S20.1: Causes and consequences of some reproductive choices by Short-tailed Shearwaters Puffinus tenuirostris
R.D. Wooller & J.S. Bradley Biological Sciences, Murdoch University, Western Australia 6150, Australia, fax 61 9 9360 6303, e-mail bradley@central.murdoch.edu.au Wooller, R.D. & Bradley, J.S. 1999. Causes and consequences of some reproductive choices by Short-tailed Shearwaters Puffinus tenuirostris. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1155-1161. Johannesburg: BirdLife South Africa.A fifty year study of a population of individually marked shearwaters has revealed no higher reproductive success in natal than in non-philopatric recruits, a slightly lower initial, but not overall, reproductive success in individuals breeding first at younger ages, and that pairs with lower reproductive success are more prone to subsequent divorce. In such ways we have attempted to evaluate the causes and consequences of decisions about where to breed, when to breed first, how frequently to breed, with whom to breed and when to take a new partner. We have also shown that the feeding regimes of nestlings are determined by parents and have assessed parental decisions about how often to feed young, and where, and how much. We suggest that many of these choices stem from the need for synchronisation between partners due to long absences from the nest-site associated with distant foraging areas. These decisions may also represent risk-minimisation strategies in the face of substantial stochastic variation in feeding conditions.
INTRODUCTION AND METHODS
The Short-tailed Shearwater Puffinus tenuirostris is a medium-sized (500g), burrow-nesting procellariiform seabird. The 23 million Short-tailed Shearwaters that breed annually in south-eastern Australia, mainly on islands around Tasmania, represent three-quarters of all the seabirds that breed around continental Australia (Ross et al. 1995). They are transequatorial migrants that spend the southern hemisphere winter in northern Pacific waters returning to their colonies of up to three million pairs about September/October and laying in late November; the single young leaves about April/May.
A small colony of 100-200 shearwaters on Fisher Island in Bass Strait has been studied annually since 1947. All individuals have been identified reliably since 1950 and their mates and reproductive performance recorded each year. Young banded as nestlings that later returned to their natal colony to breed have provided up to half the breeding population and represent a known-age component of the population. Thus, it has been possible to examine the influence of both chronological age and breeding experience upon the reproductive performances of these individuals over about fifty years (Bradley et al. 1991).
Of shearwaters that have completed their reproductive careers, 27% produced no young and 71% produced no offspring that returned to Fisher Island to breed (Wooller et al. 1988). Only 8% of these individuals produced over half of the young that later bred on Fisher Island and 26% of them produced all known reproducing offspring. If philopatry is widespread, a relatively small proportion of the breeding population thus appears responsible for much of the next generation of shearwaters. These successful individuals tend to be long-lived but have proved difficult to characterise otherwise. Short-tailed Shearwaters are very similar morphologically (Meathrel et al. 1993a). The more successful birds did not differ significantly in size from less successful individuals, although weight varies seasonally in all shearwaters. Ectoparasites and blood parasites are few and disease rare. No harvesting of young or grazing/trampling by domestic animals occurs on Fisher Island and predators have been absent until recent years.
RESULTS AND DISCUSSION
Where to breed?
Although the Fisher Island breeding population contains a large number of individuals born there, overall only 14% of all fledglings banded on the island returned there to breed. A further 27% were known to have returned to Fisher Island during their pre-breeding years but did not join the breeding population there. Some shearwaters banded as fledglings on Fisher Island were later located breeding elsewhere, mostly on adjacent islands, but no individual that has bred on Fisher Island is known also to have bred elsewhere (Wooller et al. 1990). Fledgling translocation has indicated that the fidelity of returning young to their natal colony (natal philopatry) is very locality specific in Short-tailed Shearwaters and appears to develop between hatching and the time that nestlings are abandoned by their parents (Serventy et al. 1989).
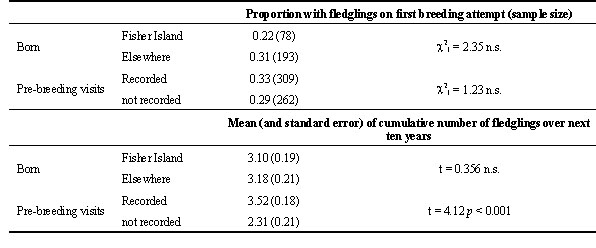
Since natal philopatry, although strong, is not complete, it is difficult to evaluate the proportion of birds from Fisher Island that survive to breed elsewhere (Gunn et al. 1998). Those that return to Fisher Island included a slight, but significant, preponderance of males (Wooller et al. 1989). There was no significant difference between the initial or subsequent reproductive success of natal and non-natal recruits (Table 1). However, whilst no difference occurred for the initial breeding attempt, there was a significant difference in the number of fledglings produced over the subsequent ten years between those birds recorded as visiting the colony before starting to breed there and those that did not (Table 1).
