S19.5: Species-specific sexual plumage: Species-isolating mechanisms or sexually selected ornaments?
Ian P. F. Owens & Sonya M. Clegg
Department of Zoology, University of Queensland, Brisbane, Queensland 4072, Australia, fax 61 7 3365 1655, e-mail iowens@zoology.uq.edu.au
Owens, I.P.F. & Clegg, S.M. 1999. Species-specific sexual plumage: Species-isolating mechanisms or sexually selected ornaments? In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1141-1153. Johannesburg: BirdLife South Africa.Why is there so much variation among species in the form of sexual plumage? The 'isolating mechanisms explanation' suggests that differences between species in sexually dichromatic plumage act as species-specific signals that allow individuals to recognise conspecifics and thereby avoid hybridisation. This view makes three predictions; that species-specific sexual plumage will be most developed in populations living in sympatry with closely-related species; that species-specific sexual plumage will break down in island-dwelling races that are isolated from closely-related species; and that species-specific sexual plumage will be very stereotyped, varying little between individuals of a species. Our comparative analyses do not support this explanation. First, species-specific sexual plumage is not more developed in populations living in sympatry with closely-related species. Indeed, sympatric pairs of populations tend to have more similar, not less similar, sexual plumage then expected by chance, probably because they often share a similar signalling environment. Second, island-dwelling forms are often more dichromatic than their mainland counterparts. Finally, sexual plumage is significantly more variable than other traits. Together, these results refute the idea that species-specific sexual plumage is, in general, a consequence of selection for species-isolation. Hence, we suggest that a few well-publicised examples of potential isolating mechanisms have clouded the overall picture that variation between bird species in the form of sexual plumage arises through variation in the form of sexual selection.
INTRODUCTION
‘Loss of sexual dimorphism...seems to occur only in localities where no other similar species exist, i.e., where a highly specific male plumage is not needed as a biological isolating mechanism between two species.’ (Ernst Mayr 1942).
‘Species isolation was over-emphasised as an explanation for secondary sex traits and mate choice during the modern synthesis of evolution... It is now clear that such an explanation is not sufficient, but it took several decades before renewed interest in sexual selection turned up important problems that had been swept under the species isolation carpet.’ (Malte Andersson 1994).
Why are there so many different forms of sexually dimorphic plumage among birds? The variation among groups is both striking and bewildering: Compare the elaborate plumes of a Peacock Parvo cristatus with the black throat patch of a male House Sparrow Passer domesticus, the white forehead patch of a male Collared Flycatcher Ficedula albicollis or the red cheeks and black chest band of a male Zebra Finch Taeniopygia guttata. Even within genera it is extremely unusual to find two species showing exactly the same form of plumage dichromatism - compare, for instance, the black belly stripe of the Great Tit Parus major with the ultra-violet crest of the Blue Tit P.caeruleus, or the black cap and chest band of the White-fronted Chat Epthianura albifrons with the red chest and cheek-patches of the Crimson Chat E. tricolor. Although a huge amount of work has been done in the last fifteen years on the function of sexual ornaments in particular species, and a fair amount of research has been directed towards explaining variation in the extent of dimorphism among species, very little effort has been devoted to explaining variation in the form of sexual dimorphism between species (Andersson 1994).
Adaptive explanations for variation in the form of sexual dimorphism, or sexual ornaments, can be divided broadly into two types. The first type of explanation stresses the idea that species-specific plumage characters are important in species-recognition and/or species-isolation. Under this scenario it is the differences between species per se that are adaptive because species-specific plumage characters allow individuals to recognise members of their own species and thereby avoid hybridisation. The second type of explanation, on the other hand, emphasises the role of sexual selection acting within species. Here, the variation among species in sexual plumage is simply a by-product of variation between species in the form of sexual selection. For instance, different species may develop different plumage ‘signals’ because they live in different light environments, or because they are signalling different sorts of information, or because of different pre-existing biases in signal perception (Owens & Hartley 1998; Price 1998).
