a peptide’s affect on behaviour in an ethologically relevant context, and that CRF but not AVT significantly inhibited territorial defence (Romero et al. 1998).
METHODS
Injection Technique
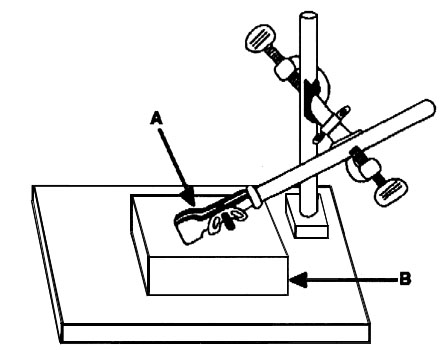
We had three design criteria for the injection apparatus. In order for the device to be useful under field conditions, it needed to be lightweight, durable, and reliable. To satisfy the first two criteria, we constructed the surgical stage out of Plexiglas (Fig. 1). We then added a raised Plexiglas platform upon which we rested the animal. This allowed a nosecone of inhalable anaesthetic (metofane from Pitman-Moore, Inc.) to be administered at beak level. A small raised platform with a hole drilled into it was then attached to the surgical stage. This allowed a removable Plexiglas post to be inserted into the hole and affixed to the platform. This post, combined with a connector, provided the scaffolding to attach a standard laboratory test tube clamp. The insides of the clamp were padded and used to hold the bird’s head stationary (the fragile skull of birds required care in providing enough pressure with the clamp to restrict head movement yet prevent damage). Using the standard laboratory test tube clamp provided three advantages: it was inexpensive; the angle and direction were adjustable so that the bird’s head could be situated optimally in relation to the nosecone; and the clamps (and the Plexiglas post) could be removed for easy transport in a field pack.
Various peptides can be injected intracerebroventricularly (i.c.v.) using the stereotaxic-like apparatus described above. After anaesthetising the birds, we plucked feathers from the rear of the head, placed the birds’ heads in the clamp, and sterilised the incision point with 95% EtOH. We then exposed the underlying skull with a scalpel and used silver nitrate (Arzol Chem. Co.) to cauterise any residual bleeding from the scalp. The sagital sinus (a ‘Y’ shaped blood vessel lying atop the brain near the back of the skull) was then identified through the skull (passerine skulls are thin enough to be moderately transparent). A 1.5 mm diameter hole was then made with a sterile hypodermic needle 1.0 mm rostral to the sinus and 0.5 mm lateral to the midline. We then injected 3 Ál of a peptide solution through a hand-held guide cannula (Plastics One, Inc.). The guide cannula was pre-cut to 1.5 mm below the pedestal and inserted into the brain at an approximately 20░ angle caudal to vertical (as determined by the plane of the skull at the injection point) until the pedestal prevented further insertion. We then inserted an injection needle (extending 0.5 mm below the end of the guide cannula), injected the peptide solution, and waited to remove both the injection needle and the guide cannula for 10-15 sec. Wounds were immediately sealed using a tissue adhesive (Vetseal, Inc.), a topical anaesthetic (Xylocaine) and antibacterial cream (neosporin) applied, and the bird placed in an opaque cloth holding bag to recover from the anaesthetic. The entire surgical procedure took 6-10 min. and birds were released within 30-40 min. of capture (20-30 min after completing the surgery).
Although many different peptides could be injected, for the first field i.c.v. administrations we injected either 25 ng ovine CRF (Sigma), 100 ng AVT (Bachem California), or lactated Ringer’s solution (LRS, Baxter) as the vehicle control (Romero, 1998). These are effective doses in other species (Dunn & Berridge 1990; Lowry et al. 1990; de Kloet et al. 1993). Peptides were dissolved in dH2O, diluted to the proper concentration in LRS, and frozen in aliquots. Each day a fresh aliquot was thawed and maintained on ice.
Simulated Territorial Intrusions (STI)
White-Crowned Sparrows provided two advantages for injecting peptides i.c.v. in the field. First, many individuals of this species breed on the arctic tundra which, with its short vegetation, provides long lines of sight without intervening trees. Territorial behaviour can thus be easily observed. Secondly, White-Crowned Sparrow males aggressively defend their territories from intrusions by other males (Wingfield 1994). We used these two advantages to test whether CRF and/or AVT could alter territorial behaviour (Romero et al. 1998).
White-Crowned Sparrows males were located on breeding territories and a live caged male decoy placed within 10 m. Every 10-15 sec a White-Crowned Sparrow song was repeated through a speaker for 10 min (caged decoys never sang). This period represents the STI. We monitored four aspects of territorial behaviour: number of songs; number of short flights (considered aggressive in this context (Wingfield 1994)); closest approach to the decoy; and time spent within 5 m of the decoy. An individual was considered to have participated in territorial defence if it responded in any of the four measures.
