S21.3: Seasonal patterns in testosterone, corticosterone and song in Reed Warblers, Acrocephalus scirpaceus
John P. Dittami 1, Eva Millesi 1, Manfred Gahr 2, Herbert Hoi 3, Josef Hemetsberger 1 , Karin Donnerbaum 3& Erich Möstl 4
1Institut für Zoologie, University of Vienna, A-1090 Vienna, Austria, fax 43 1 31336778, e-mail john.dittami@univie.ac.at; 2Max-Planck-Institut für Verhaltensphysiologie, D-80131 Seewiesen, Germany, e-mail gahr@mpi-seewiesen.mpg.de; 3 Konrad-Lorenz-Institut für Vergleichende Verhaltensforschung, A-1160 Vienna, Austria; 4 Institut für Biochemie, Veterinary Medicine University of Vienna, A-1210 Vienna, Austria
Dittami, J.P., Millesi, E., Gahr, M., Hoi, H., Hemetsberger, J., Donnerbaum, K. & Möstl, E. 1999. Seasonal patterns in testosterone, corticosterone and song in Reed Warblers, Acrocephalus scirpaceus. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1242-1250. Johannesburg: BirdLife South Africa.Reed Warblers Acrocephalus scirpaceus show pronounced changes in song behaviour and adrenal and gonadal physiology during the breeding season up to fall migration. By comparing the patterns of testosterone and corticosterone changes with song behaviour we have gained data which support a proposed model for the immediate endocrine and neuroendocrine control of the behaviour. In particular, the structure and expression of male song changes from advertisement to territorial song during breeding. Advertising song has high rates and long bouts (spontaneous) and decreases during incubation. Thereafter, rates change as male/male contact increases (territorial or reactive song). Testosterone decreases after nesting but reactive and not spontaneous song rates were still Testosterone dependent. Corticosterone levels after capture were high during nesting in adults and low thereafter, indicating decreased HPA reactivity. Individual testosterone and corticosterone levels correlated during early nesting in adult males but not after mid June. Initial data on male HVC and RA sizes paralleled changes in advertisement song and testosterone levels. We suggest that in males, testosterone decreases in combination with decreased HPA activity and perhaps vasotocin release are key factors for the change in song behaviour.
INTRODUCTION
Bird song is often used as a model to understand interactions among neural substrates, endocrine factors and the expression of behaviour (rev. Brenowitz & Kroodsma 1996; Margoliash 1997; Brenowitz et al. 1997). The fascination with the model is justifiable due to clear parallels in changes of the components. For instance, fluctuations in song nuclei, neuronal morphology and density have dramatic effects on song learning and expression in terms of bout length, element number and stereotypy. These in turn have often been linked to changes in circulating levels of gonadal steroids although the connection is still not always as clear as one would expect and indeed some aspects of song and song nuclei development have been shown to be testosterone independent (e.g. Bernard & Ball 1997; Bernard et. al. 1997). Other endocrine factors like vasotocin (Voorhuis & de Kloet 1992), and perhaps enkephalines or vasoactive intestinal peptide (VIP) (Ball et al. 1995) as well as cellular enzymatic components like aromatase (Balthazart et al. 1995) affect interactions among neural substrates, endocrine factors and behaviour.