These two types of adaptive explanation for variation among species in the form of sexual ornaments have been around for over a century (Sibley 1957; Ryan & Rand 1993; Peterson 1997). The first type of explanation - based on species recognition and/or isolation - was initially championed by Wallace (1889) and subsequently became a central concept in the Modern Synthesis view of evolution (eg, Dobzhansky 1937; Huxley 1942; Mayr 1942, 1963). Some recent work has, however, criticised the architects of the Modern Synthesis for over-emphasising the role of species isolating mechanisms at the expense of sexual selection within populations (e.g., West-Eberhard 1983; Andersson 1994). This line of reasoning leads to the second type of explanation - based on sexual selection - and is the one initially favoured by Darwin (1871). But most recently, and most explicitly, it has been developed by Zahavi & Zahavi (1997) who ridicule ‘the fallacy of species-specific signals’ as an artefact of anthropomorphic interpretation. The Zahavis use three lines of evidence to support this view. Firstly, they suggest that individual birds can easily recognise conspecifics by their overall ‘jizz’ without the need for specific recognition cues. Second, that many putative recognition cues - such as the colour of bills and eyes - are far too variable among individuals to be used in crude species recognition. And finally, that those taxonomic families with the most elaborate putative recognition cues - such as wildfowl (Anatidae), pheasants (Phasianidae), birds-of-paradise (Paradisidae) and hummingbirds (Trochilidae) - are exactly the same groups in which hybridisation is most common (see also Sibley 1957). It should be remembered, however, that this last point was fully recognised by Mayr (1942) and Lack (1968) who suggested that the high rate of hybridisation was because of the lack of a pair-bond in many species in these families - and this was why they needed such highly developed species-recognition cues. In other words, the high rate of hybridisation was the reason why such elabourate species-specific plumage had evolved in these families.
The debate over the relative importance of species-isolation type explanations versus sexual selection explanations has been intense (see Cronin 1991). Remarkably, however, there have been very few quantitative comparative tests of the relative roles of the two types of explanations. Instead, each side has tended to cite a few supportive single-species studies (but see Barraclough et al. 1995, 1998; Mitra et al. 1996; Price 1998; Møller & Cuervo 1998 for recent comparative tests of sexual selection type explanations). The overall aim of this study is, therefore, to test the power of species-isolationist types of explanation. We test three predictions. First, the sexual plumage of two closely-related species living in sympatry will be more divergent than that of two species living in allopatry because of the greater risk of hybridisation in sympatry (Dobzhansky 1940). Second, that island-dwelling forms will have less sexually dichromatic plumage characters than mainland-dwelling forms because hybridisation is less of a risk on species-depauperate islands (Mayr 1942, 1963). Finally, that there is less variation among individuals in species-specific sexually dichromatic plumage characters than there is variation among individuals in other types of plumage character because they act as stereotyped species-recognition traits (Paterson 1978, 1982). A future publication will report on a similar set of tests on predictions arising from sexual selection type explanations.
METHODS
Prediction 1: Pairs of sympatric species are more dissimilar than pairs of allopatric species
Australian birds provide an ideal opportunity for examining the effects of sympatry on divergence. Over the last few million years populations of many widely-dispersed species have been repeatedly restricted into a series of ‘refugia’ by expansions of the arid central region (reviewed in Keast 1961, 1989; Cracraft 1986; Ford 1987a,b). Such repeated sub-divisioning has led to considerable allopatric divergence among isolated populations.
Based on current taxonomies, over 100 species of Australian bird can be divided into at least two allopatric races on the basis of morphological variation (Ford 1987b). Commonly, within any one of these geographically variable species, one or more race overlaps sympatrically with another closely-related species while other races do not overlap with any con-generic species. This situation provides natural ‘matched-pairs’ of races within a single species - one of which has recently evolved in sympatry with a closely-related species (‘sympatric’ races) and one of which has recently evolved in isolation from closely-related species (‘allopatric’ races).