Presumably individuals must weigh the advantages of familiarity with the physical and social environment of an area, as well as its surrounding foraging grounds, against competition for resources and potentially deleterious inbreeding at their natal area. Burrows do not appear to be in short supply at any colony; nevertheless, larger colonies or higher density nesting areas may be more attractive. Possibly familiarity gained by prospecting in the years just prior to breeding confers advantages in reproductive output over a locale as restricted as Fisher Island whereas any familiarity gained as a nestling many years earlier may not. Also, individuals of higher quality may spend more time at the colony prospecting prior to breeding and were therefore more likely to be detected; these individuals would subsequently have a higher reproductive success.
When to breed?
In common with other procellariiforms, all Short-tailed Shearwaters lay a single egg that is not replaced if lost. Almost all eggs are laid within one week in late November throughout the species’ range (Meathrel et al. 1993b) so that seasonality of laying and clutch-size do not vary. On average, females lay eggs of increasing breadth (and hence volume) over about the first five seasons that they breed (Fig. 1; Serventy 1967); thereafter, egg dimensions change little but individuals lay eggs of very similar size each year that they breed. Although eggs differ in size by up to 40%, there is no significant association between the size of a female and the size of her egg, nor is the probability of hatching related to egg size (Meathrel et al. 1993b). Rather, exchanges between nests of eggs of different sizes have shown that breeding success is attributable to differences in parental care.
Young shearwaters often return to breeding colonies in the years before they breed for the first time at four to 15 years old; most start breeding at seven or eight years (Wooller et al. 1989). There is no suggestion that offspring of known-age parents commence breeding at an age similar to their parents (r = -0.02, n.s.), although inevitably sample sizes are small (Table 2). Individuals that start breeding at the younger ages produce fewer offspring during their first breeding attempt than those that commence when chronologically older (Wooller et al. 1990). Nonetheless, on average, early starters accumulate more total offspring during their longer breeding careers than those that delay until older ( Fig. 2).
Thus, although our data show some support for the idea that reproductive competence improves with increasing chronological age among first-breeders, we have no evidence that individuals starting early suffer consequences in terms of reduced survival. Rather, individuals that bred successfully early in their reproductive careers, whatever their chronological ages, lived longer than those that bred less successfully. A similar variation in the quality of birds has been noted in Kittiwake Gulls Rissa tridactyla (Coulson & Porter 1985), such that long-lived individuals were more productive at all stages of their breeding lifespan than those that bred only a few times. Thus, shearwaters appear to breed first at whatever age they feel able.
How often to breed?Once they have started to breed, not all individuals breed on Fisher Island every year. Checks of other islands suggest that it is improbable that these birds bred elsewhere. Most (76%) absences lasted only one year, 18% two years, 2-3% for three or four years, 1% for 5 years and none were absent more than six years (Bradley et al. 1989). Absences were more frequent during the early years of an individual’s career and became more frequent again in older birds (Fig. 3), a pattern that parallels the change in reproductive success (measured as proportion of eggs yielding fledglings) with age ( Wooller et al. 1990). Absences are slightly more common among males (11% all breeding opportunities) than females (10%) overall (Table 3; Wooller et al. 1990). The percentages of individuals known to have bred before but recorded at the colony without an egg were also highest among less experienced birds and higher in males (Table 3).
Non-breeding by adults may be widespread among long-lived seabirds but is difficult to detect without detailed, long-term studies of marked individuals. Among the Short-tailed Shearwaters studied it varied from 24% in experienced females to 43% in young males. Kittiwakes also show fewer absences among older breeding birds (Wooller & Coulson 1977). This may indicate that the first or early breeding attempts have a greater impact than later attempts or that younger birds are less prone to jeopardise by reproduction their potentially greater breeding lifespan than older individuals, or both. Young seabirds commonly have a lower breeding success than more experienced individuals so that loss of reproductive output during the early years may be offset by greater success during the longer breeding lifespan that may result from deferral of breeding. Essentially, the same factors that result in a delay before breeding for the first time may also operate in delaying the second or third attempt.
