S21.2: Seasonal endocrinology of tropical passerines: A comparative approach.
Martin Wikelski1,2,3, Michaela Hau1,2,3, W. Douglas Robinson4 & John C. Wingfield1
1Dept. of Zoology, Box 351-800, University of Washington, Seattle, WA 98195, USA; 2Smithsonian Tropical Research Institute, Apto. 2072, Balboa, Panama; 3Dept. of Ecology, Ethology and Evolution, University of Illinois at Urbana-Champaign, 515 Morrill Hall, 505 S. Goodwin Ave, Urbana-Champaign, IL 61801, USA, fax 217 244 4565, e-mail wikelski@pop.life.uiuc.edu; 4Dept. of Zoology and Wildlife Sciences, 331 Funchess Hall, Auburn University, Auburn AL 36849-5414, USA
Wikelski, M., Hau, M., Robinson, W.D. & Wingfield, J.C. 1999. Seasonal endocrinology of tropical passerines: A comparative approach. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1224-1241. Johannesburg: BirdLife South Africa.In most temperate zone birds, gonadal recrudescence, territoriality, aggression and reproduction coincide during a short window of time in spring. In contrast, scattered data on tropical birds indicated a widespread desynchronisation of gonadal growth, territoriality, and aggression. We asked how tropical birds regulate reproductive behaviour and territorial aggression if they are expressed on a seasonal basis. We investigated gonad sizes, nesting and hormone changes of males from six neotropical passerine species over one annual cycle. Our study species included purely rainforest interior birds as well as edge species, of different phylogenetic, ecological and social backgrounds. All species had regressed gonads for several months of the year. All birds increased their gonads around the beginning of the wet season, i.e. between February and April. Gonads regressed between four and nine months later. We found large differences in the pattern and levels of reproductive hormones (esp. testosterone) among species, as well as in the maximal relative testes sizes. In one focal species, the Spotted Antbird, we experimentally analysed those relationships further and found that although individuals were aggressive year-round, T levels were usually at baseline (0.2 ng/ml). Only individuals that experienced social instability had high circulating levels of T. High plasma T presumably boosted the birds' persistence during fights since experimentally elevated T increased song rate and aggression. Comparing all data available for tropical birds, we found that tropical birds (1) can be aggressive at any time of year irrespective of reproductive status and plasma T, (2) mating and social system may influence maximal gonad size and levels of plasma T secretion, and (3) plasma T levels could be high (> 1 ng/ml) despite entirely regressed gonads. Tropical birds may have generally low T levels to ameliorate the widespread costs of T.
INTRODUCTION
The development of field endocrinological techniques allowed us to explore how hormones and environmental factors interplay to orchestrate the changes that birds undergo during their annual cycle (e.g., Wingfield & Farner 1976; Wingfield & Moore 1987; Wingfield et al. 1987, 1990). However, most of this research has focused on a small subset of birds, namely north temperate passerines. Here we attempt to redirect attention toward tropical birds, which constitute the majority of avian species (Griscom 1945; Levin & Wingfield 1992). We show that by studying tropical birds we can assess the validity of our models about life history regulation in birds and perhaps vertebrates in general.
In most temperate zone birds, gonadal recrudescence, territoriality, aggression and reproduction co-occur in spring (Lofts & Murton 1973; see also Balthazart 1983; Ball 1993). Proximate control processes involve stimulatory effects of increasing day length in spring, which triggers the secretion of gonadotropins. Birds start migrating, grow their gonads and arrive at their respective breeding ground in late spring. Once they reach their final destination, they start to produce large amounts of testosterone (T), fight for territories and signal territory ownership by song. Increased circulating T levels are beneficial for birds because T boosts their persistence during territorial interactions (Hunt et al. 1997; Wingfield et al. 1997). Experimental elevation of T levels lead to an expansion of territory sizes and increased polygyny levels (for Red-winged Blackbirds, Agelaius phoeniceus, see Beletzky et al. 1989, 1992). During courtship, birds attract mating partners, begin courting and mating, lay eggs and raise young. During the parental phase, T levels decrease to allow for brood care. Finally, the breeding season is terminated when individuals regress their gonads to allow restorative processes like moult to occur. It is important to note that in this model, T, courtship and territorial aggression always co-occur. This model is perfectly applicable to the majority of temperate zone birds that are seasonal (see, e.g. Wingfield & Farner 1993).
Does this model also apply to year-round territorial birds, most of which live in tropical habitats? Dittami (1986, 1987) and Dittami & Gwinner (1990) investigated and compared six species of afro-tropical Savannah birds with regard to breeding seasons, gonad sizes, endocrinology and territorial behaviour. They suggested that the investigated avian species could be separated into strict breeders with breeding territories, opportunistic breeders with breeding territories and both strict and aseasonal breeders with all-year-round territories (Dittami & Gwinner 1990). While two species with seasonal breeding territories showed the expected seasonal changes in T levels, two species with year-round territories had elevated T levels year-round (Reichenow's weaver and Rufous sparrow versus Stonechat and Rüppel's Long-tailed Starling, respectively; for scientific names see Table 1). This seemed to confirm that T and aggression are also coupled in tropical birds. Thus, Dittami and Gwinner (1990) concluded that plasma T levels paralleled territoriality and breeding in the tropical species as it does in temperate zone birds. It remains puzzling - and is so far unresolved - how high plasma levels of T are upheld despite the fact that gonads are entirely regressed during the non-breeding season, as in the Stonechat and the Rüppel's Long-tailed Starling (Dittami and Gwinner 1990).
However, there are also several temperate zone species that are territorial year-round or territorial in non-reproductive contexts, e.g. in winter. In such species, individuals may set up new territories during autumn and defend them aggressively against conspecific intruders. Overwhelmingly it was found that although breeding territoriality is associated with high plasma T levels, winter territorial birds may have baseline plasma T. Circulating T levels do not control aggressive behaviours in winter (Silverin et al. 1989; Logan & Wingfield 1990; Schwabl 1992, Gwinner et al. 1994, Wingfield 1994; Wingfield & Hahn 1994; Soma & Wingfield 1999).