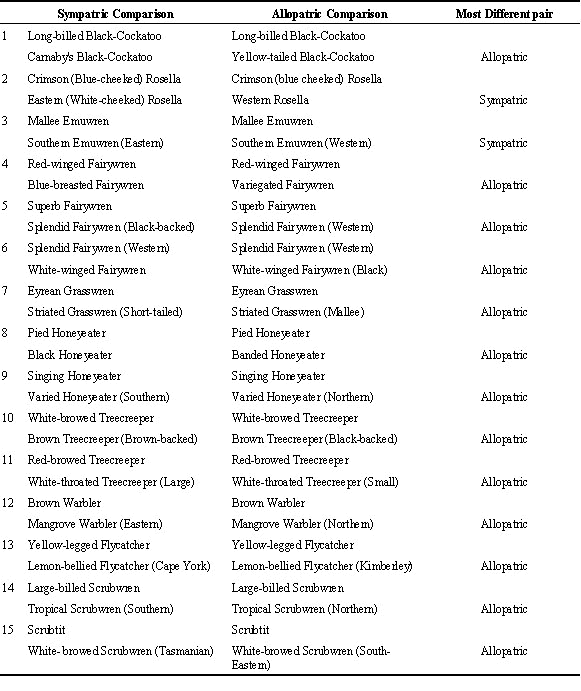
We identified 15 species or super-species which are both sexually dichromatic and had both a ‘sympatric’ and an ‘allopatric’ race with respect to a closely-related species. We then estimated the extent of overall dissimilarity between each of the two races and the closely-related species, respectively, with respect to sexually dichromatic plumage characteristics. Overall dissimilarity for each comparison between a race and a closely-related species was measured by, first, scoring the extent of dissimilarity in the dichromatic plumage of adult males in each of five body regions (0, no dissimilarity; 1, dissimilarity restricted to extent or intensity of pattern; 2 dissimilarity in overall pattern of region) and then summing the scores across all five regions. The five body regions were head, nape and throat; chest, belly and flanks; back and rump; wings; and tail.
Species-isolationist explanations predict that, for most matched pairs, the dissimilarity between the sympatric race and the closely-related species should be greater than that between the allopatric race and closely-related species. However, the null expectation is that in about half the matched pairs the sympatric race is most divergent and in about half the species the allopatric race is most divergent. We tested this null hypothesis using a two-tailed Binomial Sign test.
Prediction 2: Island-dwelling forms are less dichromatic than mainland-dwelling forms.
We tested this prediction at two different levels. First, we performed a general test between island-forms and mainland forms, then we split-up these comparisons in to different categories according to the type of island involved and analysed each category separately.
We identified 34 matched-pairs consisting of an island-dwelling form and a mainland-dwelling form. We then scored each form on a ten-point scale for sexual dichromatism (see Owens & Bennett 1994; Owens & Hartley 1998), where zero represents monochromatic plumage and ten represents maximum dichromatism. Subsequently, for each matched-pair we calculated the extent of difference between the island form and the mainland form by subtracting the plumage dichromatism score for the island form the plumage dichromatism score for the mainland score. Finally, we used a two-tailed Binomial Sign test to test the null hypothesis that it is equally likely that either the island or the mainland forms will be most divergent. We excluded those instances where the island- and mainland-forms were equally dichromatic according to our scoring method. Species-isolation type explanations predict that island forms tend to be less dichromatic.
The matched-pairs were then categorised according to whether the island in questions was a ‘Great Island’ or an ‘Oceanic Island’ (Lack 1976). Great Islands, such as the United Kingdom, Madagascar and New Zealand are isolated from the mainland but are sufficiently large to support almost the same diversity of species as the mainland itself. Oceanic Islands are small and very depauperate in terms of species diversity. Two-tailed Binomial tests were then repeated for each type of island separately. Here, species-isolationist explanations predict that island-dwelling forms of Oceanic Islands should be particularly likely to loose dichromatic plumage.
Prediction 3: Species-specific dichromatic plumage traits are unusually invariable among individuals.
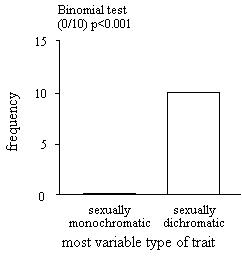
We found ten sexually dichromatic species for which the Queensland Museum held at least ten non-moulting, undamaged, adult male specimens that were collected in the last twenty years. For each species we identified a pair of comparable plumage traits - one of which was dichromatic and the other of which was putatively monochromatic. We then measured the size of each of these traits for each of the ten male specimens and subsequently calculated the coefficient of variation among individuals for each trait. Finally, for each species we calculated which trait was most variable by subtracting the coefficient of variation for the monochromatic trait from the coefficient of variation for the dimorphic trait. The null expectation was that, across species, it is equally likely that either dichromatic or the monochromatic trait should be most variable. We tested this expectation using a two-tailed Binomial test.