There are compounding issues in the understanding of this system like annual fluctuations in physiological processes of endocrine factors where differing seasonal and even breeding phase expressions are often still linked to the same factor. It is possible that a better understanding of birds exposed to these changes in causes and effects, indeed living in natural circumstances, may add significant information to the model and the variability of neural and behavioural plasticity itself. Indeed, after some pioneer work on the varying seasonal control of song and aggression (e.g. Schwabl & Kriner 1991; Wingfield & Monk 1992; Wingfield 1994a) recent studies have begun to concentrate on the role of neural substrate changes in these seasonal behaviours in wild birds (e.g. Gulledge & Deviche 1997; Smith et al. 1997a, 1997b). We have started parallel investigations on a long distance migrant the Reed Warbler Acrocephalus scirpaceus. This species differs from previously studied birds in that Reed Warblers are exposed to extreme temporal and physiological constraints due to long distance migration. It may also serve as a good model because much is known about the structure and function of Reed Warbler song. For instance, males have three characteristic songs: an advertisement song, a conversational or subsong and a territorial song (rev. in Cramp et al. 1992). The first is different from the other two with regard to bout length but not element number. The latter two differ in intensity (amplitude). Advertisement song begins shortly after arrival and continues until mating where conversational song is prevalent. Territorial song is found throughout the season but is the predominant nesting, late breeding and postbreeding form. Only a small percent of females sing (about 10%) and their song is reported to be territorial, in particular as a response to human nest disturbances (Dittami et al. 1991; Cramp et al. 1992). Juveniles do not sing on the breeding grounds. In addition, we have reported that song frequency and testosterone levels decrease from early nesting to feeding in the first brood period and that song activity increases again after testosterone implantation without regaining prenesting levels (Dittami et al. 1991). We continued this work here by examining endocrine, neural and behavioural aspects of song control later in the season using individual focus and censusing techniques.
METHODS
The work was carried out at Lake Neusiedl from 1989 to 1997 with two different approaches. Up to 1994, work concentrated on individual behaviour and physiology using challenge experiments and focal protocols for behaviour and focal capture techniques described in Dittami et al. (1991) and Hoi et al. (1995). Song activity was determined in one hour focal observations with one/zero sampling in minute intervals or 10 minutes prechallenge with 15 seconds intervals. Challenges were five x one minute playbacks with one minute pauses. The same segment of spring song was repeated using a Sony walkman with AIWA portable speakers mounted on a post in the centre of the movement range of the observed individual. The sound threshold of the song was over 50 dB at 10 m distance. Later we expanded censusing techniques for the number of singing individuals, point transect techniques as used in Hoi et al. (1991), with a form of constant effort netting (three x three m nets; five whole day intervals/month with hourly collection rates for two years (1996 and 98). Brains of four males were collected in late April, late May and late June to examine changes in the song control system premating, during the first brood, and late nesting (a second or replacement brood). Brains were placed in 4 % formaldehyde solutions and song nuclei sizes analysed according to the techniques described in Gahr (1990). Testosterone and corticosterone were measured in
20 m of plasma after a 3 ml diethyl ether extraction using EIA techniques with specific antisera (Palme & Möstl 1993). Intra- and interassay variation was controlled to remain under 15%. Corticosterone levels represent stressed levels in individuals because they were always taken at least ten minutes after capture. They therefore are not a measure of tonic states but more one of adrenal reactivity.
RESULTS
Song and population demography
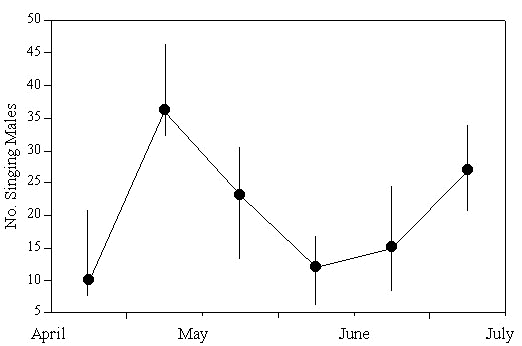
The song censuses showed that there were high numbers of singing individuals in early May which decreased in June and increased again in July (Fig.1). Preliminary data indicate that song activity in August returned to the June levels. In order to understand the causes for these changes we compared song behaviour with censusing data from constant effort netting. Adult and juvenile numbers, sex components and indications of nesting activity (brood patches) were of initial interest. The results demonstrate that the numbers of adults caught had seasonal trends (Fig. 2). For instance, after prenesting, there was a pronounced increase in adult numbers in late June and early July. When one examines the male component of the captured population it is evident that the increase was due to a change in male numbers (Fig. 2). At the same time it occurred at the end of the breeding peak (estimated by brood patch presence, Fig. 2). The increase in male capture rates could have two causes: first, there may have been a post mating flux of individuals into the area. We doubt this because breeding migration has not been described for this species. A more likely explanation would be that the resident males near the capture sights increased their home ranges to collect food for the young. It is clear from the literature (see below) that males feed more and travel farther to forage than females during early post hatch. The foraging and territorial data from English, German and Polish Reed Warbler populations support this hypothesis (rev. in Cramp et al. 1992). For instance it is known that during the nestling period, male foraging areas increase up to five-fold although a site tenacity remains until late July when migration begins (Berthold et al. 1991).