The uncoupling of T and aggression makes good sense for birds that are territorial in non-sexual contexts (e.g. in winter, non-breeding season), because many studies have shown that elevated T levels are costly. It has been suggested that T suppresses the immune system (Zuk 1996; Hillgarth & Wingfield 1997; Hillgarth et al. 1997) and lowers overwinter survival rate (Dufty 1989; Ketterson et al. 1992). Extending this argument to tropical birds, one would expect that year-round territorial individuals should keep plasma T as low as possible (Wingfield et al. 1990; Levin & Wingfield 1992). By comparing data on territoriality and T for various avian taxa along a latitudinal gradient, Wingfield et al. (1997; Fig. 1) documented that aggression and territoriality are more likely uncoupled in sedentary avian species. Thus, they expected that in many year-round territorial tropical avian species T and aggression are not correlated.
Other than Dittami & Gwinner (1990), only few studies dealt with year-round territoriality and hormone-behaviour relationships in tropical birds. Territorial aggression was uncoupled from T in afro-tropical subequatorial White-browed Sparrow Weavers Plocepasser mahali (Wingfield et al. 1991, 1992; Wingfield & Lewis 1993). These group-living birds are territorial year-round, but show practically no increase in T except for a short and modest rise in mid breeding season. Even simulated territorial intrusions using an entire group of conspecifics did not increase plasma T levels in White-browed Sparrow Weavers, although aggression strongly increased in challenged birds. Similarly, although the removal of a territorial male led to a strong increase in fights among subordinate males, this did not enhance T levels (Wingfield et al. 1992). Along the same lines, Levin and Wingfield (1992) reported that neotropical Bay Wrens were highly aggressive year-round, but never had measurable T levels. As in the White-browed Sparrow Weaver, simulated territorial intrusion had no measurable effect on T levels in Bay Wrens (Levin 1996).
In an attempt to resolve the relationship between year-round territoriality, T and aggression, we extended the data base on tropical species by investigating annual hormone levels, gonad sizes, breeding seasonality and territoriality in six neotropical bird species. Our aim was to identify possible causal relationships between these four factors. We mainly targeted rainforest bird species because most data on afro-tropical birds came from species inhabiting savanna habitats (Dittami & Gwinner 1990). Furthermore, we also tried to include species with diverse mating systems and social systems. We compare bird species with different phylogenetic backgrounds under the premise that the vertebrate endocrine system is highly conserved. This notion is supported by the fact that for example gonadotropins stimulate the secretion of gonadal steroids in all tetrapods and T is the same molecule and has similar functions in most vertebrates (e.g., Hadley 1992). For the purpose of the present comparison we suggest that (1) deviations from the general vertebrate endocrine system are potential adaptations to specific ecological circumstances, and that (2) phylogeny does not pose major constraints on the evolution of hormone-behaviour relationships within the generally established hormonal framework.
METHODS
We studied six neotropical species between November 1996 and December 1997 in Soberania National Park and adjacent woodlands around the small village of Gamboa, in the Republic of Panama. The Soberania National Park represents a lowland 22000 ha moist forest (Holdridge 1967) in central Panama (9° N 79° W) bordering the Panama canal (Leigh et al. 1982). The forest consists of a mixture of secondary and primary forest. There is about 2600 mm of rain per year, with 90% falling during the late April to mid-December wet season (Windsor 1990, Robinson et al. in press). Mean daily low temperatures are 23 oC, and highs average 29 oC in the wet season, 32 oC in the dry season.
Physiological measurements
Birds were captured in mistnets either passively or with the help of playback. Individual birds were only investigated if the time from capture to subsequent blood sampling was less than about 30 min. Plasma levels of gonadal steroids usually remain unchanged during this time. Bird were weighed to the nearest 0.5 g. Upon capture, birds were banded, blood samples were collected by puncturing the alar wing vein with a 26-gauge needle. Blood was collected in heparinized microcapillary tubes and kept cool (4 oC) until centrifugation. Length and width of the left testis and diameter of the largest follicle were measured to the nearest 0.1 mm below 1 mm length and to the nearest 0.2 mm above 1 mm length by unilateral laparotomy under Isoflurane anesthesia (for details on standard laparotomy procedures see Wingfield & Farner 1976; . Testis volume was calculated using a formula for ellipsoid cylinders (4/3p a²b, where a is half the testis length and b half the width). Relative testes volume was calculated by dividing mean monthly testis volume by mean body mass. To calculate gonad volumes for afro-tropical birds we used published data on gonad width and subsequently estimated gonad length as width times 1.2. This factor was the mean length to width ratio among our study birds (unpubl. data). If body mass was not given in the literature (Dittami 1986, 1987; Dittami & Knauer 1986; Dittami & Gwinner 1990) we used the mean body masses for those species provided by Dunning (1993).