RESULTS
Prediction 1: Pairs of sympatric species are more dissimilar than pairs of allopatric species
In only two (13%) of the 15 matched pairs was the sympatric race most dissimilar from the closely-related species (Table 1). As predicted by the species-isolation type explanation, this pattern is significantly different from what would be expected to occur by chance (Binomial test, p < 0.01). However, the direction of the relationship is opposite from that predicted by species-isolation type explanations (Fig. 1). Hence, we found no evidence for the prediction that, in general, sympatric races should be more dissimilar from the closely-related species than allopatric races. Rather, allopatric forms are more different from one another than expected by chance.
Prediction 2: Island-dwelling forms are less dichromatic than mainland-dwelling forms.
In 10 (29%) of the 34 matched-pairs of island- and mainland-dwelling forms there was no difference between the forms in the extent of dichromatism (Table 2). Out of the remaining 25 cases, there were just 2 instances where the mainland form was more dichromatic than the island form. Hence, island-dwelling forms were less dichromatic than mainland forms in only 8% of cases where there was a difference in dichromatism (Table 2). Again, as predicted, this pattern is significantly different from what would be expected by chance (Binomial test, p < 0.005). However, the direction of the relationship is once again opposite to that predicted, with island-dwelling species being more dichromatic (Fig 2a).
When we split-up the island-mainland comparisons into Great Island and Oceanic Islands the results were qualitatively the same in each category. Thirteen of the 34 comparisons involved an Oceanic Island race, of which 6 showed the same degree of dichromatism as the mainland-form and seven showed more dichromatism than the mainland-form. In no case did an Oceanic Island-form show less dichromatism than the mainland comparison (Fig 2b). Similarly, of the 21 comparisons that involved a race from a Great Island, 4 showed the same amount of dichromatism as the mainland form, 15 were more dichromatic than the mainland-form whereas in only two cases was the island form less dichromatic than the mainland-form (Fig 2c). Hence, for both types of island, where island- and mainland-forms differ in dichromatism it is the island-form that tends to be more dichromatic (Binomial tests: Oceanic Islands, p < 0.05; Great Islands p < 0.01).
Prediction 3: Species-specific dichromatic plumage traits are unusually invariable among individuals.
In all ten species examined the sexually dichromatic plumage trait was less variable than the monochromatic trait (Table 3). This proportion is significantly different from that predicted by chance (Binomial test p < 0.01) but yet again the direction of the relationship is opposite to that predicted by the species-isolation hypothesis (Fig 3). Individuals appear more variable with respect to sexually dichromatic plumage traits than they are with respect to monochromatic traits.
DISCUSSION
Our analyses did not support the idea that variation among bird species in the form of sexual dichromatism is an adaptive consequence of selection for species-recognition or species-isolation.
Populations living in sympatry with closely-related species, and therefore vulnerable to hybridisation, did not have more distinctive sexual plumage than populations living in isolation from closely-related species. Indeed, populations living in sympatry tended to have more similar, not less similar, sexual plumage than expected by chance. Second, island-dwelling forms, living in the absence of closely-related species, did not possess less well developed sexual ornaments than mainland-dwelling forms which do face the risk of hybridisation with congeneric species. In fact, island races tended to be more dichromatic than mainland races. And finally, sexually dichromatic plumage characters were not more standardized across individuals of a species than other types of plumage character - rather, sexually dichromatic traits were significantly more variable than comparable monochromatic traits.
