S20.3: The role of body condition on breeding and foraging decisions in albatrosses and petrels
Henri Weimerskirch
CEBC-CNRS, 79360 Beauvoir, France,
fax 33 5 4909 6526, e-mail henriw@cebc.cnrs.fr Weimerskirch, H. 1999. The role of body condition in breeding and foraging decisions in albatrosses and petrels. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1178-1189. Johannesburg: BirdLife South Africa.Petrels and albatrosses are central place foragers that infrequently provision their single offspring because of the distance from the nest to feeding zones. They are long-lived and according to life-history theory they should limit the risk of increased mortality during a breeding event. I show that they work between a high mass threshold that determines breeding decision and a lower mass threshold that determines foraging decision and resource allocation. By comparing 14 species with different foraging ranges, their response to changing environmental conditions and to experimental manipulation, I investigate the connections between foraging behaviour and allocation processes, and especially the role of body condition in allocation decisions when birds are provisioning their chicks. For species where feeding zones allowing a positive yield are located at more than 300 km, a specific provisioning strategy is adopted where a foraging zone close to the colony is used alternately to increase the energy flux to the chick resulting in a negative yield. Their body condition allows them to regulate breeding effort. These pelagic species behave as prudent parents in never allowing their body condition to deteriorate under natural conditions as under experimental situations.
INTRODUCTION
The concept of reproductive effort is based on the idea of the existence of a trade-off between present and future reproduction, with the assumption that resources are limited in the environment (Williams 1966). Therefore at any time during a reproductive event individuals face allocation decisions. Allocation of resources into present reproduction is easily measured with provisioning parameters, whereas allocation into future survival is more difficult to appreciate. Allocation decisions are possibly related to the state of the parent's body reserves (Drent & Daan 1980) and the amount of energy stored by the individual, measured as the body condition or more simply the mass of the individuals, is probably a good appraisal of resources allocated into future reproduction. Recently MacNamara and Houston (1996) have proposed that these decisions are probably more dependent on the individual physiological state, and especially the body condition, than on age related factors. In long-lived species such as seabirds the risk of increased mortality incurred during a breeding attempt should be reduced because of their high residual reproductive value (Goodman 1974), i.e. they should therefore behave as prudent parents (Drent & Daan 1980) and therefore the body condition could play a central role in allocation processes in these species.
The changes in body mass are generally related to the use or storage of energy reserves, in particular fat reserves (Blem 1990). The storage of body reserves is associated with benefits, but also with costs (Witter and Cuthill 1993). In seabirds that store at some stage large amounts of fat (Groscolas 1990) changes in body mass of breeding birds can be extensive. The storage of energy reserves is generally related to the subsequent use of these reserves to cover period of fast due to incubation tasks (Groscolas 1990), night time when feeding is not possible (King 1974), increased energy expenditure due to breeding (Tuomi et al. 1983) or risks of mass loss due to environmental condition such as cold (Lima 1986). However the decrease in mass could also be considered as a purely adaptive answer that enhance breeding success, for example by reducing foraging costs (Norberg 1981, Freed 1981). Decreases in mass have often been related to a reproductive stress and to what extent could the use of body reserves alter survival, either directly, or indirectly by higher susceptibility to predators, diseases or other environmental factors is not well known (see Tuomi et al. 1983). Furthermore there is little direct evidence for a physiological cost leading to increased mortality (Partridge & Harvey 1985). This is possibly because the use of body reserves or their allocation towards reproduction may not automatically result in survival costs, with the existence of threshold values above which somatic costs do not result in costs to survival (Tuomi et al. 1983).
The aim of this work is to examine in a group of long-lived seabirds, albatrosses and petrels (order Procellariiformes) the role of body reserves in allocation decisions, and to consider the extent to which these birds behave as prudent parents.
Terminology
In the text I have used average values for the actual body mass, and not body condition, i.e. the mass corrected by structural size. This is acceptable in petrels and albatrosses where the variance in the size of the animals for a particular species is not extensive, except for the species with a high sexual dimorphism where I have separated the two sexes.