Testosterone data showed a pattern of changes in males which paralleled that known for many other species (Fig. 3). There was however a high variance among individuals with regard to their circulating levels. Prenesting and mating levels were elevated, they decreased thereafter. The lowest levels were found in July . These low levels were comparable to individual feeding testosterone levels we had previously reported for males of the first brood (Dittami et al. 1991). Corticosterone also showed a seasonal pattern and a high degree of variance especially in May (Fig. 3). The circulating levels decreased after May and were lowest in July. This means that the adrenal (HPA) reaction to the stress of being captured and hanging in the nets for up to an hour decreased during nesting in June and was lowest in July. Because of the parallel character of corticosterone and testosterone changes we looked at possible individual correlation’s between the two factors (Pearson corrected by Bonferroni). Male testosterone and corticosterone levels were positively related in May (r = 0.390, P < 0.03, n = 30) but not at any other time in the season.
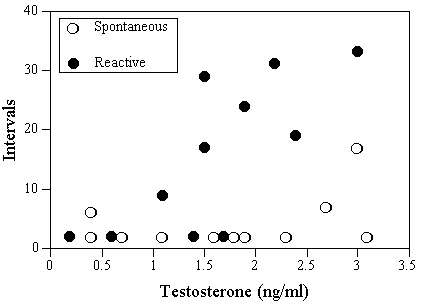
Our next step was to examine how testosterone and song behaviour interacted. We had previously shown that preincubation and early brood (until mid June) levels of spontaneous song covaried with testosterone and were effected by T- implantation. At this time spontaneous song was frequent: 50.5 ± 3.8 (mean ± S. D.) minute intervals per hour. Later in the season at the end of June and in July we re-examined individual song behaviour with a slightly different technique before and during the challenge experiments with 15 sec intervals. Results of the latter work demonstrated that spontaneous and reactive song during late breeding differed significantly in frequency: 4.8 ± 1.5 intervals for spontaneous (n = 10) and 15.6 ± 3.8 intervals for reactive (n = 11), (Mann-Whitney U-Test, Z = 2.028, P = 0.04). The early breeding data are not completely comparable here due to methodological differences, however, 84 % of the time intervals had spontaneous song early in the season and only 24 % later. In addition, reactive song to confrontation was observed in 78 % of the time intervals after song playbacks (challenge experiments). In total, it appears that seasonal changes in testosterone paralleled spontaneous but not reactive song. To examine possible late testosterone effects in more detail we looked at testosterone-song interactions among the birds in challenge studies (Fig. 4). Correlation statistics (Pearson corrected by Bonferroni) were highly significant for reactive song (r = 0.745, P < 0.01) and not significant for spontaneous song (r = 0.547, P = 0.32).
Lastly, we decided to see if these changes in song and testosterone were related to any gross morphological changes in the song nuclei, in particular RA and HVC sizes. We compared individuals in April (19, n = 3) which were migratory or recently arrived with early breeding (May 24, n = 3) and late breeding (June 30, n = 3) individuals . HVC and RA were well defined in Nissl-stained brain section of the second group but was ambiguous in Nissl-stained sections of the first group. Measurements of the neuron soma size in RA showed an increase from the first to the second group and a decrease thereafter. Soma size was 55 ± 14 µm3 in April, 72 ± 18 µm3 in May and 63±7 µm3 in June. The anatomical data indicate seasonal changes in the HVC and RA neuroanatomy but more animals need to be analyzed. This indicates that testosterone increases around spring arrival can contribute to the song nuclei development. The idea is supported by the observation that April birds had low testosterone levels of less than 1 ng/ml (n = 8) compared with later data. Another aspect is that despite the decreases in testosterone and spontaneous song in late June the nuclei were smaller but still robust.