Given such clear results, it seems appropriate to ask why species-isolation type explanations for variation in the form of sexual ornaments were ever applied to the plumage of birds. The answer to this question is at least twofold. The most important reason must be that the Modern Synthesis’ prime challenge was to explain speciation and population divergence, rather than morphological diversity per se. Hence, for this central objective the concept of isolating mechanisms was a novel and valuable tool - which, like any new tool, was initially applied to any job that presented itself. Sexual selection was either ignored or actively dismissed (Huxley 1942; Mayr 1942, 1963; Lack 1968; see discussion in Mayr 1992). Neverthless, we feel that another reason that species-isolation type explanations have been so widely applied to bird plumage is that a rather small number of supportive examples have been repeatedly used as evidence without ever checking quantitatively whether these examples were actually representative of the overall picture. For example, to support the idea that sympatric overlap leads to sexual divergence, two examples have been used with particular regularity. First, the observation that, in areas where Red-winged Agelaius phoeniceus and Tricolored Blackbirds A. tricolor, overlap, the form of the shoulder patches of these species is particularly divergent (see Hardy & Dickerman 1965). Second, that the males of both Pied Ficedula hypoleuca and Collared Flycatchers F. albicollis tend to be black and white in areas where the two species do not overlap but male pied flycatchers tend to be brown and white in areas of sympatry (eg, Røskaft et al. 1986; Sætre et al. 1997). Similarly, the widespread belief that island-dwelling birds are generally less sexually dimorphic than their mainland counterparts can be traced back to three cases: Lack’s (1940, 1968) observations on Darwin’s Galapagos finches (Geospizidae); Murphy and Chapin’s (1929; Murphy 1938) data on the Azores race of bullfinch Pyrrhula; and Mayr’s (1942) comments on particular island-races of Golden Whistler Pachycephala pectoralis and Scarlet Robin Petroica multicolor.
In recent years, however, the supportive power of all these well-used examples has been called in question by several workers (eg, Grant 1965; Butcher & Rohwer 1989; Grant & Grant 1992; Andersson 1994). Hence, it should not be surprising that our analyses suggest that, although these examples are well-used by advocates of species-isolationist views, they are not representative of the overall picture. This illustrates a potential strength of modern, quantitative comparative analyses over ‘traditional’ comparative methods - namely, that modern quantitative methods are less easily biased by preconceived ideas.
So, if variation in the form of sexual ornaments in birds isn’t an adaptive consequence of selection for species recognition or species isolation, why do closely-related species often have such divergent sexual plumage? What alternative explanations exist and how can they be tested? A common alternative that has already been mentioned is based on the various models of sexual selection. The most obvious form of this argument is that the exact form of sexual selection may vary between populations. For instance, different light environments may favour the use of different colours in signals (Endler 1990, 1992; Zahavi & Zahavi 1997), or different levels of parasitism may lead to differential use parasite-based mate choice criteria in divergent populations (Hamilton 1990). These sorts of argument can, therefore, be tested by looking for covariation between variation in the form of signals and variation in the environment. However, there is another type of explanation linking sexual selection models to variation in the form of sexual ornaments. These explanations are based on Fisherian runaway models of sexual selection which assume that sexual ornaments are designed to take advantage of capricious pre-existing female mating criteria, rather than to signal any sort of phenotypic or genotypic ‘quality’ (Lande 1981, 1982). Hence, the form of sexual ornament will simply track changes in female mate choice criteria, which, under some circumstances, are thought to change very rapidly under runaway selection (Iwasa & Pomiankowski 1995). It is much harder to test the power of this sort of explanation for variation in sexual ornaments. The most straightforward test would be to look for an association between ornament diversity and the likelihood of runaway sexual selection. For instance, Møller and Pomiankowski (1996) found that ‘multiple’ sexual ornaments were particularly common in polygynous species and Mitra et al. (1996) suggested that polygynous taxa were more species-rich than monogamous ones. Similarly, both Barraclough et al. (1995, 1998) and Møller and Cuervo (1998) have shown that, across lineages, prominant sexual dimorphism is associated with species richness. However, in reality, it is still unclear in which species runaway selection should be most common and there seems little prospect of performing a direct test. It is more likely, therefore, that tests of the runway hypothesis will have to be indirect and arise a posteriori from failures to find support for other hypotheses - for instance, failure to find a correlation between variation in sexual ornaments and any environmental factors.
