S19.3: Assessing the role of sexual selection in adaptive radiation of the auklets (Alcidae, Aethiini)
Ian L. Jones
Department of Biology, Memorial University of Newfoundland, St. John’s, Newfoundland, A1B 3X9 Canada, fax 709 737 2430, e-mail ijones@morgan.ucs.mun.ca
Jones, I.L. 1999. Assessing the role of sexual selection in adaptive radiation of the auklets (Alcidae, Aethiini). In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1115-1125. Johannesburg: BirdLife South Africa.The auklets provide an enigmatic comparative example of sexual selection, with expression of ornamental traits among these five small socially monogamous, sexually monomorphic plankton-eating seabird species ranging from extremely subdued to the most elaborate of any marine bird. This ornamental diversity among auklet species relates to aspects of their biology (e.g., nocturnal/diurnal, colonial/non-colonial, and to other factors). Molecular evidence indicates that parts of this group radiated explosively about five million years BP during the late Pliocene, a possible example of peripatric speciation. Experimental evidence and observations indicate that mutual sexual preferences of both sexes favour naturally occurring ornaments, that some aspects of their variable ornaments and plumage are signals of social dominance, that different ornaments vary in their relationship to age and condition, that sperm competition is an important factor in some species, and that at least one species possesses heterospecific mating preferences for an ornament, yet there are few clues as to which mechanism(s) of sexual selection (viability indicator, Fisherian, sensory exploitation, or species isolation signal) are responsible for the evolution of their elaborate ornaments. Recently diverged species have more elaborate ornaments than older species, consistent with a link between sexual ornamentation and speciation.
INTRODUCTION
Some avian radiations have involved groups that include many strikingly ornamented species. Examples of such groups include the include the herons (Ardeidae), pheasants (Phasianidae), some auks (Alcidae), parrots (Psittacidae), bee eaters (Meropidae), barbets (Capitonidae), toucans (Ramphastidae), hummingbirds (Trochilidae), manakins (Pipridae), pittas (Pittidae), birds of paradise (Paradisaeidae), North American warblers (Parulidae), tanagers (Icteridae) and waxbills (Estrildidae). An important question concerns whether members of these groups are unusually ornamented because of factors directly related to their rapid radiations per se, or alternatively whether their showiness is simply coincident with aspects of their breeding systems that promote sexual selection but are unrelated to speciation and radiation. Avian radiations would tend to favour the evolution of elaborate display traits if these served as species isolating or species recognition mechanisms (Dobzhansky 1937; Mayr 1963). Thus we might expect to find groups of bird species displaying ornamental traits in the present day that at least formerly served as isolating or recognition mechanisms during the adaptive radiation of closely related species but now represent evolutionary relics of rapid speciation. An alternative to the possibility that radiation produces a fertile environment for sexual selection is that groups of birds in which ecological conditions have favoured sexual selection are inordinately likely to undergo adaptive radiation (i.e., that sexual selection drives speciation and radiation rather than the other way around). For example, if a species’ breeding system leads to the evolution of elaborate display traits through sexual selection, these traits might secondarily result in reproductive isolation of geographically isolated populations, facilitating speciation. Deriving an understanding of which came first, sexual selection or radiation, is likely to be problematic, but a confirmation of a relationship between sexual selection and radiation in avian groups is likely to be a helpful first step.