Birds were kept for approximately 5-10 minutes after the laparotomy to allow recovery before release. Plasma was separated from red blood cells by centrifugation at 6000 r/min. for 4 min and treated with
b-propiolactone to kill viruses. Samples were then stored at -20° C and transported to Seattle on dry ice for hormone analysis under permits from Panamanian and US authorities.Radioimmunoassays
Plasma levels of T, oestradiol (E2), androstendione (A4), 5
a-dihydrotestosterone DHT and progesterone (P) were measured with a direct RIA after separation of hormones on a chromatography column . Water blanks were taken through the entire assay procedure and were usually below detection limit. The accuracy of the hormone standards was 6.9%. Intra-assay variation was 1.7% (mean of 4 assays). Inter-assay variation was 4.6% (between n=6 assays). Assay sensitivity was at 0.2±0.06 ng/ml (see Wingfield & Farner 1975 for methods). This provided a conservative estimate for statistical comparisons.Breeding seasons and behavioural observations
As part of an ongoing study on nest predation rates (Robinson et al., in press), we recorded territorial tenure and whether nesting occurred in a certain month. We either used active nests or active brood patches to assess breeding activities. To determine the relationship of plasma T levels and social challenges in our focal species, the Spotted Antbird, we categorised the reproductive state of birds as follows: (1) the non-breeding season was defined as the time when all birds had small gonads (September to February); (2) pre-breeding phase was the time when gonads increased in size but no breeding took place (up to half maximal gonad size, March to April); (3) courtship phase was the time when birds had half maximal to maximal gonad sizes, but no active nests were found or no birds were captured with active brood patches; (4) the subsequent parental phase covered most of the wet season until gonad sizes shrank again to half maximal sizes in about August/September. We assumed that birds experienced periods of social instability whenever we captured more than two Spotted Antbirds on the territory of a known pair on the same day when we captured the territory owner(s).
To study whether T influenced aggressiveness and/or song in a tropical bird species, we caught several groups of male Spotted Antbirds and kept them in captivity in individual cages (approx. 40x30x50 cm). We implanted individuals with either empty silastic tubes (controls), or tubes filled with crystalline T (10 mm), or tubes filled with substances to block T action (16 mm ATD tubes, 20 mm Flutamite tubes; for details on methods see Searcy & Wingfield 1980). We performed staged encounter experiments during which the cages of two males were placed face-to-face for 30 minutes. The behaviour and the vocalisations of birds were continuously recorded during these experiments.
Data were processed with SPSS for Windows. Two-tailed test statistics were used. Data are given as means ± 95% confidence interval.
RESULTS
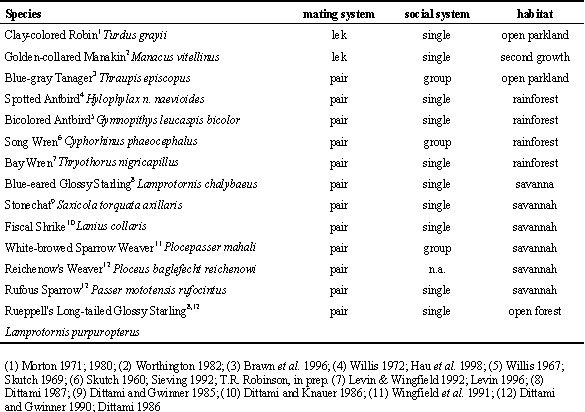
We concentrated on males because our data sets were more complete for males than for females. We compared our results against published data from 6 afro-tropical and one neo-tropical bird species (Table 1).
Gonad changes
All species showed seasonal changes in gonad sizes (Fig. 2). Clay-colored Robins, Golden-collared Manakins and Blue-gray Tanagers started to increase their gonads during the dry season (January) and reached their peak testes size in April and May. Thereafter, gonads regressed again entirely. Spotted Antbirds showed a similar pattern of gonad recrudescence and total regression, but the start of gonadal growth and the peak in maximal gonad size occurred approximately 2 months later. Both Bicolored Antbirds and Song Wrens had a prolonged period with increased gonad sizes which stretched from approximately March until October.
The absolute values of gonad sizes were different between the investigated bird species. Clay-colored Robins, Blue-gray Tanagers and Golden-collared Manakins had the largest relative testes size (in this order), while Bicolored Antbirds, Song Wrens and Spotted Antbirds had much smaller relative testes sizes.
Hormonal changes
All values for E2 were close to the detection limit and did not show any seasonal pattern. Therefore, we do not consider E2 in this contribution. The values of DHT and A4 were always lower than those of T and usually paralleled T (Wikelski et al. submitted). Therefore we do not report values for DHT and A4 here. Clay-colored robins showed a marked increase in T at the beginning of their territorial period in February (Fig. 2). T peaked during April and decreased along with the decrease in gonad size toward June. Golden-collared Manakins, on the other hand, established their lekking territories in February without any detectable T. T only peaked during the onset of the mating season about end of March, and stayed elevated until the end of the lekking season in about July/August. Blue-gray tanagers had baseline T throughout the year, despite the fact that the gonad sizes were amongst the largest we found for neo-tropical birds. Spotted Antbirds had very low T levels throughout the year, despite the fact that they were highly territorial and aggressive year-round. The pattern of T were overall similar in Song Wrens, who were also territorial throughout the year. The plasma T levels of Bicolored Antbirds were slightly elevated during the early wet season (Fig. 2).
Comparisons between tropical bird species
To create testable hypotheses about the influence of environmental and social factors on the reproductive physiology of tropical birds, we compared the pattern found in afro-tropical species with those found in our study on neo-tropical birds. If testes sizes were important for T secretion, we would expect higher maximal T values and larger T amplitudes in species with larger gonads. This was not the case (Fig. 3a, Fig 3b). Furthermore, if T was important for some aspects of territoriality, we would expect a correlation between time with territory and time with elevated T. For example, species with year-round territoriality might have low T amplitudes and generally low T values. No such relationships were found (Fig. 3c, Fig. 3d). However, there was a relationship between habitat type and the maximal relative gonad volume (Fig. 4, upper left panel). No such association was found between habitat type and amplitude in T levels (Fig. 4, upper right panel). Lek mating species appeared to have higher amplitudes in T and had also larger gonad sizes (Fig. 4, middle panel). Species living in groups had similar gonad sizes than those not living in groups, but their amplitudes in T levels were much lower (Fig. 4, lower panel).
Influence of T on behaviour
To test whether T had biologically important actions in tropical birds with year-round low T levels, we selected the Spotted Antbird as a focal species. Implantation of T-filled silastic tubes immediately increased spontaneous song rate strongly over that of controls (Hau et al. unpubl. data). Similarly, the number of aggressive calls during staged encounters were much higher in T-implanted compared to control-implanted birds. The opposite result was found when T conversion and T reception was blocked pharmacologically (Hau et al. unpubl. data). From this we concluded that T had important biological actions in Spotted Antbirds.