Of course, there is another explanation for a failure to find a link between dichromatic and environmental variation. As for any phenotypic trait, variation in sexual ornaments may not be adaptive at all. That is, variation in sexual ornaments may be due to neutral processes, such as founder effects or drift, rather than any form of selection (see Mayr 1963). Again, non-adaptationist explanations are difficult to test directly and, in the near future, any support is likely to come indirectly from failures to corroborate the predictions of other hypotheses. However, in the case of the neutralist explanation, the extent of divergence between species in terms of sexual ornaments should be strongly associated with the extent of divergence at neutral genetic markers. This is a prediction not shared with the runway sexual selection hypothesis.
We have already performed some preliminary comparative tests of the predictions outlined above. These tests will be written-up in detail elsewhere but it seems appropriate to report here the current overview. We have found several environmental covariates of the colour of ornaments (see Hausmann 1997; Olson & Owens 1998). For example, there are strong relationships between variation in the colour of ornaments and variation in the type of light environment in which the species display. (This is the reason why, contrary to species-isolationist explanations, species living in sympatry often had more similar sexual plumage than species living in allopatry - sympatric species often share a similar light environment, whereas the allopatric species more often experience very different light environments.) For instance, sexual plumage tends to be black or white in open-dwelling species, yellow in canopy-dwelling species, and rufous in birds that live below the canopy (see also Zahavi & Zahavi 1997). The exact form of the sexual display also influences colour. The addition of active movement into the sexual display, for example, is often associated with iridescent plumage (Hausmann 1997; see also Zahavi & Zahavi 1997).
Other analyses have, however, yielded no significant correlations. We have found no associations between environmental variation and the overall form of ornaments. Similarly, among closely related taxa, we have found no consistent relationships between the ornamental variation and the extent of neutral genetic divergence. Thus, based on our current evidence we would suggest that variation in sexual ornaments can be due to several different factors, depending on the level of variation in question. Differences between species in the exact colour of a particular type of sexual ornament are determined by differences in the environmental and the form of display. Differences in the overall form of ornament, on the other hand - such as whether it is on the forehead or throat - seem likely to be due to pre-existing biases in sensory perception.
ACKNOWLEDGMENTS
We thank The Queensland Museum for access to its collection, Peter Bennett, Francisca Hausmann, Jiro Kikkawa, Justin Marshall, Valérie Olson, Hugh Paterson and Amotz Zahavi for discussing the problem of variation in sexual dichromatism. Ben Sheldon and Marion Petrie provided useful comments on the manuscript.
REFERENCES
Andersson, M. 1994. Sexual Selection. Princeton University Press: 207, 437-438.
Barraclough, T.G., Harvey, P.H. & Nee, S. 1995. Sexual selection and taxonomic diversity in passerine birds. Proc. Roy. Soc. Lond. B 259: 211-215.
Barraclough, T.G., Vogler, A.P. & Harvey, P.H. 1998. Revealing the factors that promote speciation. Phil. Trans. Roy. Soc. Lond. B 353: 241-249.
Butcher, G.S. & Rohwer, S. 1988. The evolution of conspicuous and distinctive coloration for communication in birds. Curr.Ornithol. 6, 51-108.
Cracraft, J. 1986. Origin and evolution of continental biotas: speciation and historical congruence within the Australian avifauna. Evolution 40: 977-996.
Cronin, H. 1991. The Ant And The Peacock. Cambridge.
Darwin, C. 1871. The Descent of Man, And Selection In Relation To Sex. Murray: London.
Dobzhansky, T.T. 1937. Genetics And The Origin Of Species. Columbia University Press.
Endler, J. 1990. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 41, 315-352.
Endler, J. 1992. Signals, signal conditions and the direction of evolution. Amer. Nat. 139: s125-s153.
Ford, J. 1987a. Minor isolates and minor geographic barriers in avian speciation in continental Australia. Emu 87: 90-102.
Ford, J. 1987b. Hybrid zones in Australian birds. Emu 87: 158-179.
Grant, P.R. 1965. Plumage and the evolution of birds on islands. Syst. Zool. 14: 47-52.
Grant, P.R. & Grant, B.R. 1992. Hybridisation of bird species. Science 256: 193-197.