When captured in a colony the mass of a bird is composed of the structural mass of the animal, i.e. the mass without any body reserves, plus a certain amount of body reserves, and finally to the stomach content, especially when the birds are feeding chicks. The following terms are used:
'Working mass': mass of birds during the different stages of the breeding season, at the start or end of incubation shifts or brooding shifts, or after delivery of a meal during the chick rearing period. The working mass used in this work therefore does not include the meal brought to the chick. During the incubation fast, or when provisioning chicks, birds use their body reserves. Changes in mass are therefore representative of changes in body reserves.
'Lower threshold mass': mass at which birds stop breeding, i.e. when eggs or chicks are abandoned, generally because of the depletion of body reserves (Chaurand and Weimerskirch 1994a) . During incubation, this occurs after fasting for extended periods.
'Critical mass': this is a physiological measure that corresponds to a state when fat stores are critically depleted. During a long incubation fast birds use mainly their fat stores but also a significant amount of protein and the specific daily body mass loss remains constant (Cherel et al. 1988, Groscolas 1990). When they reach this critical mass the specific daily body mass loss increases steadily and birds start to increase their use of protein, probably because their fat reserves are close to depletion (Groscolas 1990). It has been suggested that this critical mass induces a signal for refeeding (Robin et al. 1998), i.e. it is likely to be associated with the lower threshold mass.
'Lethal mass': mass when all body reserves are depleted leading to the death of the animal.
'Upper threshold mass': mass of bird with body reserves that allow the onset of breeding.
All these values of mass do not include the food transported in the stomach.
'Maximum mass': mass of the animal including the body reserves and food transported in the stomach. This mass is probably close to the working mass when adults return to the nest to start a new incubation shift, and to the working mass added to the meal mass when adults rear a chick. When meal mass or body reserves are maximised, the overall mass of birds is likely to be close to a maximum loading capacity, after which the bird cannot fly without high costs for aerodynamic reasons (see Pennycuick 1989).
The Blue Petrel Halobaena caerulea
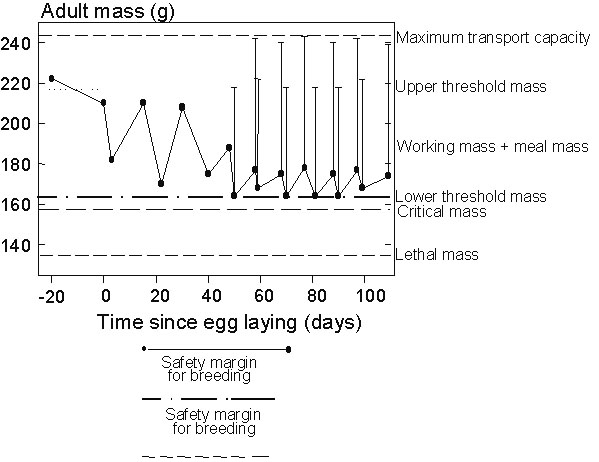
The body mass of Blue Petrels shows considerable variation throughout the breeding season (Fig. 1). The highest values are attained when the birds return to the colonies one month before laying, the lowest when birds rear their chick. In this species the mass of the bird one month before laying will influence whether it will breed or not during this season. If birds have not attained an upper threshold body mass corresponding to a mass of 220 g (Chastel et al. 1995a) they will not breed one month later. For the birds that attempt to breed, those whose breeding fails have a significantly lower mass during the pre-laying period than those that will subsequently hatch a chick (Chastel et al.1995a). During incubation, parents alternate periods of fast on the nest and periods of feeding at sea. The time spent by the incubating bird is mainly determined by the foraging bird, since birds generally leave the nest only when they are relieved. However a significant proportion of birds spontaneously desert the nest, generally because of the prolonged stay at sea of the partner. The time elapsed between the return of the bird and the desertion is inversely related to the mass of the bird when it returns from the sea. This indicates that foraging success will determine the fasting ability of the bird. The consequence is that birds desert when they have attained a lower threshold mass of 164 g on average (Chaurand & Weimerskirch 1994b). This mass is 4-14 g higher than the critical mass (150-160 g, Ancel et al. 1998) when birds have almost depleted their fat reserves and extensive use of protein, mostly muscle tissues, starts. It appears therefore that birds desert before they have depleted their muscle mass to a significant extent, thereby before the mortality risks increase significantly. This is what could be expected from a long-lived seabird that should limit the risks of an increased mortality. The lag between the lower threshold mass and the critical mass could allow birds to reach the feeding zones that are likely to be distant in this species (Chaurand and Weimerskirch 1994a). The lethal mass of Blue Petrels is around 130g (unpublished) and corresponds to birds that are completely emaciated: it is 20-30 g lower than the critical mass. The difference between the lower threshold mass and the critical mass or the lethal mass could be considered as a 'safety margin for survival'.