One possible explanation for the decrease in absences with increasing age is that birds with a higher frequency of absences also had a lower survival and were thus selected out of the cohort. However, when absences are examined in a group of birds that survived at least twelve years after starting to breed, the same decreasing frequency is apparent, indicating a change in the behaviour of individuals with age. The cumulative total of fledglings produced is reduced with increasing absences (Fig. 4), not surprisingly since the seasons available to produce offspring are thereby reduced. If absence represents a risk minimisation strategy to conserve resources for future breeding attempts, we might then expect that birds with absences would have a higher success in those years when they do attempt to breed, thereby compensating for their reduced number of attempts. However, there was a significant (regression t = -3.499, p = 0.001) decrease in the proportion of eggs producing fledglings in those birds with a higher number of absences (Fig. 5). This strongly suggests that intermittent breeding is characteristic of birds with a lower competence in incubation or chick rearing. The question remains as to whether this reduced efficiency is innate to the birds or, rather, is characteristic of the pair bonds in which they find themselves.
Those individuals absent more often were also less likely to be recorded with the same mate during those years that they attended the colony (Fig.5 ). Thus, intermittent breeding appears associated with a reduced ability to acquire a mate and raise offspring rather than representing a trade-off between continual reproduction and reduced breeding lifespan. Again, it appears that individuals of higher quality are able to breed more frequently than others without any compensatory reduction in their annual breeding success or overall breeding lifespan.
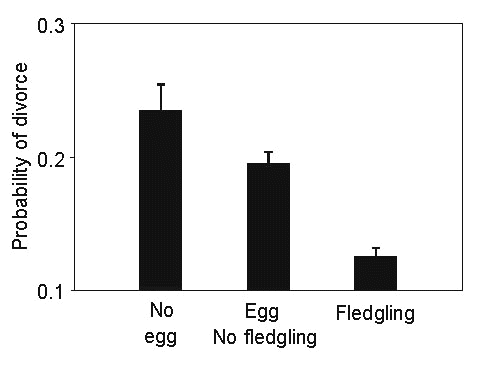
With whom to breed?Among breeding Short-tailed Shearwaters, 30% had mates with identical breeding experience and 60% had mates whose breeding experience differed from theirs by two years or less (Bradley et al. 1995). In part, this situation results from prolonged pair-bonds between the same experienced individuals and, partly, from the large number of inexperienced individuals available as potential mates (Wooller & Bradley 1996). Nonetheless, weak assortative mating based on breeding experience does occur (Bradley et al. 1990; Wooller & Bradley 1996). Divorce was more likely if a pair failed to produce young during the preceding season (Fig. 6) or in pairs where one partner had been absent in some breeding season (Bradley et al. 1990; Fig. 5). Divorce had a cost in terms of a lower reproductive success by newly-established pairs but this may have been offset by acquisition of a partner that was more behaviourally compatible or more vigorous or healthy than the previous mate. Thereafter, reproductive success increased in sequential years as a pair remained together, except in older birds whose success was already high. This increasing reproductive success with increasing mate familiarity was additional to an increase in reproductive success associated with cumulative breeding experience overall.
Thus, long-lived individuals often associate early and accumulate substantial reproductive output during their lifetimes together. Birds of higher quality may have a higher reproductive success early in life than shorter-lived individuals and this may allow potential partners to evaluate each others ability. Divorce provides a mechanism for reassortment but carries a short-term cost that must be offset against the potential long-term gain.
Feeding regimes of nestlings
For a few days after hatching, Short-tailed Shearwater nestlings are guarded by at least one parent and fed daily for the first ten days. Like most petrels, they are then left in their burrow for 90-100 days until fledging. The nestling peaks at 140-150% of the weight of an adult about 50-70 days after hatching but is not fed for the last two weeks of burrow life and fledges at little more than adult weight (Lill & Baldwin 1983; Hamer et al. 1997). Between 10 days and fledging, less than half of the nestlings, on average, were fed each night and intervals between feeds could be as long as 13 nights. The variability in feeding frequency, as well as the interval between feeds, are greater than in other species of shearwater (Hamer et al. 1997).