Testosterone and social instability
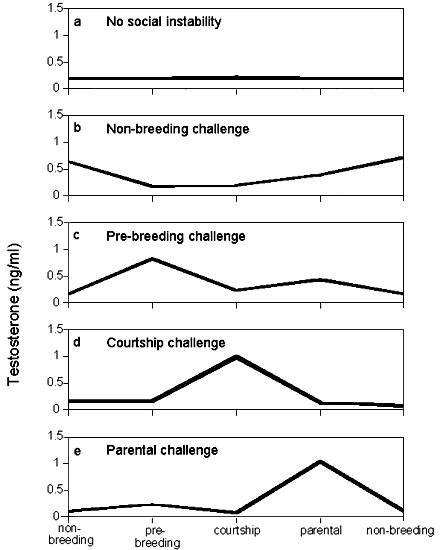
Although most Spotted Antbirds had very low plasma T levels year-round, some individuals showed peak T levels of up to 1.6 ng/ml during certain times of the year (Wikelski et al. submitted). We tested whether instances of social instability might contribute to these enhanced T values. For six individual male Spotted Antbirds we could not detect any instance of social instability throughout the year (Fig. 5a). Those males had baseline T levels year-round although they were aggressively reacting to playback and although they were breeding (and thus had enlarged gonads). Two males endured social instabilities during the non-breeding season (Fig. 5b), while three males each faced social instabilities during the pre-breeding or the courtship season, respectively (Fig. 5c and Fig. 5d). Another three males experienced social instabilities during the parental stage (Fig. 5e). Thus, all males for which we had enough repeated recaptures only showed T increases during times of social instability.
DISCUSSION
Our data showed that neo-tropical birds are in their majority seasonal breeders and do not maintain their gonads in near-functional state year-round. As a consequence of seasonal reproduction, many tropical birds will face the problem that they have to be territorial at times when their gonads are entirely regressed. Naively, one would expect that no T can be produced during those times. Contrary to those expectations, tropical birds could have elevated T levels during times when their gonads were entirely regressed. Even more puzzling, tropical birds could be aggressive with baseline T levels. At least one species, the neo-tropical Spotted Antbird, only had high plasma T during times of social instability. Experiments showed that T had a behavioural role in boosting aggression and song in Spotted Antbirds. We will review the evidence for seasonal breeding in tropical birds, compare hormonal and gonadal changes between species differing in habitat type, mating and social system and then discuss the physiological implications of a social modulation of T. Furthermore, we will discuss our data in light of the challenge hypothesis (Wingfield et al. 1990).
Study species and problems for comparisonsThe bird species investigated here and earlier are certainly far from being a random or representative sample of the tropical avifauna. Rather, they were opportunistically selected because of their local abundances, the ease with which they could be captured, and their body size (allowing endocrinological investigations). However, despite these potential limitations of the present comparison, we use these data to create testable hypotheses about the influence, and interaction of, territoriality and hormones in tropical birds. Another major drawback of almost all (including our) physiological studies on tropical birds is that data were only taken on the population level (Dittami & Gwinner 1990). This precludes a detailed analysis of seasonal reproductive pattern, because individual pairs may be out of synchrony with each other. Thus, pattern found on the population level may appear opportunistic when instead the real pattern is strict seasonal breeding. This may happen when pairs breed out of synchrony with other pairs.
The 14 study species discussed here come equally from the afro- and the neo-tropical regions of the world. The species differ in (1) habitat type, (2) social system and (3) mating system.
(1) Habitat type: The afro-tropical species included mostly birds that live in open forests or savanna type habitat (Dittami & Gwinner 1990). To the contrary, 4 of our neo-tropical species and the Bay Wren investigated by Levin (Levin & Wingfield 1992; Levin 1996) inhabit mostly forested habitats. (2) Social system: Our data set contained group-living afro-tropical White-browed Sparrow Weavers and neo-tropical Song wrens and possibly Blue-gray tanagers. There is only little known about the sociobiology of the latter species, but all data indicate that birds always travel in groups, have no territories in the usual sense and may have some form of pair-bond within the group (E. S. Morton, pers. comm.; pers. obs. W. D. Robinson). (3) Mating system: While the afro-tropical bird species were all socially monogamous, we included two neo-tropical species with lek mating systems in our comparison (Table 1).
Seasonal gonadal changesTo our large surprise, all species had entirely regressed gonads during some part of the year, even on the population level (Fig. 2). For the Song wren, this period was only very short, about 2-3 months. For several other species gonads were entirely regressed for up to half a year, e.g. for the Spotted Antbirds, the Blue-gray Tanager, the Clay-colored Robin or the Stonechat. The gonads usually recrudesced in the first months of the year in equatorial or northern tropical birds. Patterns were the converse for the White-browed Sparrow Weaver (studied at 13 oS latitude) in that birds had large gonads during the southern spring months (Wingfield et al. 1991). Several non-exclusive reasons may ultimately contribute to this widespread synchronisation of gonad recrudescence in spring: i) migrants from north temperate zones could increase competition for food during the northern winter months and thus preclude gonadal recrudescence (e.g., Moreau 1950; Morton 1980). However, in Panama the total density of migrants wintering at sites in Soberania National Park is less than 3% of the total avian density (Robinson et al. in press), therefore competition seems unlikely the reason for restricting food available for resident birds; ii) the climate pattern might be such that rainy seasons are more expressed after the first months of the year in the north-tropical areas and thus birds cue in on increased food abundance during these times (Wolda 1989; Windsor 1990), or iii) predation pressure may be different during different times of the year (Morton 1980; Robinson et al. in press). This factor may act in concert with the food abundance, e.g. if mammalian predators are highly food limited during the dry seasons in the first months of the year or reptilian predators are more active during the hot dry seasons at the beginning of each year (Huey & Slatkin 1976).