Thus it appears that Blue Petrels are 'working' during the breeding season between a lower and a upper threshold mass. The difference between the working mass and the lower threshold mass, that we call hereafter 'safety margin for breeding' is very small during chick rearing, whereas during incubation it is slightly higher. The lower mass of birds during the chick-rearing period compared to the incubation has been interpreted as either the result of the stress of reproduction, or as an adaptation to reduce flight costs (e.g. Norberg 1981). Clearly seabirds undergo large changes in body mass during incubation. When we consider only the lowest values as measured at the end of incubation shifts, and compare them with those measured during chick rearing, there is a significant decrease. One advantage of this mass decrease in the Blue Petrel would be to allow the transport of a large meal size, as the birds are not weighed down by their own reserves. Indeed Blue Petrels can transport meals as heavy as 83 g (i.e. 50% of their lower threshold mass), with average values of 32 to 40% of the lower threshold mass (Chaurand & Weimerskirch 1994). This meal size is therefore extremely high for a flying bird (Pennycuick 1989) and a maximum values of 250 g (adult mass + meal size) is likely to represent the maximum loading capacity of this bird that possibly correspond to a threshold value with respect to the wing loading and flight performance of the birds.
Relationship between lower threshold mass and critical mass
Only one other study has been able to relate the 'lower threshold mass' or mass at desertion under natural circumstances and the critical mass (Olsson 1997). The extensive literature available on emperor penguins (review in Groscolas 1990 and Robin et al. 1998) surprisingly does not provide values of mass or body condition index of birds naturally deserting their egg or chick. Olsson gave for a small sample of adult king penguins naturally deserting their egg a mass that is lower than the critical mass measured by Cherel et al. (1988), i.e. the converse situation to that of the Blue Petrel. However a bias could have occurred in king penguins because very large and fat birds were selected to study the changes in body mass during long fasts (Y. Cherel pers. com.), biasing the value of critical mass towards high values. The attainment of a critical mass is associated with a change in the metabolite and hormonal composition (Cherel et al. 1998) that could produce a change in behaviour, an hence trigger desertion (Robin et al. 1998). Therefore a simple mechanistic explanation could support the hypothesis of critical mass being higher than lower threshold mass as suggested by results on king penguins. In Blue Petrels results suggest that birds desert before they attain the critical mass, i.e. before they start using protein to a large extent, hence depleting muscular mass: in an ecological perspective this could be interpreted as a 'prudent behaviour'. With only two species where both critical mass and 'lower threshold mass' have been measured, with contrasting results, it is difficult to decide whether one occurs before or after the other, and more accurate data in natural conditions are necessary. The critical mass is difficult to measure because it corresponds to a progressive change in the physiological state of the animal, whereas the lower threshold mass is much easier to estimate.