One explanation for such variable and long absences by parents is that they feed on prey taken in Antarctic waters (Skira 1986). There is increasing evidence that substantial numbers of Short-tailed Shearwaters feed at latitudes from 50 to 65o S, a long way south and west of their breeding colonies around Tasmania (Kerry et al. 1983; Veit & Hunt 1991). Satellite-tracking of breeding individuals has confirmed these movements, which coincide with the known distribution of high densities of Krill Euphausia superba (Nicholls et al. 1998; N.I. Klomp. pers comm.).
Shearwaters are very efficient fliers (Spear & Ainley 1997a & b) and have been documented moving more than 1000 km in less than 24 hours (Nicholls et al.1998). Nonetheless, such long-distance travel to rich food sources may carry risks for both parents and nestlings. Weimerskirch (1998) has proposed that shearwaters exploit distant food sources during long absences from the nest, then return to feed their young from local sources, often squid, on several successive nights before returning to the distant source. Parents are thought to gain body condition in Antarctic waters before returning to feed the nestling stomach oil rich in energy. However, parents may lose condition during the time that they feed their young, rather than themselves, from local sources, often squid. In contrast, the nestling is likely to grow most actively as a consequence of these consecutive days of local feeding.
Thus, parents must balance the need to sustain their own body condition against their considerable investment in their single slow-growing young. More experienced and better quality parents, often paired, may be faster or more efficient at travelling to and from distant sources, or may be better able to locate and exploit the rich food sources once there. Any subsequent reduction in the interval between long (distant) and short (local) absences would enhance nestling growth during the cyclic alternation of increase and decrease in chick body mass. Even if both parents feed their young independently, any reduction in long absences will reduce the risk of a nestling remaining unfed so long that it becomes inviable. Successful parents may have feeding schedules that result in young being fed not only as often as possible but also as regularly as possible. They may, indeed, be able to remain at the colony feeding their young each night from local sources for longer than less successful parents. It would be necessary to monitor the feeding regimes of individuals whose breeding histories are known to test this proposition.
If alternation of long and short intervals between parental visits to their nestling is a result of feeding at distant food sources, then tropical shearwaters, too far from rich, high latitude waters should not show this pattern. We have recently started to study the Wedge-tailed Shearwater Puffinus pacificus, a more tropical species and the second most abundant seabird in Australia. Although only 60% of the body mass of the Short-tailed Shearwater, the Wedge-tailed Shearwater has a wing area 150% of its congener. In consequence, Wedge-tailed Shearwaters have an aspect ratio 80% and a wing loading only 40% of that in Short-tailed Shearwaters and feed much more at the surface rather than underwater. Wedge-tailed Shearwaters thus appear to be efficient harvesters of low density food, probably in the vicinity of their colonies, and are not long-distance migrants (Marchant & Higgins 1990).
Our preliminary data (Nicholson et al. 1998) indicate that Wedge-tailed Shearwaters feed their young more frequent but smaller meals than Short-tailed Shearwaters. Wedge-tailed Shearwaters were also markedly less variable in their feeding intervals than Short-tailed Shearwaters and continued to feed their young until it fledged. Similar patterns of chick provisioning were documented for Wedge-tailed Shearwaters on the Hawaiian islands by Pettit et al. (1984). Thus, 66% of young Wedge-tailed Shearwaters were fed each night (Pettit et al. 1984) in contrast to only 49% of Short-tailed Shearwater nestlings (Hamer et al. 1997). On average, Short-tailed Shearwaters delivered 140 g meals every 1.3 days, whereas Wedge-tailed Shearwaters delivered 42 g meals every 0.9 days (Nicholson et al., 1998). Short-tailed Shearwater nestlings peak at 140-150% adult weight around 50-70 days after hatching compared with 128% adult weight about 72-87 days after hatching for Wedge-tailed Shearwater young (ibid). These differences in provisioning rates and growth patterns of young presumably reflect the additional distant source of food used by the Short-tailed Shearwater. It is not yet clear whether differences between other aspects of the life histories of these congeneric species (e.g. in survival rates or divorce rates) also reflect these differences in foraging.