The different species had large absolute differences in gonad size, which presumably reflected the habitat and the mating system (Stutchbury & Morton 1995). The year-round pair-bonded Spotted Antbirds showed the smallest relative gonad volumes of all species (ca. 0.3 mm3 per g body mass), while the lek mating Clay-colored Robins and Golden-collared Manakins had the largest gonads (Fig. 2). Astonishingly, group-living White-browed Sparrow Weavers and possibly group-living Blue-gray Tanagers also had relatively large gonad volumes. This might be caused by high sperm competition in those groups, but such notion is presently largely speculative (see Stutchbury and Morton 1995).
Hormonal changes and regulation of territoriality
We concentrate here on T because all other measured androgens paralleled T and were found at much lower concentrations. In addition, we demonstrated for one tropical species, the Spotted Antbird, that T was effective in changing aggressive and singing behaviour, at least under laboratory conditions (Hau et al.unpubl. data). It has also been documented that T accumulates in the brain of Bay Wrens, implying that it serves important behavioural functions in these tropical wrens (Brenowitz & Arnold 1985).
Territorial aggression could occur despite baseline T leves in most tropical birds except for Stonechats, which had continuously elevated plasma T levels (Dittami & Gwinner 1985). Spotted Antbirds reacted aggressively toward playback during any time of the year, even when they had no detectable plasma T (Wikelski et al. submitted). Similarly, Bay Wrens were aggressive year-round with low plasma T (Levin & Wingfield 1992), as were White-browed Sparrow Weavers (Wingfield et al. 1991). Furthermore, the duration of territorial aggressiveness within the year was independent of plasma T levels and the amplitude of plasma T changes. Overall, this supports the independence of plasma T and aggression.
There was also no relationship between mean maximal gonad volume and plasma T levels. Thus, large gonads did not secrete more T than small gonads. This relationship is not too surprising, since only a small portion of the gonads, the Leydig cells, produce T. The largest tissue fraction of active gonads are seminiferous tubules that are responsible for spermatogenesis (Lofts & Murton 1973). In light of this finding it is conceivable that although Blue-gray Tanagers had the second largest gonads of all species, their plasma T levels remained at baseline throughout the year. This result is interesting in light of the hypothesis that in group-living species, gonads may be large due to sperm competition (cf. Stutchbury & Morton 1995).
Over and above the uncoupling of aggression from plasma T levels and the uncoupling of plasma T levels from gonad volumes, there was also a temporal uncoupling of gonad sizes and territoriality in tropical birds. There is a striking flexibility in patterns of territoriality among tropical birds. Some birds were territorial outside of the breeding season (e.g., Spotted Antbird, Stonechat). Other species were not territorial at all even during their breeding season, i.e., when they had highly enlarged gonads. This was most clear in the Blue-gray tanager, for which no territories nor territorial fights are demonstrated (E. S. Morton, pers. comm., and pers. obs.).
If plasma T is not related to aggression per se nor to gonad size or territoriality, but it could enhance aggression (Wingfield et al. 1990, 1997), is it used in this context in tropical birds? We tested whether social challenges may induce an increase in plasma T in Spotted Antbirds (Wikelski et al. submitted). We could only perform this test in one species due to the elaborate protocol. Individual birds had to be recaptured during different phases of their breeding cycle and we had to observe whether a social challenge had occurred around the same time. Spotted Antbirds that were unchallenged had baseline plasma T levels throughout the year. To the contrary, individuals that experienced social instability - like the intrusion of two or more males into their territory - increased their plasma T levels. This suggested that Spotted Antbirds relied on T during challenging situations, i.e., when T boosts the persistence during fights (cf. Beletzky et al. 1989). It is so far unclear where high plasma T levels come from when gonads are entirely regressed. It could be that (1) Leydig cells are still active and secrete T in otherwise 'regressed' gonads, (2) T is produced in other tissues like the adrenal, or (3) that T-precursor molecules are enzymatically converted to T by target tissues, like the brain (cf. Soma & Wingfield 1999). These possibilities are presently under investigation. In light of the above results it is also possible that the constantly elevated plasma T levels of year-round territorial Stonechats resulted from constant territorial challenges in this species. Such social interactions may be more frequent in the open savanna habitat where Stonechats occur. Furthermore, if birds in open habitats have generally higher levels of social interactions, this might explain the generally higher plasma T levels in afro-tropical compared to neo-tropical birds (Fig. 2).
In summary, our data supports the 'challenge hypothesis' (Wingfield et al. 1990) in situations where life history stages appear in 'unusual' combinations, such that territoriality, gonad growth and T production do not occur at the same time. Questions that could be addressed by future research are: (1) is T secreted from the gonads when they are entirely regressed, (2) why do most tropical birds recrudesce their gonads in spring, (3) how do tropical birds time their life history, i.e. how do they learn about the seasonality in their environment, (4) how common is a social modulation of plasma T levels in birds, (5) how do tropical birds defend huge territories with low T levels, and (6) is the low song rate of tropical birds causally linked to low T levels? These questions would greatly benefit from the inclusion of a more diverse array of tropical bird species from different phylogenetic backgrounds into the analysis. The methods to address these questions are available and it remains exciting to address such problems in wild birds, especially in the tropical habitats of the world that harbour the majority of avian species.