Safety margins and working mass in other ProcellariiformesLower threshold masses have been measured in natural conditions in 5 other species in addition to the Blue Petrel, and it appears that the relative safety margin for breeding increases with the size of the different species during incubation as well as during the chick rearing period (Fig. 2a and Fig 2b). In small species such as Blue Petrels and prions birds work close to the lower threshold mass, especially during the chick rearing period, whereas large species have an extensive safety margin, with male wandering albatrosses carrying body reserves which constitute an additional 50% of their lower threshold mass during chick rearing (Fig. 2b). This is particularly surprising because birds generally decrease their working mass during chick rearing, possibly allowing reduced foraging costs (Norberg 1981) and a greater load-carrying capacity (see Blue Petrels) to deliver more food to their chick. When the mass of the meal is considered, it appears that the mass of feeds delivered to chicks relative to the threshold mass also decreases with respect to mass of the species (Fig. 2c). One factor responsible for the reduction in the meal size could be related to the reduction of the ability to lift loads in large species, i.e. a consequence of scale effects on the power required to fly (Pennycuik 1975). However a linear relationship such as this is not expected by Pennycuick et al. (1984) who considered that the problem of weigh limitation, during take off especially, is mainly for very large species such as wandering albatross. Thus the relationship suggests that the amount of food delivered may be not only related to flight constraints, but also to other factors, for example to the energy requirements of the chick. Smaller species have a higher relative metabolic rate thereby requiring more energy per unit of time. If the relative mass of the meal is added to the relative working mass of the adult, the difference between the threshold mass and the maximum mass increases with size, although this effect is less marked during the chick rearing period (compare Fig. 2b and Fig 2c). This presents a paradox in terms of flight energetics: large species carry relatively heavy loads compared to smaller species, due to the large amount of body reserves. This is probably made possible because the specific cost of transport for flying decreases with increasing body size (Schmidt-Nielsen 1984).
Why are larger species carrying such a large amount of body reserves? The working mass of species is probably the result of a complex of trade-offs between the energetic constraints of flight, energy requirements of chicks but also other factors. For example the predictability of resources may also play a significant role in the storage of body reserves (e.g. Rogers 1987) and the greater safety margin for breeding of larger Procellariiform species could confer on them a greater margin for survival of environmental extremes (Blem 1990). This is consistent also with the suggestions by several authors who have investigated the selective advantage of the evolution of increased size in birds (e.g. Calder 1984).
Chick provisioning and distance to feeding zones
When provisioning chicks Procellariiform parents forage independently (Warham 1990). Adults visit their chick irregularly to deliver meals and until recently the duration of foraging trips was estimated from the feeding frequency at which chicks are fed, using average flight speeds (e.g. Pennycuick 1984). However, recently, the duration of foraging trips of adults provisioning chicks has been studied for several species at the individual level and has resulted in the discovery of a specific provisioning strategy whereby in several species adults alternate trips of short duration and of longer duration (Weimerskirch et al. 1994). The use of satellite telemetry, or the analysis of prey brought after trips of short or long duration have shown that short trips are performed in waters close to colonies, generally, but not always, on the shelf surrounding the colonies, and long trips in more distant oceanic waters (e.g. Weimerskirch & Cherel 1998, Weimerskirch et al. 1997b). The energy acquired at sea per unit of time during a foraging trip is always higher for long trips than for short trips, and the yield of foraging trips is on average positive during long trips and negative during short trips. The use of a two fold strategy is related to the reliance of the population to distant foraging zones: those species relying on close feeding zones do not have the bi-modal distribution of the duration of foraging trips (Fig. 3) and several species have a positive yield when foraging in waters close to the colonies. Recent results show also that within the same species population-specific strategies are evident with some populations relying on distant resources using a two-fold strategy, whereas others where resources are closer perform only trips of short duration (Waugh et al. in press).
The distance of food resources from the breeding grounds probably has a major influence on the evolution of breeding strategies in seabirds (Lack 1968) and therefore on acquisition and allocation processes. One central prediction of the optimal foraging theory is that in species foraging at long distance from the colonies such as Procellariiformes meal mass should be maximised (Lessells and Stephens 1983). This is apparently not the case since for every species of petrels or albatrosses studied the modal values of meal mass are never close to the maximum values observed, especially in the larger species. Additionally there is no significant relationship between the maximum foraging range and the relative meal size delivered for 9 species of Procellariiformes studied (r=0.044, P>0.1). Since petrels and albatrosses are able to concentrate energy into stomach oil (Warham et al. 1976) the energy delivered to the chick rather than the meal mass should be considered to examine this question. The relationship between the maximum foraging range and the energy delivered is still not significant (r=0.320, n=6, P>0.1). Species using a two-fold strategy spend a varying amount of time in short or long trips, because of the inter-species differences in the duration of long or short foraging trips (Fig. 3), but also because of the amount of time spent in both types of trip, i.e. in trips with either positive or negative yield. This measure appears important and is closely related to the maximum range (Fig. 3). It is also significantly related to the meal size or the amount of energy delivered (r=0.616, n=14, P=0.01 and r=0.9063, n=6, P>0.001). The time spent in long trips also allows us to predict that adult petrels and albatrosses would not be able to rely on feeding zones farther than 3000 km to provision their chick because they would spend all their foraging time in long trips (Fig. 3), i.e. they would not be able to increase the provisioning rate to the chick with short trips and therefore chicks would not receive a sufficient energy flow per unit of time to grow.