Hamilton, W.D. 1990. Mate choice near or far. Amer.Zool. 30: 341-352.
Hardy, J.W. & Dickerman, R.W. 1965. Relationships between two forms of the red-winged balckbird in Mexico. Living Bird 4: 107-129.
Hausmann, F. 1997. Evolutionary Ecology of Ultraviolet Reflectance and Fluorescence in Birds. Unpubl. Hons. Thesis, University of Queensland.
Huxley, J. 1942. Evolution, The Modern Synthesis. Allen & Unwin: London.
Iwasa, Y. & Pomiankowski, P. 1995. Continual change in mate preferences. Nature 377: 420-422.
Keast, A. 1961. Bird speciation on the Australian continent. Bull. Mus. Comp. Zool. Harv. Uni. 123: 305-495.
Keast, A. 1989. Evolutionary biogeography of Australian birds. In: Keast, A. (ed.) Ecological Biogeography of Australia. 1585-1635. Junk: The Hague, Netherlands.
Lack, D. 1940. Evolution of the Galapagos finches. Nature 146: 324-325.
Lack, D. 1968. Ecological Adaptations for Breeding in Birds. Methuen: London.
Lack, D. 1976. Island Biology: Illustrated By the Land Birds of Jamaica. Blackwell Scientific Publications: Oxford.
Lande, R. 1981. Models of speciation by sexual selection on polygenic characters. Proc. Nat. Acad. Sci.USA 78: 3721-3725.
Lande, R. 1982. Rapid origination of sexual isolation and character divergence in a cline. Evolution 36: 213-223.
Mayr, E. 1942. Systematics And The Origin Of Species. Columbia University Press. 49-50.
Mayr, E. 1963. Animal Species And Evolution. Harvard University Press.
Mayr, E. 1992. Controversies in retrospect. Oxf. Surv. Evol. Biol. 8: 1-34.
Mitra, S., Landel, H. & Pruett-Jones, S. 1996. Species richness covaries with mating system in birds. Auk 113: 544-551.
Møller, A.P. & Cuervo, J.J. 1998. Speciation and feather ornamentation in birds. Evolution 52: 859-869.
Møller, A.P. & Pomiankowski, A. 1996. Why have birds got multiple sexual ornaments? Behav. Ecol. Sociobiol. 32: 167-176.
Murphy, R.C. 1938. The need of insular exploration as illustrated by birds. Science 88: 533-539.
Murphy, R.C. & Chapin, J.T. 1929. A collection of birds from the Azores. Amer. Mus. Novit. 384.
Olson, V. & Owens, I.P.F. 1998. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. In press.
Owens, I.P.F. & Bennett, P.M. 1994. Mortality costs of parental care and sexual dimorphism among birds. Proc. Roy. Soc. Lond. B 257: 1-8.
Owens, I.P.F. & Hartley, I.R. 1998. Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proc. Roy. Soc. Lond. B 265: 397-407.
Paterson, H.E.H. 1978. More evidence against speciation by reinforcement. S. Afr. J. Sci. 74: 369-371.
Paterson, H.E.H. 1982. Perspective on speciation by reinforcement. S. Afr. J. Sci. 78: 53-57.
Peterson, A.T. 1997. Geographic variation in sexual dichromatism in birds. Bull. Br. Orn. Club 116: 156-172.
Price, T. 1998. Sexual selection and natural selection in bird speciation. Phil. Trans. Roy. Soc. Lond. B 353: 251-260.
Sætre, G.P., Moum, T., Bures, S., Kral, M., Adamjan, M. & Moreno, J. 1997. A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387: 589-592.
Sibley, C.G. 1957. The evolutionary and taxonomic significance of sexual dimorphism and hybridisation in birds. Condor 59: 166-191.
Røskaft, E., Järvi, T., Nyholm, N.E., Virolainen, M., Winkel, W. & Zang, H. 1986. Geographic variation in the secondary sexual plumage colour characteristics of the male pied flycatcher. Ornis.Scand. 17: 293-298.
Ryan, M.J. & Rand, A.S. 1993. Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47: 647-657.