DISCUSSION
Although this study combines a number of different methods for examining song behaviour and the possible physiological causes, a few general conclusions can be made. From these we would like to develop a working hypothesis for future research. To begin with, it is clear that a resurgence of song occurred post mating during nesting. On the basis of playback experiments (Dittami et al. 1991) and estimates of male numbers or movements from netting it can be concluded that the song behaviour was a reaction to territorial intrusion and not part of a tutoring program. Secondly, although the latter song has been characterised as having shorter phrase and bout lengths (rev. in Cramp et al. 1992) little can be said about the absolute amount of song behaviour except for the fact that the numbers of birds censused singing was lower than during mating. The role of testosterone in the expression of song is evident from various reasons. On the basis of earlier work we had established that mating song activity covaried with circulating testosterone levels (Dittami et al. 1991). Song activity and song control nucleus size also increased post migration with testosterone. To this, the depression of song during incubation was paralleled by testosterone decreases (Dittami et al. 1991). Lastly, during postincubation, the amount of reactive but not spontaneous song covaried with testosterone plasma titres. Neuroanatomically, the data were also quite interesting in that RA and HVC volume changes were pronounced early in the season with song development but not later with the change from spontaneous to reactive song. A change, as mentioned accompanied by decreased song bout length.
Another ‘wild card’ in the development of working hypotheses for us was the consideration of a possible role of HPA axis changes in patterns of song behaviour. Our evidence for a change in HPA reactivity is circumstantial, however the same paradigm has been used with other species producing similar results (e.g. Wingfield 1994b, Wingfield et al. 1995). During netting procedures with birds hanging in the nets for up to an hour we found systematic changes in the resulting corticosterone plasma titres. These changes indicated that after mating and during feeding, there was a down regulation of HPA reactivity. A result which is consistent with a number of other avian species (rev. in Wingfield et al. 1997). Following this, the hypothesis for the endocrine/neuroendocrine control of song could have two aspects. One, there could be threshold effects of testosterone in that over a certain concentration bout length and activity increase spontaneously to produce advertisement song. Unpublished data from testosterone-implant studies late in the season do not support this theory. We found no effects of implants on the frequency of spontaneous song or male feeding components in late broods. The second aspect could be that changes in the HPA or a related factor could modulate activity without impinging on song diversity or the neural control centres used for its production. Direct corticosterone, ACTH or CRH effects may be possible, however because of the multiplicity of their physiological actions possible mechanisms of action are difficult to localise. There is, however a related factor, arginine vasotocin, which is known to have highly synergistic effects with the HPA, at least in mammals. It is known to be a secretagogue of ACTH and have situation specific importance in regulating adrenal activity (rev. in Sapolsky 1991). To this it effects bird song and reproductive behaviour (rev. in Voorhuis & de Kloet 1992; Maney et al. 1997) and has direct input into the RA (Voorhuis et. al. 1991).
There are some other previous results which shed light on this hypothesis. In starlings, for instance, it has be shown very elegantly that bout length varies on an interindividual level with changes in RA size (Bernard et al. 1996). Like in Reed Warblers this was shown to occur independent of element diversity. Hence, changes in this area may be directly related to bout length and overall song activity. Still, in this study we were looking at rather short term intraindividual behavioural changes. It is therefore not certain whether these should or would be directly expressed in neuroanatomical correlates. For this reason, a neuroendocrinological modulation of behaviour like that of vasotocin might be a more propitious explanation. The neuromodulatory effects of vasotocin or perhaps even CRF, ACTH and corticosterone may have a very important role in the immediate control of song behaviour. Fluctuations, especially during different nesting phases, are rapid and very functional and may occur in this manner without overt changes in the control nuclei. In our opinion, these aspects of song control have not received as much scientific attention as they deserve.
REFERENCES
Ball, G.F., Absil, P. & Balthazart, J. 1995. Assessment of volumetric differences in the song control nuclei HVC and RA in Zebra Finches by immunochemistry for methionine enkephalin and vasoactive intestinal polypeptide. Brain Research 699: 83-96.