Regulation of body mass and provisioning with respect to the lower mass threshold
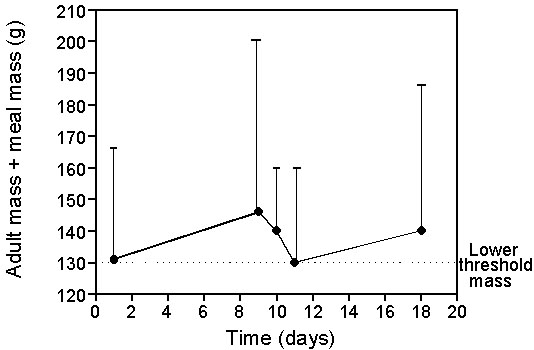
The decision to start a long or a short trip has been shown to depend only on the body condition of the adult and not on the condition of the chick or on the time spent foraging, suggesting that birds do not have a fixed schedule of foraging (Weimerskirch 1998). The negative yield of short trips is due to the use of adult body reserves to cover the foraging costs. This can be schematised for a thin-billed prion provisioning its chick (Fig. 4). The parent stores body reserves during long trips to use them during short trips. After one or several short trips, the mass of the parent comes close or reaches the lower threshold mass and the bird starts then a long trip, whereas after long trips adult body mass is much higher than the threshold mass. When they return from a long trip the body condition of the adult is positively correlated to the level of prolactin, a hormone associated with parental care (Weimerskirch & Cherel 1998). The use of body reserves to cover foraging costs allow pelagic species to exploit resources close from the colonies that provide a relatively low profitability for foragers, yet enable an increase in the rate of food provisioning to the chick. The storage of energy thus appears to have a functional role in the short term and loss of mass during breeding should be considered as a reproductive tactic rather than a simple physiological cost of reproduction. In species that are not using a two fold strategy no influence of adult condition on the provisioning strategy was found (Weimerskirch et al. 1997b, unpublished). Therefore the distance at which birds are foraging for prey where a positive yield results may influence the role of body reserves in allocation decision.
Data on wandering albatrosses, which have an extensive safety margin, indicate that together with a short term regulation of body mass, birds modify the amount of body reserves in the longer term, throughout the chick rearing period. They reach the lowest values at the end of the brooding period, i.e. at the end of the most energetically constraining period (Salamolard & Weimerskirch 1993). When the chick is left alone on the nest, i.e. when energetic constraints are not as strong as during brooding and when conditions in terms of food availability are favourable, birds restore their body reserves to a high level. When feeding conditions deteriorate, from late August, birds reduce their safety margin (Weimerskirch & Lys in press a). Similar results are observed in medium sized albatrosses such as yellow-nosed albatrosses that are able to temporarily lower their body mass when feeding conditions become unfavourable, but restore it as soon as conditions improve (Weimerskirch et al. submitted). This is confirmed by experimental manipulations of foraging costs that show that when foraging costs are increased, adults temporarily decrease their working mass (Weimerskirch et al. in press b).
In small species, probably because of the absence of a safety margin, there is no long term change in body mass throughout the chick rearing period and birds never decrease their working body mass when environmental conditions deteriorate, or when foraging costs are experimentally increased (Weimerskirch et al. 1995, in press). The absence of safety margin appears therefore to handicap small birds in adjusting their provisioning effort. This could be a reason why for example large species such as wandering albatrosses have a very low variance in breeding success, especially in fledging success (Weimerskirch and Jouventin 1997) because they can buffer any low foraging success or longer term decrease in food availability with their extensive safety margin. Conversely changes in food availability for small species are much more likely to induce immediately egg desertion or a reduction in chick provisioning and therefore these species have a more variable breeding success (see e.g. Chastel et al. 1995a).