CONCLUSION
Our studies have shown that the reproductive output of Short-tailed Shearwaters improves with their breeding experience before declining in older birds towards the end of their reproductive career. However, we have found little evidence of extensive trade-offs between current and future reproductive output and subsequent survival, at least during the earlier part of an individual’s career. For instance, no penalty appears incurred by starting to breed relatively young or breeding in most years thereafter - indeed, there is some overall advantage in doing both. Rather, we have found that some individuals are characterised from the outset by a suite of associated traits, such as longer reproductive careers during which they are absent and change mates less often and manifest a high annual reproductive success. In an environment dominated by substantial stochastic variation, especially during the crucial chick-feeding phase, such individuals may be better able to surmount the difficulties presented. The role of trade-offs, even in such long-lived iteroparous birds may be largely reduced to risk minimisation in the face of unforcastable, chance processes, especially once the first breeding years have passed.
Whilst our study is based on an unmanipulated population, and thus the absence of substantial trade-offs cannot be demonstrated by experiment, a number of unmanipulated bird studies have demonstrated significant negative correlations between reproductive effort and subsequent survival (see Stearns, 1992). In addition, Bradley et al.(1989) demonstrated such a significant negative correlation in this species between reproductive output in the first 15 years of the breeding career and survival subsequent to this, indicating the existence of such a trade-off in the later breeding careers of long-lived individuals; in the earlier years of breeding the correlation was positive. Thus, if substantial trade-offs occurred in short-tailed shearwaters, we feel that our study would have provided more evidence for them than it has done.
REFERENCES
Bradley, J.S., Wooller, R.D., Skira, I.J. & Serventy, D.L. 1989. Age-dependent survival of breeding Short-tailed Shearwaters Puffinus tenuirostris. Journal of Animal Ecology 58 : 175-188.
Bradley, J.S., Wooller, R.D., Skira, I.J. & Serventy, D.L. 1990. The influence of mate retention and divorce upon reproductive success in short-tailed shearwaters Puffinus tenuirostris. Journal of Animal Ecology 59 : 487-496.
Bradley, J.S., Skira, I.J. & Wooller, R.D. 1991. A long-term study of Short-tailed Shearwaters Puffinus tenuirostris on Fisher Island, Australia. Ibis 133 : 55-61.
Bradley, J.S., Wooller, R.D. & Skira, I.J. 1995. The relationship of pair-bond formation and duration to reproductive success in short-tailed shearwaters Puffinus tenuirostris. Journal of Animal Ecology 64 : 31-38.
Coulson, J.C. & Porter, J.M. 1985. Reproductive success of the Kittiwake Rissa tridactyla : the roles of clutch size, chick growth rates and parental quality. Ibis 127 : 450-466.
Gunn, B.M., Skira, I.J., Bradley, J.S. & Wooller, R.D. 1998. Age-dependent prospecting and recruitment to a breeding population of Short-tailed Shearwaters Puffinus tenuirostris. Ibis (in press)
Hamer, K.C., Nicholson, L.W., Hill, J.K., Wooller, R.D. & Bradley, J.S. 1997. Nestling obesity in procellariiform seabirds - temporal and stochastic variation in provisioning and growth of Short-tailed Shearwaters Puffinus tenuirostris. Oecologia 112 : 4-11.
Kerry, K.R., Horne, R.S.C. & Dorward, D.F. 1983. Records of the Short-tailed Shearwater Puffinus tenuirostris in Antarctic waters. Emu 83 : 35-37.
Lill, A. & Baldwin, J. 1983. Mass changes and the mode of depot fat accumulation in migratory Short-tailed Shearwaters. Australian Journal of Zoology 31 : 891-902.
Meathrel, C.E., Skira, I.J., Bradley, J.S. & Wooller, R.D. 1993a. The influence of egg-size, mass and composition upon hatching success in the Short-tailed Shearwater Puffinus tenuirostris. Journal of Zoology, London 230 : 679-686.
Meathrel, C.E., Bradley, J.S., Wooller, R.D. & Skira, I.J. 1993b. The effect of parental condition on egg-size and reproductive success in Short-tailed Shearwaters Puffinus tenuirostris. Oecologia 93 : 162-164.
Nicholls, D.G., Stampton, P., Klomp, N.I. & Schultz, M. 1998. Post-breeding flight to Antarctic waters by a Short-tailed Shearwater Puffinus tenuirostris. Emu 98 : 79-82.
Nicholson, L.M., Nicholson A.B. & Bradley J.S. 1998. Automatic measurement of chick provisioning rates in wedge-tailed shearwaters Puffinus pacificus using an integrated ultrasonic burrow monitoring system. Poster abstract. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban. Ostrich 69: 330.