ACKNOWLEDGMENTS
We thank K. Soma, E. Gwinner, W.D. & T. R. Robinson, A.S. Rand, N.G. Smith, B. Poulin, G. Lefebvre, E.S. Morton and the staff at STRI for help during the study. Special thanks to Lynn Erckman for invaluable help throughout. Thanks to K. Soma for comments on previous versions of this manuscript. IN.RE.NA.RE. kindly permitted our work in Soberanía National Park, Republic of Panamá. This study was supported by grants from the Deutsche Forschungsgemeinschaft, the Deutsche Ornithologen-Gesellschaft, the Smithsonian Tropical Research Institute and the Max-Planck-Gesellschaft to M.H., by the Alexander-von-Humboldt Society and the Smithsonian Tropical Research Institute to M.W., by the Dissertation Completion Fellowship from the University of Illinois to W.D.R. and by the National Science Foundation to J.C.W.
REFERENCES
Ball, G.F. 1993. The neural integration of environmental information by seasonally breeding birds. American Zoologist 33:185-199.
Balthazart, J. 1983. Hormonal correlates of behaviour. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology VII. New York; Academic Press: 221-365.
Beletsky, L.D., Orians, G.H. & Wingfield, J.C. 1989. Relationships of steroid hormones and polygyny to territorial status breeding experience and reproductive success in male red-winged blackbirds. Auk 106: 107-117.
Beletsky, L.D., Orians, G.H. & Wingfield J.C. 1992. Year-to-year patterns of circulating levels of testosterone and corticosterone in relation to breeding density experience and reproductive success of the polygynous red-winged blackbird. Hormones & Behaviour 26: 420-432.
Brawn, J.D., Collins, T.M., Medina, M. & Bermingham, E. 1996. Associations between physical isolation and geographical variation within 3 species of neotropical birds. Molecular Ecology 5: 33-46.
Brenowitz, E.A. & Arnold, A.P. 1985. Lack of sexual dimorphism in steroid accumulation in vocal control brain regions of duetting song birds. Brain Research 344: 172-175.
Dittami, J.P. 1986. Seasonal reproduction, moult and their endocrine correlates in two tropical Ploceidae species. Journal of Comparative Physiology B. 156: 641-647.
Dittami, J.P. 1987. A comparison of breeding and moult cycles and life histories in two tropical starling species: the blue-eared glossy starling Lamprotornis chalybaeus and Rüppell's Long-tailed Glossy Starling L. purpuropterus. Ibis 129: 69-85.
Dittami, J.P. & Gwinner, E. 1985. Annual cycles in the African Stonechat Saxicola torquata axillaris and their relationship to environmental factors. Journal of Zoology London 207: 357-370.
Dittami, J.P. & Gwinner, E. 1990. Endocrine correlates of seasonal reproduction and territorial behaviour in some tropical passerines. In: M. Wada (ed.) Endocrinology of birds: molecular to behavioural. Tokyo/Berlin; Japan Scientific Society Press/Springer: 225-233.
Dittami, J.P. & Knauer, B. 1986. Seasonal organization of breeding and moulting in the Fiscal Shrike (Lanius collaris). Journal für Ornithologie 127: 79-84.
Dufty, A.M. 1989. Testosterone and survival. Hormones and Behaviour 23: 185-193.
Dunning, J.B. Jr. 1993. CRC handbook of avian body masses. Boca Raton: CRC Press.
Griscom, L. 1945. Modern Bird Study. Cambridge Mass: Harvard Univ Press.
Gwinner, E., Rödl, T. & Schwabl, H. 1994. Pair territoriality of wintering stonechats - behaviour, function and hormones. Behavioural Ecology and Sociobiology 34: 321-327.
Hadley, M.E. 1992. Endocrinology. Englewood Cliffs, NJ: Prentice Hall.
Hau, M., Wikelski, M. & Wingfield, J.C. 1998. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proceedings of the Royal Society London B 265: 89-95.
Hillgarth, N., Ramenofsky, M. & Wingfield, J.C. 1997. Testosterone and sexual selection. Behavioural Ecology 8: 108-109.
Hillgarth, N., & Wingfield, J.C. 1997. Parasite-mediated sexual selection: endocrine aspects. In: Clayton, D.H. & Moore, J. (eds) Host-parasite evolution. Oxford: Oxford University Press: 78-104.
Holdridge, L.R. 1967. Life zone ecology. Occasional papers of the Tropical Science Center. San Jose, Costa Rica.
Huey, R.B. & Slatkin, M. 1976. Cost and benefits of lizard thermoregulation. Quarterly Review of Biology 51: 363-384.
Hunt, K.E., Hahn T.P. & Wingfield J.C. 1997. Testosterone implants increase song but not aggression in male Lapland longspurs. Animal Behaviour 54: 1177-1192.
Ketterson, E.D., Nolan, V.Jr., Wolf, L. & Ziegenfus, C. 1992. Testosterone and avian life histories: effects of experimetally elevated testosterone on behaviour and correlates of fitness in the dark-eyed junco (Junco hyemalis). American Naturalist 140: 980-999.
Leigh, E.G., Rand, A.S. & Windsor, D.M. 1982. The ecology of a tropical forest. Washington, D.C.: Smithsonian Press.
Levin, R.N. 1996. Song behaviour and reproductive strategies in a Duetting Wren, Thryothorus nigricapillus: I. Removal experiments. Animal Behaviour 52: 1093-1106.
Levin, R.N. & Wingfield, J.C. 1992. The hormonal control of territorial aggression in tropical birds. Ornis Scandinavica 23: 284-291.
Lofts, B. & Murton, R.K. 1973. Reproduction in birds. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology Vol III. New York; Academic Press: 1-107.
Logan, C.A. & Wingfield, J.C. 1990. Autumnal territorial aggression is independent of plasma testosterone in mockingbirds. Hormones & Behaviour 24: 568-581.
Moreau, R.E. 1950. The breeding seasons of african birds. 1. Land birds. Ibis 92: 223-267.
Morton, E.S. 1971. Nest predation affecting the breeding season of the Clay-colored Robin. Science 181:920-921.
Morton, E.S. 1980. Adaptations to seasonal changes by migrant land birds in Panama Canal Zone. In Keast, A. & Morton, E.S. (eds) Migrant birds in the Neotropics: ecology, behaviour, distribution and conservation. Washington D.C.; Smithsonian Institution Press: 437-453.