CONCLUSIONS
Because Procellariiformes are long-lived they are expected to store body reserves not only for reproducing but also to limit the risk of increased mortality due to breeding. We have seen here that this is probably one reason why large species store very large body reserves, but that smaller species, even though long-lived, are working with low levels of body reserves. None probably reaches critical masses in natural condition, always keeping sufficient reserves to limit the risk of increased mortality. The storage of body reserves not only has a role in limiting mortality risks but has a major functional role in behavioural decisions. This is made possible by the existence of threshold masses that induce decisions. Earlier studies on fowls had shown the existence of flexible endogenous thresholds for behavioural decisions (Mrosovsky & Sherry 1980). The mechanisms involved in these decisions are possibly related to complex interactions between the levels of body reserves and hormonal and metabolite levels (Cherel et al. 1988, Robin et al. 1998). The lower threshold mass appears to trigger the desertion of the egg for refeeding and during the chick rearing period the start of a long trip, i.e. the restoration of adult body condition or feeding for themselves. It is probably less flexible in small species than in large ones. In larger species where desertion is triggered when reserves are very low, the decision to start a long trip, i.e. for adults to store body reserves, is made at a much higher level than the lower threshold mass, suggesting that the lower threshold mass for foraging decisions may be different or flexible during the chick rearing period. Decisions in these large species could be related not only to the body mass of the adult but to interactions between the body mass of the adult and the needs of the chick. The distance at which birds are foraging significantly modifies the role of the lower threshold mass in allocation decisions and this aspect should be investigated further. Indications of upper threshold shows that decisions to start breeding are related to the level of body reserves in the most pelagic species, but not in coastal species (Chastel et al. 1995a).
ACKNOWLEDGEMENTS
The studies in the French Antarctic and sub-Antarctic Territories were funded by IFRTP, programme n°109 directed by P. Jouventin. I thank the various people involved in the studies on the field as well as colleagues that have helped at different stages and with whom discussions on the subject have been helpful, T. Chaurand, Y. Cherel, O. Chastel, S. Waugh.
REFERENCES
Ancel, A., Petter, L. & Groscolas R. 1998. Changes in egg and body temperature indicate triggering of egg desertion at a body mass threshold in fasting incubating Blue Petrels (Halobaena caerulea). J. Comp. Physiol. B168:533-539.
Blem, C.R. 1990. Avian energy storage. Current Ornithology 7: 59-113.
Calder, W.A.,III 1984. Size, function and life history. Harvard Univ. Press, Cambridge, Mass.
Charlesworth, B. 1980. Evolution in age-structured populations. Cambridge University Press, Cambridge.
Chastel, O., Weimerskirch H. & Jouventin P. 1995. Body condition and seabird reproductive performance: a study of three petrel species. Ecology 76: 2240-2246.
Chaurand, T. & Weimerskirch, H. 1994a. The regular alternation of short and long trips in the Blue Petrel Halobaena caerulea: a previously undescribed strategy of food provisioning in a pelagic seabird. J. Anim. Ecol. 63:275-282.
Chaurand T. & Weimerskirch H. 1994b. Incubation routine, body mass regulation and egg-neglect in the Blue Petrel Halobaena caerulea. Ibis 136:285-290.
Cherel, Y., Robin, J.P., Walch, O., Karmann, H., Netchitailo, P. & LeMaho, Y. 1988. Fasting in King Penguins. I. Hormonal and metabolic changes during breeding. Am. J. Phys. 254: R170-177.
Drent, R. & Daan, S. 1980. The prudent parent: energetic adjustment in avian breeding. Ardea 68: 225-253.
Groscolas, R., Schreiber, L. & Morin, F. 1991. The use of tritiated water to determine protein and lipid utilisation in fasting birds: a validation study in incubating Great-winged Petrels Pterodroma macroptera. Physiol Zool 64: 1217-1233.
Kacelnick, A. & Cuthill, I. 1990. Central place foraging in starlings (Sturnus vulgaris). II. Food allocation to chicks. J. Anim. Ecol. 59: 655-674.
King, J.R. 1972. Adaptive periodic fat storage by birds. Proceed. 15th Int. Orntihol. Congr., Leiden, Netherlands: 200-217.