West-Eberhard, M.J. 1983. Sexual selection, social competition and speciation. Q.Rev.Biol. 58: 155-183.
Wallace, A.R. 1889. Darwinism. 2nd edn. Macmillan: London.
Zahavi, A. & Zahavi, A. 1997. The Handicap Principle: 43-60. Oxford University Press.
Table 1. Comparison of relative difference in sexual ornaments between sympatric pairs versus allopatric pairs of populations.

Table 2. Comparison of relative extent of sexual dichromatism shown by mainland- versus island-dwelling forms.
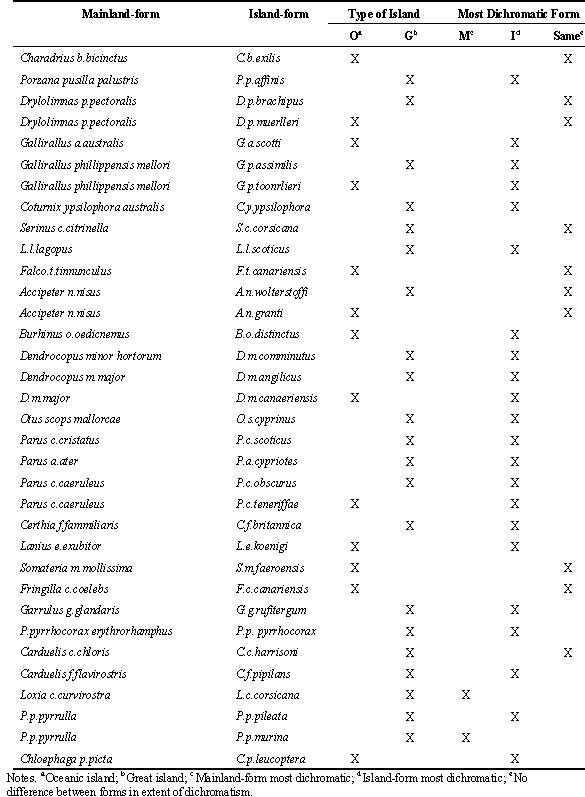
Table 3. Comparison of relative extent of size variation in dichromatic versus monochromatic traits.
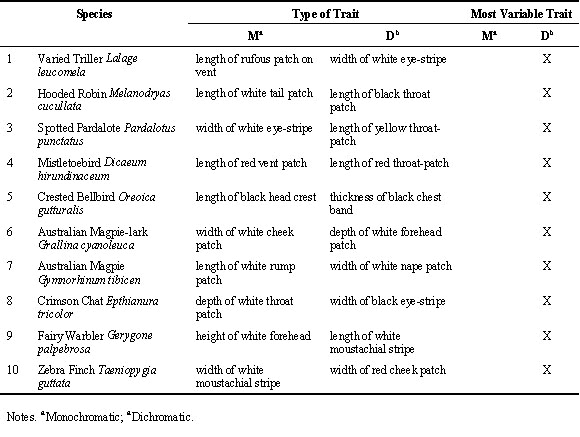
Fig. 1. Effect of patterns of sympatry and allopatry on divergence in sexually dichromatic plumage.
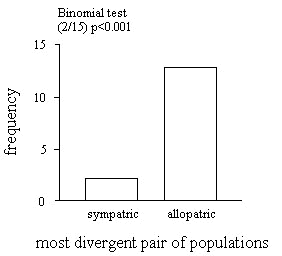
Fig. 2. Comparative dichromatism shown by mainland- and island-races of bird (a) among all islands, (b) among Oceanic islands only, and (c) among Great islands only.
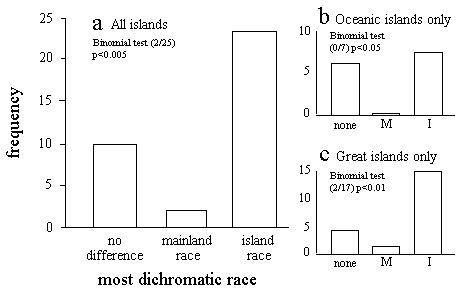
Fig. 3. Relative variation among sexually dichromatic and sexually monochromatic plumage characters.