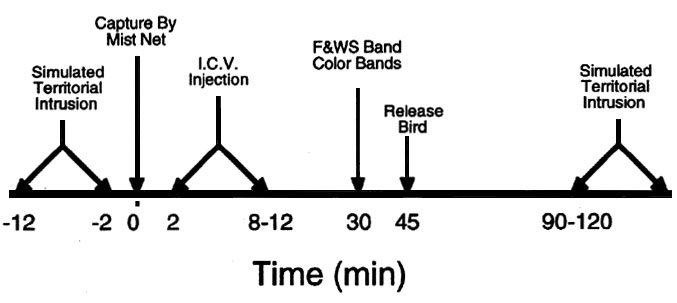
After the STI, we continued the taped songs until capturing the bird (3-6 min) using a Japanese mist net (Avinet). We immediately initiated surgery to inject the peptides, waited 30-40 min to allow recovery, and released the bird after placing a U.S. Fish & Wildlife Service band on one leg and a unique sequence of colour bands on the other leg (Fig. 2). We then left the area and returned 90-120 min after the injection to initiate a second STI. This period was chosen in order to maximise recovery time from the surgery, yet remain within the reported effective time course (Lowry et al. 1990; Korte et al. 1993).
RESULTS
Using the stereotaxic-like technique to inject peptides into the lateral ventricles was successful in two species. In the laboratory, injections in House Sparrows Passer domesticus had a 90% success rate (Romero & Wingfield 1997) and all four White-Crowned Sparrow brains injected in the field with india ink had labelled lateral ventricles (Romero et al. 1998).
I.c.v. injections of CRF into free-ranging white-crowned sparrows significantly inhibited territorial defence (Romero et al. 1998). Whereas 65% of LRS-injected birds (the controls) responded to a second STI, only 10% of CRF-injected birds responded. AVT, on the other hand, had no effect, with 50% of the birds responding. This technique also did not have drastic long-term affects on the birds. We were able to visually identify (via their unique colour bands) 23 of 26 birds defending their territories 1-2 weeks after surgery, and nearly 14% of these birds survived the winter and returned to their breeding sites (Romero et al. 1998), a higher return rate than previously reported for this species (Hahn et al. 1995).
DISCUSSION
These data show that peptides can be injected into the brains of small passerine birds under field conditions, and that ethologically relevant behaviours can be affected by those peptides. Capture and surgery are, of course, very stressful to the birds (Wingfield 1994; Romero et al. 1997). Rapid surgeries, however, mean birds are anaesthetised for relatively short periods of time. Inhalable, rather than injectable anaesthetics, can then be used, resulting in finer control of dose and rapid recovery. This speed allows birds to be released soon after surgery, a crucial factor in getting them to respond to a second STI before the peptide effects wear off. It is likely that not all birds recover sufficiently from the stress of capture and surgery to resume territorial defence, leading to the low response rate in the vehicle-injected controls (however, even uncaptured birds have a lowered response rate (approximately 90%) to a second STI (Romero et al. 1998)). Territorial defence is a robust enough behaviour, however, that 65% of controls do resume territorial defence. This is a high enough response rate from controls to allow detection of alterations in behaviour due to peptide effects.
Injecting into the lateral ventricles is not ideal. Reproducible injections into small discrete brain nuclei is now a standard technique in neuroscience, allowing anatomical precision in studies of brain function. These studies are difficult, however, without stereotaxic brain atlases. These atlases have been produced for a variety of species (e.g. (Karten 1967; Pellegrino & Cushman 1967; Stokes et al. 1974), but differences in both brain sizes and brain orientation in the skull generally make these atlases of little use for other species. Only one stereotaxic atlas exists for a passerine, the canary Serinus canaria (Stokes et al. 1974), and none are available for potential subjects for studies of ethologically relevant behaviours. A recent technique for third ventricle cannulations of small passerines (Richardson & Boswell 1993) can provide access to a more discrete area in small avian brains. Third ventricle cannulation, however, requires a lengthy surgery and is potentially more damaging to the brain than lateral ventricle cannulations. These two drawbacks suggest that third ventricle cannulations would not be an optimal choice for injecting peptides i.c.v. in free-ranging birds. Injecting into the lateral ventricles also results in fairly rapid transport into the third ventricle (Romero & Wingfield 1997; Romero et al. 1998), so that this area is accessible using this technique as well. This emphasises, however, that peptide effects are not particularly localised in the brain. Using the lateral ventricles as a target, although not ideal, can thus provide reproducible access to the brain for studies of global effects of peptides in the brain.
Although several studies have established techniques for injecting into the lateral ventricles of large species such as turkeys and pigeons (Buntin & Tesch 1985; Denbow 1985; Youngren et al. 1991), these techniques preclude studies on smaller birds. The technique presented here should prove valuable, therefore, since many ethological studies on birds have focused on small passerines. We thus hope that this technique will finally make possible studies addressing the neurophysiology of ethologically relevant behaviours in free-living and freely-behaving passerines.
ACKNOWLEDGEMENTS
The studies reviewed here were made possible by National Science Foundation (USA) grants OPP9300771 to JCW and BIR9406842 to LMR.