Lessells, C.M. & Stephens, D.W. 1983. Central place foraging: single prey again. Anim. Behav. 31: 238-243.
Lima, S.L. 1986. Predation risk and unpredictable feeding conditions: determinant of body mass in birds. Ecology 67: 377-385.
Lorentsen, S.H. 1996. Regulation of food provisioning in the Antarctic Petrel Thalassoica antarctica. J. Anim. Ecol. 65: 381-388.
MacNamara, J.M. & Houson, A.I. 1996. State dependent life histories. Nature 380: 215-221.
Norberg, B.A. 1981. Temporary weight decrease in breeding birds may result in more fledged young. Am. Nat. 118: 838-850.
Olsson, O. 1997. Clutch abandonment: a state-dependent decision in King Penguins. J. Avian Biol. 28:264-267.
Partridge, L. & Harvey, P. 1988. The ecological context of life history evolution. Science 241: 1449-1455.
Pennycuick, C.J. 1975. Mechanics of flight. In: Avian Biology, Vol. 5, (Farner, D.S. & King, J.R.), Academic Press, New York, pp 1-75.
Pennycuick, C.J. 1989. Bird flight performance. A practical calculation manual. Oxford University Press, Oxford.
Pennycuick, C.J., Croxall, J.P. & Prince, P.A. 1984. Scaling of foraging radius and growth rate in petrels and albatrosses (Procellariiformes). Ornis Scandinavica 15: 145-154.
Ricklefs, R.E. 1992. The role of parent and chick in determining feeding rates in Leach's Storm Petrels. Anim. Behav. 43: 895-906.
Robin, J.P., Boucontet, L., Chillet, P. & Groscolas, R. 1998. Behavioral changes in fasting emperor penguins: evidence for a 'refeeding signal' linked to a metabolic shift. Am. J. Physiol. 274: R746-R753.
Rogers, C.M. 1987. Predation risk and fasting capacity: do wintering birds maintain optimal body mass? Ecology 68: 1051-1061.
Schmidt-Nielsen, K. 1984. Scaling: why is animal size so important? Cambridge Univ. Press, Cambridge UK.
Stearns, S.C. 1992. The evolution of life histories. Oxford, Oxford University Press.
Tuomi, J., Hakala, T. & Haukioja, E. 1983. Alternative concepts of reproductive effort, costs of reproduction, and selection in life-history evolution. Amer. Zool. 23: 25-34.
Warham, J. 1990. The petrels. Their Ecology and breeding systems. Academic Press, London.
Warham, J. 1996. The behaviour, population biology and physiology of the petrels. Academic Press, San Diego.
Waugh, S., Weimerskirch, H., Cherel, Y., Shankar, U., Prince, P.A. & P.M. Sagar. Submitted. The exploitation of the marine environment by two sympatric albatrosses in the Pacific Southern ocean.
Weimerskirch, H. 1998. How can a pelagic seabird provision its chick when relying on a distant resource? Cyclic attendance, foraging decision and body condition in Sooty Shearwaters. J. Anim. Ecol. 67: 99-109.
Weimerskirch H. & Cherel Y. 1998. The feeding ecology of the Short-tailed Shearwaters: breeding in Tasmania and foraging in the Antarctic? Mar Ecol Progr Ser. 167: 261-274.
Weimerskirch, H. & Jouventin, P. 1997. Changes in population size and demographic parameters of 6 albatross species breeding in the French sub-antarctic slands. In: Robertson, G. & Gales, R. (eds) Albatross Biology and Conservation. Surrey Beatty & Sons, Sydney, Australia: 84-91
Weimerskirch, H., Chastel, O., Chaurand, T., Ackerman, L., Hindermeyer, X. & Judas, J. 1994. Alternate long and short foraging trips in pelagic seabird parent. Anim. Behav. 47: 472-476.
Weimerskirch, H., Chastel, O. & Ackermann, L. 1995. Adjustment of parental effort to manipulated feeding ability in a pelagic seabird, the Thin-billed Prion Pachyptila belcheri. Behav. Ecol. Sociobiol. 36: 11-16.
Weimerskirch, H., Cherel, Y., Cuenot Chaillet, F. & Ridoux, V. 1997a. Alternative foraging strategies and resource allocation by male and female Wandering Albatrosses. Ecology 78: 2051-2063.