Pettit, T.N., Byrd, G.V., Whittow, G.C. & Seki, M.P. 1984. Growth of the Wedge-tailed Shearwater in the Hawaiian islands. Auk 101 : 103-109.
Ross, G.J.B., Burbidge, A.A., Brothers, N., Canty, P., Dann, P., Fuller, P.J., Kerry, K.R., Norman, F.I., Menkhorst, P.W., Pemberton, D., Shaughnessy, G., Shaughnessey, P.D., Smith, G.C., Stokes, T. and Tranter, J. 1995. The status of Australia’s seabirds. In: Zann, L. and Kailola, P. (eds). Technical Annex 1, State of the Marine Environment Report of Australia. Great Barrier Reef Marine Park Authority, Townsville : 167-182.
Serventy, D.L. 1967. Aspects of the population ecology of the Short-tailed Shearwater. Proceedings of the 14th International Ornithological Congress : 165-190.
Serventy, D.L., Gunn, B.M., Skira, I.J., Bradley, J.S. & Wooller, R.D. 1989. Fledgling translocation and philopatry in a seabird. Oecologia 81 : 428-429.
Skira, I.J. 1986. Food of the Short-tailed Shearwater, Puffinus tenuirostris, In Tasmania. Australian Wildlife Research 13 : 481-488.
Spear, L.B. & Ainley, D.G. 1997a. Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis 139 : 221-233.
Spear, L.B. & Ainley, D.G. 1997b. Flight speed of seabirds in relation to wind speed and direction. Ibis 139 : 234-251.
Stearns, S.C. 1992. The evolution of life histories. Oxford; Oxford University Press 249pp
Veit, R.R. & Hunt, G.L. 1991. Broadscale density and aggregation of pelagic birds from a circumnavigational survey of the Antarctic Ocean. Auk 108 : 790-800..
Weimerskirch, H. 1998. How can a pelagic seabird provision its chick when relying on a distant food reserve? Cyclic attendance at the colony, foraging decision and body condition in sooty shearwaters. Journal of Animal Ecology 67 : 99-109.
Wooller, R.D. & Coulson, J.C. 1977. Factors affecting the age of first breeding of the Kittiwake Rissa tridactyla. Ibis 119 : 339-349.
Wooller, R.D., Bradley, J.S., Skira, I.J. & Serventy, D.L. 1988. Factors contributing to reproductive success in Short-tailed Shearwaters (Puffinus tenuirostris) In: Ouellet, H. (ed) Proceedings of the 19th International Ornithological Congress. University of Ottawa Press, Canada : 848-856.
Wooller, R.D., Bradley, J.S., Skira, I.J. & Serventy, D.L. 1989. Short-tailed Shearwater. In: Newton, I. (ed) Lifetime reproduction in birds. London; Academic Press : 405-417.
Wooller, R.D., Bradley, J.S., Skira, I.J. & Serventy, D.L. 1990. Reproductive success of short-tailed shearwaters Puffinus tenuirostris in relation to their age and breeding experience. Journal of Animal Ecology 59:161-170.
Wooller, R. & Bradley, S. 1996. Monogamy in a long-lived seabird : the Short-tailed Shearwater. In: Black, J. (ed) Partnerships in birds. Oxford University Press: 233-234.
Table 1. Comparison of initial and subsequent breeding success between birds fledged from Fisher I. and others, and between birds recorded as making prebreeding visits to Fisher I. and others. Subsequent reproductive success was measured by the cumulative number of fledglings produced ten years after the commencement of breeding, irrespective of how long the bird survived. Birds that commenced breeding after 1984 were excluded.

Table 2. Age at which breeding commenced for parents and for their returning progeny. The start of breeding is defined as the first observation with an egg.
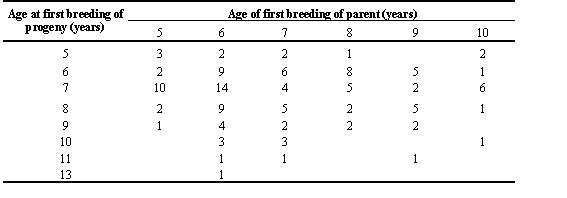
Table 3. Non-attendance and non-breeding by male and female Short-tailed Shearwaters on Fisher Island, Tasmania.