REFERENCES
Antoni, F.A. 1993. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Frontiers of Neuroendocrinology 14: 76-122.
Buntin, J.D. & Tesch, D. 1985. Effects of intracranial prolactin administration on maintenance of incubation readiness, ingestive behavior, and gonadal condition in ring doves. Hormones and Behavior 19: 188-203.
de Kloet, ER., Elands, J. & Voorhuis, D.A.M. 1993. Implication of central neurohypophyseal hormone receptor-mediated action in the timing of reproductive events: evidence from novel observations on the effect of a vasotocin analogue on singing behaviour of the canary. Regulatory Peptides 45: 85-89.
Denbow, D.M. 1985. Food and water intake response of turkeys to intracerebroventricular injections of angiotensin II. Poultry Science 64: 1996-2000.
Dunn, A.J. & Berridge, C.W. 1990. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Research Reviews 15: 71-100.
Hahn, T.P., Wingfield, J.C., Mullen, R. & Deviche, P.J. 1995. Endocrine bases of spatial and temporal opportunism in arctic-breeding birds. American Zoologist 35: 259-273.
Karten, H.J. 1967. A stereotaxic atlas of the brain of the pigeon, Columba livia. Baltimore, Johns Hopkins Press.
Korte, S.M., Bouws, G.A.H. & Bohus, B. 1993. Central actions of corticotropin-releasing hormone (CRH) on behavioral, neuroendocrine, and cardiovascular regulation: brain corticoid receptor involvement. Hormones and Behavior 27: 167-183.
Lowry, C.A., Deviche, P. & Moore, F.L. 1990. Effects of corticotropin-releasing factor (CRF) and opiates on amphibian locomotion. Brain Research 513: 94-100.
Menzaghi, F., Heinrichs, S.C., Pich, E.M., Weiss, F. & Koob, G.F. 1993. The role of limbic and hypothalamic corticotropin-releasing factor in behavioral responses to stress. Annals of the New York Academy of Sciences 771: 142-154.
Owens, M.J. & Nemeroff, C.B. 1991. Physiology and pharmacology of corticotropin-releasing factor. Pharmacological Reviews 43: 425-473.
Pellegrino, L.J. & Cushman, A.J. 1967. A stereotaxic atlas of the rat brain. New York, Appleton-Century-Crofts.
Richardson, R.D. & Boswell, T. 1993. A method for third ventricular cannulation of small passerine birds. Physiology and Behavior 53: 209-213.
Romero, L.M., Dean, S.C. & Wingfield, J.C. 1998. Neurally-active stress peptide inhibits territorial defense in wild birds. Hormones and Behavior 34: 239-247.
Romero, L.M., Ramenofsky, M. & Wingfield, J.C. 1997. Season and migration alters the corticosterone response to capture and handling in an arctic migrant, the white-crowned sparrow (Zonotrichia leucophrys gambelii). Comparative Biochemistry and Physiology 116C: 171-177.
Romero, L.M. & Wingfield, J.C. 1997. A novel stereotaxic-like method for injecting into the lateral ventricles of small passerine birds. Physiology and Behavior 62: 1109-1112.
Sahgal, A. (ed.) 1993. Behavioural Neuroscience: A Practical Approach.New York, Oxford Univ Press.Stokes, T.M., Leonard, C.M. & Nottebohm, F. 1974. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. Journal of Comparative Neurology 156: 337-374.
Wingfield, J.C. 1994. Control of territorial aggression in a changing environment. Psychoneuroendocrinology 19: 709-721.
Wingfield, J.C. 1994. Hormone-behavior interactions and mating systems in male and female birds. The Difference Between the Sexes. Short, R. V. & E. Balaban. London, Cambridge University Press: 303-330.
Wingfield, J.C. 1994. Modulation of the adrenocortical response to stress in birds. Perspectives in Comparative Endocrinology. Davey, K. G., R. E. Peter & S. S. Tobe. Ottawa, national Research Council of Canada: 520-528.
Wingfield, J.C., Ball, G.C., Dufty, A.M., Hegner, R.E. & Ramenofsky, M. 1987. Testosterone and aggression in birds. American Scientist 75: 602-608.
Wingfield, J.C. & Ramenofsky, M. 1985. Hormonal and environmental control of aggression in birds. Neurobiology. Gilles, R. & J. Balthazart. Berlin, Springer-Verlag: 92-104.
Youngren, O.M., El Halawani, M.E., Silsby, J.L. & Phillips, R.E. 1991. Intracranial prolactin perfusion induces incubation behavior in turkey hens. Biology of Reproduction 44: 425-431.
Fig. 1. Diagram of the surgical apparatus. (A) padded test tube clamp. (B) raised surgical platform. (Reprinted from Romero & Wingfield 1997, with permission from Elsevier Science).

Fig. 2. Time line detailing protocol for field injections of CRF and AVT. F&WS = U.S. Fish and Wildlife Service aluminium leg bands.