Robinson, W.D., Brawn, J.D. & Robinson, S.K. in press. Structure of a forest bird community in central Panama: Influence of spatial scale and biogeographic history. Ecological Monographs.
Robinson, W.D., Robinson, T.R., Brawn, J.D. & Robinson, S.K. in press. Nesting success of understory forest birds in central Panama. Journal of Avian Biology.
Schwabl, H. 1992. Winter and breeding territorial behaviour and levels of reproductive hormones of migratory European Robins. Ornis Scandinavica 23: 271-276.
Searcy, W.A. & Wingfield, J.C. 1980. The effects of androgen and antiandrogen on dominance and aggressiveness in male red-winged blackbirds. Hormones and Behaviour 14: 126-135.
Sieving, K.E. 1992. Nest predation and differential insular extinction among selected forest birds of central Panama. Ecology 73:2310-2328.
Silverin, B., Viebke, P.A. & Westin, J. 1989. Hormonal correlates of migration and territorial behaviour in juvenile Willow tits during autumn. General and Comparative Endocrinology 76, 148-156.
Skutch, A.F. 1969. Life histories of central American birds. Pacific Coast Avifauna 35: 248-269.
Soma, K. & Wingfield, J.C. 1999. Endocrinology of aggression in the non-breeding season. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1606-1620. Johannesburg: BirdLife South Africa.
Stutchbury, B.J. & Morton, E.S. 1995. The effect of breeding synchrony on extra-pair mating systems in songbirds. Behaviour 132: 675-690.
Wikelski, M., Hau, M. & Wingfield, J.C. submitted. Social instability increases plasma testosterone in a year-round territorial neotropical bird. Proceedings of the Royal Society London B.
Willis, E.O. 1967. The behaviour of Bicolored Antbirds. University of California Publications in Zoology 79: 1-132.
Willis, E.O. 1972. The behaviour of Spotted Antbirds. Ornithological Monographs 10. American Ornithological Union.
Windsor, D.M. 1990. Climate and moisture variability in a tropical forest: long-term records from Barro Colorado Island, Panamá. Washington, D.C; Smithsonian Institiution Press.
Wingfield, J.C. 1994. Regulation of territorial behaviour in the sedentary song sparrow, Melospiza melodia morphna. Hormones & Behaviour 28: 1-15.
Wingfield, J.C. & Farner, D.S. 1975. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steroids 26: 311-327.
Wingfield, J.C. & Farner, D.S. 1976. Avian endocrinology - field investigations and methods. Condor 78, 571-573.
Wingfield, J.C. & Farner, D.S. 1993. Endocrinology of reproduction in wild species. In Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology IX. New York; Academic Press: 163-327.
Wingfield, J.C. & Hahn T.P. 1994. Testosterone and territorial behaviour in sedentary and migratory sparrows. Animal Behaviour 47: 77-89.
Wingfield, J.C. & Lewis, D.M. 1993. Hormonal and behavioural responses to simulated territorial intrusion in the cooperatively breeding White-browed Sparrow Weaver, Plocepasser mahali. Animal Behaviour 45: 1-11.
Wingfield, J.C., Ball, G.F., Dufty, A.M., Hegner, R.E. & Ramenofsky, M. 1987. Testosterone and aggression in birds. American Scientist 75: 602-608.
Wingfield, J.C., Hegner, R.E., Dufty, A.M. & Ball, G.F. 1990. The ‘Challenge Hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist 136: 829-846.
Wingfield, J.C., Hegner, R.E. & Lewis, D.M. 1991. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding White-browed Sparrow Weaver, Plocepasser mahali. Journal of Zoology London 225: 43-58.
Wingfield, J.C., Hegner, R.E. & Lewis, D.M. 1992. Hormonal responses to removal of a breeding male in the cooperatively breeding White-browed Sparrow Weaver, Plocepasser mahali. Hormones & Behaviour 26: 145-155.
Wingfield, J.C., Jacobs, J. & Hillgarth, N. 1997. Ecological constraints and the evolution of hormone-behaviour interrelationships. In: Carter, C.S., Lederhendler, I.I. & Kirkpatrick, B. (eds) The integrative neurobiology of affiliation. Vol. 807. New York; New York Academy of Sciences: 22-41.
Wingfield, J.C. & Moore, M.C. 1987. Hormonal, social, and environmental factors in the reproductive biology of free-living male birds. In: Crews, D. (ed.) Psychobiology of reproductive behaviour: an evolutionary perspective. New Jersey; Prentice Hall: 149-175.
Wolda, H. 1989. Seasonal cues in tropical organisms. Rainfall? Not necessarily! Oecologia 80: 437-442.
Worthington, A. 1982. Populations sizes and breeding rhythms of two species of manakins in relation to food supply. In: Leigh, E.G.Jr., Rand, A.S. & Windsor, D.M. (eds) The biology of a tropical forest. Washington, D.C.; Smithsonian Inst Press: 213-225.
Zuk, M. 1996. Disease, endocrine-immune interactions, and sexual selection. Ecology 77: 1037-1042.
Table 1. Scheme of life history traits of tropical bird species for which annual cycles of gonad sizes and hormonal changes have been measured in the field. The species investigated during our study are highlighted by bold letters. Literature describing aspects of the ecology of these species is indicated by superscripts.

Fig 1. Schematic representation of the latitudinal changes in territorial behaviour (shaded areas) and of circulating T (black curves) in a variety of bird taxa (redrawn after Wingfield et al. 1997). When birds live in highly seasonal habitats, T is related to territorial behaviour and aggression (top three panels). In the two species depicted in the lower two panels, territoriality and aggression is not strictly seasonal or entirely aseasonal. Accordingly, T levels are not related with the behaviour, especially outside of the breeding season.