Balthazart, J., Absil, P., Fiasse, V. & Ball, G.F. 1995. Effects of the aromatase blocker R76713 on sexual differentiation of brain and behaviour in Zebra Finches. Behaviour 131: 225-260.
Bernard, D.J., Eens, M. & Ball, G.F. 1996. Age and behavior related variation in volumes of song control nuclei in male European Starlings. Journal of Neurobiology 30: 329-339.
Bernard, D.J. & Ball, G.F. 1997. Photoperiodic condition modulates the effects of testosterone on song control nuclei volumes in male European Starlings. General and Comparative Endocrinology 105: 276-283.
Bernard, D.J., Wilson, F.E. & Ball, G.F. 1997. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American Tree Sparrows (Spizella aborea). Brain Research 760: 163-169.
Berthold, P., Fliege, G., Heine, G., Querner, U. & Schlenker, R. (eds). 1991. Autumn migration, resting behaviour, biometry and moult of small birds in Central Europe. Journal of Avian Biology, suppl. 36.
Brenowitz, E.A. & Kroodsma, D.E. 1996. The neuroethology of bird song. In: Kroodsma, D.E. & Miller, E.H. (eds.) Ecology and evolution of acoustic communication in birds. Comstock, New York, Ithaca: 285-304.
Brenowitz, E.A., Margoliash, D. & Nordeen, D.W. 1997. Introduction to bird song and the avian song system. Journal of Neurobiology 33: 495-500.
Cramp, S., Brooks, D.J., Dunn, E., Gillmor, R., Hall-Craggs, J., Hollom, P.A.D., Nicholson, E.M., Ogilvie, M.A., Roselaar, C.S., Sellar, P.J., Simmons, K.E.L., Snow, D.W., Vincent, D., Voous, K.H., Wallace, D.I.M. & Wilson, M.G. (eds.). 1992. Handbook of the birds of Europe the Middle East and North Africa. Vol. 6; Oxford, UK; Oxford University Press: 193-212.
Dittami J.P., Hoi M. & Sageder G. 1991. Parental investment and territorial/sexual behaviour in male and female Reed Warblers: are they mutually exclusive? Ethology 88: 249-255.
Gahr, M. 1990. Delineation of brain nucleus: comparisons of cytochemical, hodological and cytoarchitectural views of the song control nucleus HVC of the adult canary. Journal of Comparative Neurology 294: 30-36.
Gulledge, C.C. & Deviche, P. 1997. Androgen control of vocal control region volumes in a wild migratory songbird (Junco hyemalis) is region and possibly age dependent. Journal of Neurobiology, 32: 391-402.
Hoi, H., Eichler, T. & Dittami, J.P. 1991. Territorial spacing and interspecific competition in 3 species of Reed Warblers. Oecologia 87: 443-448.
Hoi, H., Kleindorfer, S., Ille, R. & Dittami, J.P. 1995. Prey abundance and male parental behaviour in Acrocephalus Warblers. Ibis 137: 490-496.
Maney, D.L., Goode, C.T. & Wingfield, J.C. 1997. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. Journal of Neuroendocrinology 9: 487-491.
Margoliash, D. 1997. Functional organization of forebrain pathways for song production and perception. Journal of Neurobiology 34: 671-693.
Palme, R. & Möstl, E. 1993. Biotin- strepavidin enzyme immunoassay for the determination of oestrogens and adrogens in boar faeces. In: S. Görög (ed) Proceedings of the 5th symposium on the analysis of steroids. Szombathely, Hungary: 123-145.
Sapolsky, R. 1991. Stress, the aging brain and mechanisms of neuron death. Cambridge, USA; MIT Press; 350 pp.
Schwabl, H. & Kriner, E. 1991. Territorial aggression and song of male European Robins (Erithacus rubecula) in autumn and spring: effects of antiandrogen treatment. Hormones and Behavior 25: 180-194.