Weimerskirch, H., Mougey, T. & Hindermeyer, X. 1997b. Foraging and provisioning strategies of Black-browed Albatrosses in relation to the requirements of the chick: natural variation and experimental study. Behav. Ecol. 8: 635-643.
Weimerskirch, H., Zimmermann, L. & Prince, P.A. submitted. Annual and seasonal variation in provisioning parameters and adult mass in Yellow-nosed Albatrosses: flexible foraging effort in a variable environment?
Weimerskirch, H. & Lys, P. in press a. Parental care in the wandering albatross: sex differences in growth and survival of chicks in relation to provisioning behavior of male and female parents.
Williams, G.C. 1966. Natural selection, the costs of reproduction and a refinement of Lack's principle. Am. Nat. 100: 687-690.
Witter, M.S. & Cuthill, I.C. 1993. The ecological costs of avian storage. Phil. Trans. R. Soc. Lond. B 340: 73-92.
Fig. 1. Changes in the average body mass (black circles, the bar indicates the size of meals) of Blue Petrels throughout the breeding season, and threshold values

Fig. 2. Relationship between body size, meal mass, working mass and maximum mass in several species of petrels and albatrosses. The dotted line indicates the lower threshold mass (BLP Blue Petrel, TBP Thin-billed Prion, CDP Common Diving Petrel, SNP Snow Petrel, GWP Great-winged Petrel, YNA Yellow-nosed Albatross, MWA and FWA male and female Wandering Albatross, SOS Sooty Shearwater, STS Short-tailed Shearwater, WCP White-chinned Petrel, BBAk or c Black-browed Albatross at Kerguelen or Campbell, LMSA Light-mantled Sooty Albatross, SOA Sooty Albatross).
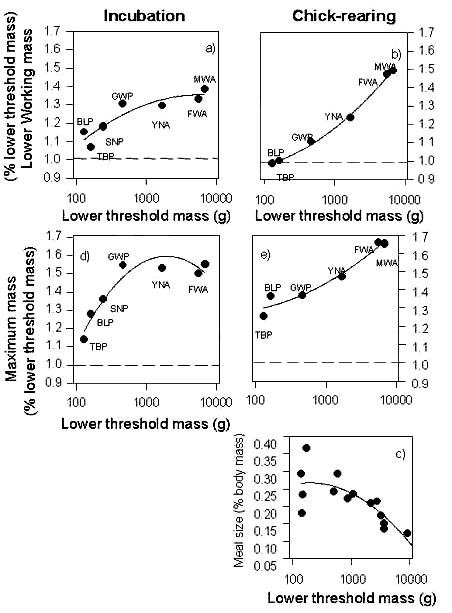
Fig. 3. Distribution of the duration of foraging trips in several species of albatrosses and petrels (short trips in black, long trips in grey) and relationship between the foraging range and the percentage of time spent in long trips, the duration of long trips and the mass of adults.
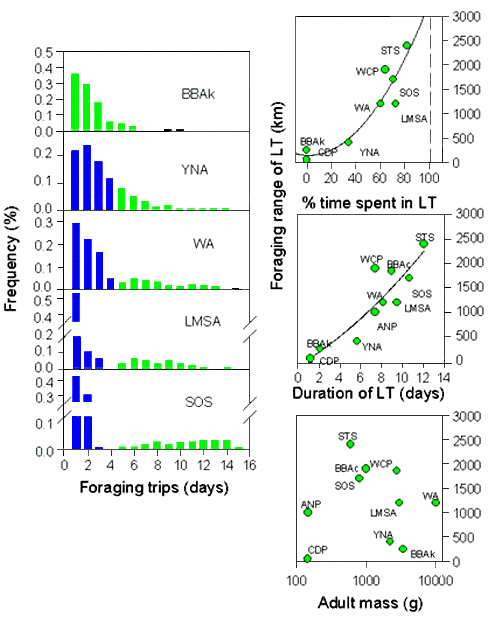
Fig. 4. Change in body mass (circle) and meal size (bar) in relation to the lower threshold mass in a thin-billed prion provisioning its chick