Explanations for the evolution of elaborate sexually selected display traits have more recently focused on mechanisms not directly related to adaptive radiation at all. Indeed, the relevance of mechanisms of the evolution of elaborate traits and mating preferences for them to adaptive radiation has not been thoroughly evaluated. Nevertheless, such mechanisms include: (1) a ‘Fisherian’ runaway process driven by a genetic link between an ornamental trait possessed by males and a mating preference for the ornament possessed by females (Fisher 1930; Lande 1980; Lande 1981; Kirkpatrick 1982); (2) a ‘viability indicator’ (also known as ‘good genes’, or ‘handicap’) process driven by benefits to individuals choosing ornamented partners (e.g., Andersson 1986; Pomiankowski 1988); and (3) sexual selection for sensory exploitation (Ryan 1990; Ryan & Rand 1993) in which a mating preference arises due to natural selection for sensory biases unrelated to mate choice, followed by the appearance of a display trait that can 'exploit' the pre-existing sensory biases and subsequently be favoured by inter-sexual selection driven by mating preference that result from the sensory biases. Extensive research has not provided an answer as to which of these mechanisms is most important in the evolution of ornaments, but for a variety of reasons, conditions for each of these mechanisms might be enhanced by speciation events. For example, a Fisherian runaway process might operate more quickly and completely in a small founder population of an incipient species because preference and ornament expression genes would go rapidly to fixation (Lande 1981). Similarly, individuals of a recently evolved species occupying a new niche (e.g., colonisation of an island) would be expected to have greater genetic variation for traits related to fitness because of the lesser opportunity for natural selection to eliminate unfavourable genotypes in the new environment, providing an ideal setting for the evolution of viability indicating sexually selected traits. Even sexual selection for sensory exploitation could be enhanced by speciation if selection against conspicuous display traits was relaxed (e.g., related to an absence of predators) in incipient species occupying new habitats.
The auklets (family Alcidae, tribe Aethiini) provide an example of a limited (five species) adaptive radiation of a group of bird species, some of which display elaborate sexually selected ornamental traits that have been well documented by field observations and experiments. The purpose of this paper is in part to evaluate whether auklets’ adaptive radiation could have directly favoured the evolution of their present ornaments. Specifically, I evaluated whether the function and pattern of occurrence of present day auklet ornaments is consistent with the hypothesis that they evolved as species recognition mechanisms. An association of ornament evolution with adaptive radiation would be supported if the following hypotheses could be tested: (1) auklet ornaments play a central role in species recognition during mate choice; (2) auklet species that occur sympatrically with other species are more ornamented than auklets occurring with few or no other species; (3) the most recently split or speciated auklet species are the most ornamented, since these would have the greatest need for conspicuous isolating mechanisms; and (4) auklets that breed together in the same colonies should have the greatest interspecific differences in ornaments.
The objectives of this paper were: (1) to review the available evidence regarding the form and function of ornaments and display traits of the auklets (Alcidae, tribe Aethiini); (2) to evaluate the evidence regarding the role of auklet ornaments as species isolating or species recognition mechanisms, and (3) to consider the evidence for a relationship between rapid speciation and the relative ornamentedness of auklets compared with other species in the family Alcidae.
RESULTS and DISCUSSION
Phylogeny
It has long been recognised that the auklets form a monophyletic group (tribe Aethiini). Reconstructing phylogenetic relationships within this group has been problematic. Strauch (1985) suggested that Cassin’s Auklets Ptychoramphus aleuticus are distinct from the remaining four species but recommended that Parakeet Auklets be placed within the genus Aethia. Strauch (1985) was unable to resolve relationships among species in this Parakeet-Least-Crested-Whiskered group. Recently there have been several attempts to reconstruct a phylogeny of the auklets using molecular approaches. Work by Moum et al. (1994) and Friesen et al. (1996) appear to confirm that Cassin’s Auklets are distinct from the remaining species, but provide few clues as to the relationships among the remaining species. Nevertheless, Whiskered Aethia pygmaea and Crested Auklets A. cristatella may be sister species, based on shared derived characters including their distinctive citrus-like plumage odour, forehead crests and vocalisations (Gaston and Jones 1998). Taken together, the molecular evidence suggests an extremely rapid adaptive radiation of the Parakeet-Least-Crested-Whiskered group about 5 million years BP (Moum et al. 1994). I consider the auklets’ diversification to represent an example of ‘adaptive’ radiation because the extant species seem to fill a range of compatible niches while exploiting zooplankton as a food source.