Smith, G.T. Brenowitz, E.A. & Wingfield, J.C. 1997a. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. Journal of Neurobiology 32: 426-442.
Smith, G.T., Brenowitz, E.A., Beecher, M.D. & Wingfield, J.C. 1997b. Seasonal changes in testosterone, neural attributes of song control nuclei and song structure in wild songbirds. Journal of Neuroscience 17: 6001-6010.
Voorhuis, T.A.M. De Kloet, E.R., & De Wied, D. 1991. Effect of a vasotocin analogue on singing behavior in the canary. Hormones and Behavior 25: 549-559.
Voorhuis, T.A.M., & De Kloet, E.R. 1992. Immunoreactive vasotocin in the Zebra Finch brain (Taeniopygia guttata). Brain Research Dev. Brain Res. 69:1-10.
Wingfield, J.C. 1994a. Regulation of territorial behavior in the sedentary Song Sparrow Zonotrichia melodia morphna. Hormones and Behavior 28: 1-15.
Wingfield, J.C. 1994b. Modulation of adrenal cortical response to stress in birds. In: Davey, K.G., Peter, R.E. & Tobe, S.S. (eds) Perspectives in comparative endocrinology. Ottawa; National Research Council: 520-528.
Wingfield, J.C. Breuner, C. & Jacobs, J. 1997. Corticosterone and behavioral responses to unpresictable events. In: Harvey, S. & Etches, R.J. (eds) Perspectives in avian endocrinology. Bristol; Society for Endocrinology: 267-278.
Wingfield, J.C. & Monk, D. 1992. Control and context of year-round territorial aggression in the non-migratory Song Sparrow Zonotrichia melodia morphna. Ornis Scandinavica 23: 298-303.
Wingfield, J.C., O’Reilly, K.M. & Astheimer, L.B. 1995. Ecological basis of the modulation of adrenocortical response to stress in arctic birds. American Zoologist 35: 285-294.
Fig. 1. Seasonal changes in song activity of male Reed Warblers. Total number of singing males along a 2 km point transect (200 m census, collected 3x/week; medians and ranges are shown; for detail see Hoi et al. 1991).

Fig.2. Population demography data from different trapping dates in 1996 to 1997: (A) Number of captured adult (closed symbols) and juvenile (open symbols) Reed Warblers.(B) Sex ratio (percent males) of the captured individuals. (C) Percentage of captured females with brood patches.
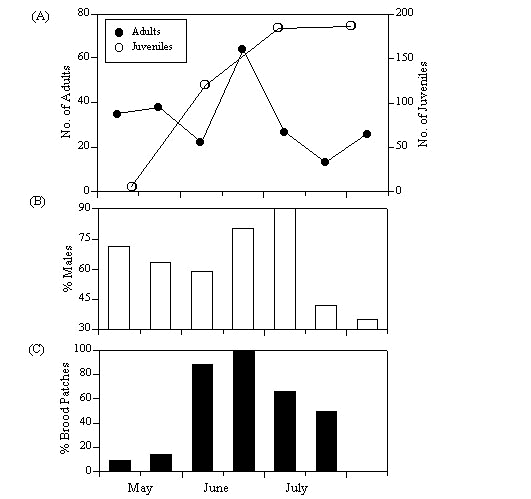
Fig.3. Seasonal patterns of testosterone (Testo, upper graph) and corticosterone (Cort, lower graph) levels in adult males (medians and ranges are shown, sample sizes: Testo/Cort: May 25/11, June 16/8, July 8/6, August 5/5).
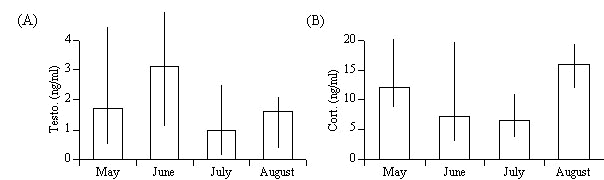
Fig. 4. Male Reed Warbler testosterone levels and number of observed time intervals with spontaneous song (open symbols) and reactive song, (challenge experiments, closed symbols) during late breeding.