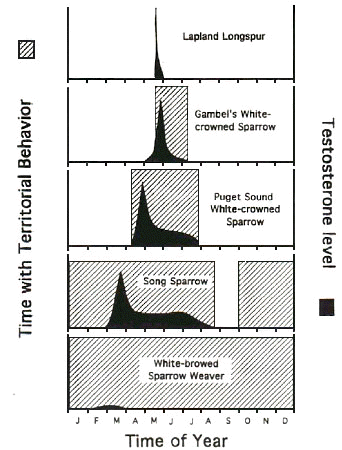
Fig 2. Schematic representation of the changes in gonad volumes (black curve, left panels), breeding times (shaded areas, left panels), and plasma T levels (black curves, right panels) and territoriality (shaded areas, right panels) for 12 tropical bird species (see Table 1 for references on published data and scientific names). Data for all neo-tropical species except Bay Wrens were collected during this study (a), while data for afro-tropical birds were compiled from published data only (b). No gonad data are available for Bay Wrens. Rainfall data for both afro-tropical and neo-tropical areas are provided in the lowest panel (from Dittami & Gwinner 1990 and Wikelski et al. submitted). For the White-browed Sparrow Weaver (b, 4th panel from top), the only south-tropical bird species investigated, we shifted the annual cycle by 6 months to ease comparisons. T data given for Rüppel's Starling (b, second lowest panel) were taken from both Dittami 1987 (D 87, monthly means for wild individuals) and Dittami & Gwinner 1990 (D&G 90). The latter include data for captive birds held in aviaries. The territoriality pattern of neo-tropical Bicolored Antbirds are 'transient' or 'nomadic'. They do not fit current definitions and were thus depicted in black shading.
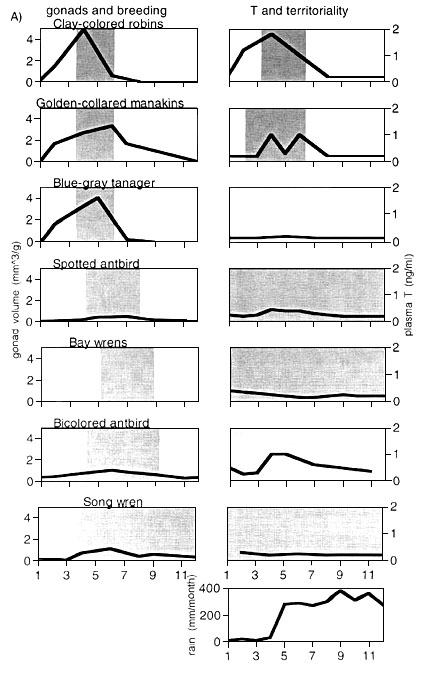
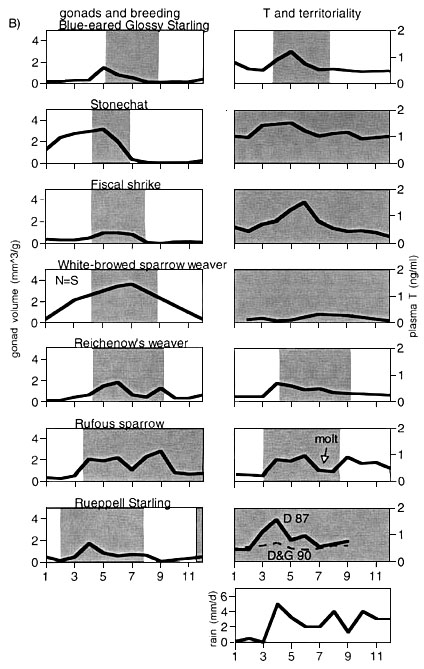
Fig 3. Comparison between morphological, physiological and ecological parameters for the 14 tropical bird species. For this comparison, we assume phylogenetic independence. There was no relationship between the maximal mean monthly gonad volume and the mean monthly amplitude of circulating plasma testosterone (T; in a), nor between gonad volume and maximal mean monthly T (b), nor between the duration of territoriality and both amplitude and maximal monthly T. Each square represents one species.
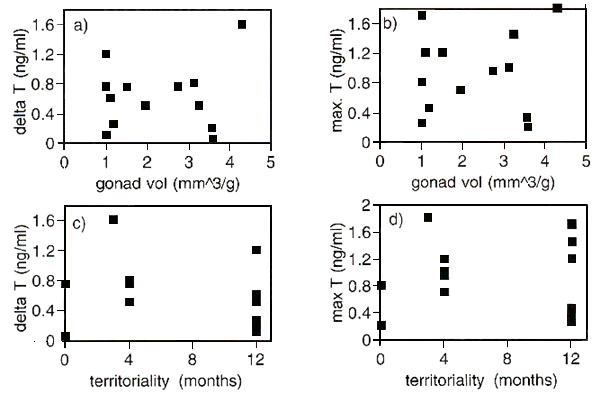
Fig 4. Repeated comparisons among the 14 tropical bird species with regard to the mean monthly amplitude in maximal monthly gonad volume and circulating plasma testosterone (T). Bars represent means and 95% confidence intervals. Numbers below panels indicate the number of species in each column (see also Table 1 for categorisations). No statistics are presented because of problems of phylogenetic pseudoreplication. Comparisons should instead be regarded as exploratory.
Fig 5. Scheme of plasma levels of testosterone (T) during 4 life history stages of the annual reproductive cycle of male Spotted Antbirds (redrawn after Wikelski et al. submitted). Repeated measurements of individual birds are averaged for each life history stage and connected by lines. Maximal T levels were only found during times of social challenges. The different panels depict differences in the time of social instability experienced by those males: (a) males never experienced social instability during their annual cycle (n=6); (b-e) males were caught in socially unstable situations either during the non-breeding season (n=2; b), the pre-breeding season (n=3; c), the courtship phase (n=3; d) or during the parental phase (n=3, e).