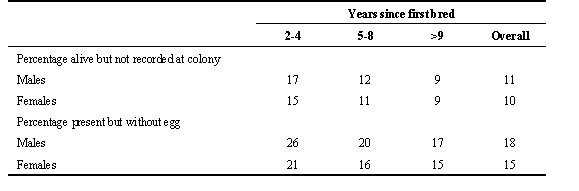
Fig 1. Change in egg breadth with the increasing age of the female since first breeding.
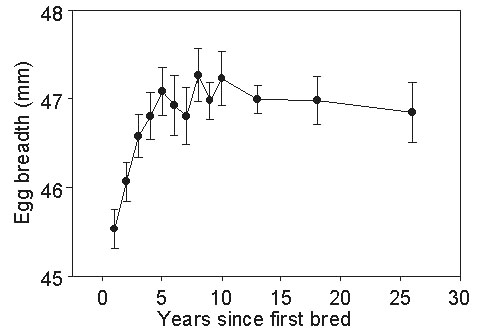
Fig 2. Reproductive success of known-age birds in relation to their age at first breeding. The circles represent the proportion of eggs yielding fledglings at the time of first breeding. The triangles represent the mean cumulative number of fledglings 12 years after the bird's birth date, and the squares the same value 20 years after the birth date. To be included, individuals survived to breed but were not required to have survived 12 or 20 years.
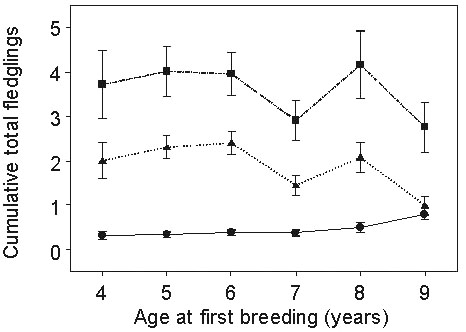
Fig 3. The change in proportion of birds attending the colony with increasing time since first-breeding.
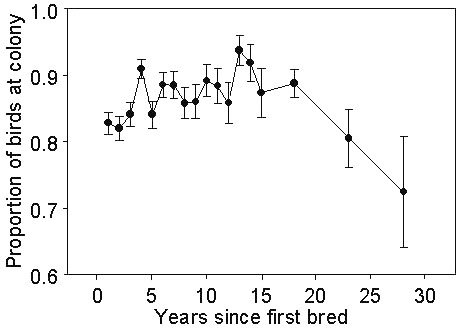
Fig 4. The change in mean cumulative reproductive success (circles represent number of eggs, squares numbers of fledglings) with increasing numbers of years absent in birds that survived twelve years from commencement of breeding. The results of the first breeding occasion are omitted since, by definition, first breeding birds must be associated with an egg.
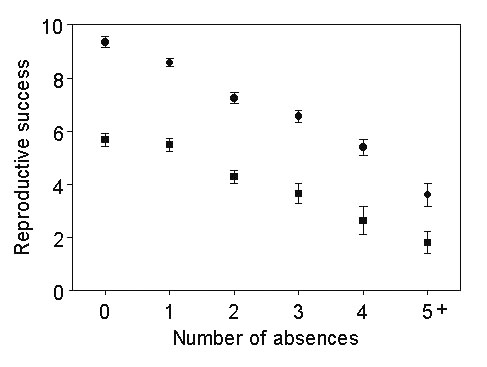
Fig 5. The change in the proportion of occasions an egg produced a fledgling (squares, lower line), or a bird retains the same mate (circles, upper line), with increasing numbers of years absent. The birds included have survived twelve years from commencement of breeding.
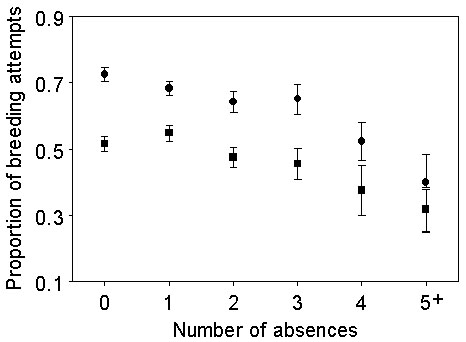
Fig 6. The change in probability of divorce (defined as the proportion of occasions a bird mates with a different individual than its previous mate, when the previous mate also remates with another individual before the death of the original bird), with differing outcomes from the previous breeding attempt.