Behaviour
All auklet species are colonial nesters nesting in rock crevices (all species) or earth burrows (Cassin’s Auklet). All species are socially monogamous (Gaston & Jones 1998). Parakeet Cyclorrhynchus psittacula, Least Aethia pusilla and Crested Auklets have diurnal colony attendance, with courtship and agonistic behaviour taking place on display rocks on the colony site surface (Jones 1993a, 1993b). Cassin’s and Whiskered Auklets have nocturnal colony attendance; their courtship activity on the colony surface and within their enclosed nest sites is accompanied by intense vocal displays (Thoresen 1964; Manuwal & Thoresen 1993; I.L. Jones & F.M. Hunter unpublished data). All auklet species except Cassin’s Auklets attend and engage in courtship at a staging area at sea nearby their colonies (Gaston & Jones 1998). Taken together, these features of auklet behaviour suggest that mate choice is based on: (1) diurnal visual and vocal courtship displays on the sea and at the colony site by Parakeet, Least and Crested Auklets; (2) diurnal visual and vocal courtship displays on the sea and nocturnal vocal displays at the colony site by Whiskered Auklets; and (3) nocturnal vocal displays only at the colony site by Cassin’s Auklets. In all auklet species, males perform distinctive vocal advertising displays to attract mates. Similar advertising displays have been described for only one species (Ancient Murrelet, Synthliboramphus antiquus among the remaining 18 extant alcid species (Gaston & Jones 1998).
Ecology and geographic range
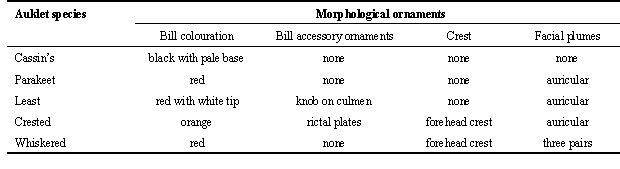
Auklets are pursuit-diving planktivorous seabirds, the smaller species specializing on copepods (Neocalanus) while the larger species feed principally on euphausiids (Euphausia and Thysanoessa) and amphipods (Parathemisto; Gaston & Jones 1998). Auklets range throughout the Bering and Okhotsk Seas and adjacent parts of the North Pacific (Fig. 1; Gaston & Jones 1998). One species, the Cassin’s Auklet, ranges southwards along the west coast of North America to the coast of Baja California in northwestern Mexico (Manuwal & Thoresen 1993). Three or more auklet species occur sympatrically throughout the entire Bering and most of the Okhotsk Seas, four or more species co-occur throughout the Aleutian Islands and northern Kurile Islands, and all five extant auklet species co-occur in the central and western Aleutian Islands (Fig. 1). All five auklet species coexist at Buldir, a small island in the western Aleutians (Byrd & Day 1984). Least and Crested Auklets’ ranges overlap very closely and the two species occur together at most of their large isolated colony sites (e.g., Least Auklets coexist with Crested Auklets at 33 of 38, or 87%, of their colonies in Alaska; Jones 1993b). Most Cassin’s Auklets nest in colonies without other auklet species present. Whiskered Auklets have the most restricted range of any auklet species; they occur only in the Aleutian and Kurile Islands (Williams & Byrd 1994), where auklet diversity is greatest.
Morphology of ornaments
The Alcidae divide phylogenetically into two major groups, an auklet-puffin group and a second group including the auks, murres, murrelets and guillemots (Gaston & Jones 1998). Among the former group, spectacular ornaments (crests, plumes, brightly coloured bills and feet, contrasting eye colour and facial wattles) are virtually the rule, especially among the auklets, whereas among the latter species ornaments are relatively subdued or lacking.
Adults of all the auklet species have conspicuous white irides, relatively dull overall plumage (black, gray, and white), and dull bluish gray feet. Ornaments consist of plume and crest feather ornaments on the face, orange or red bill colouration, and accessory plates or knobs on the bill (Table 1; Gaston & Jones 1998). Least Auklets display three forms of ornamentation as well as conspicuous polymorphism of overall plumage. Their ornaments include: white facial plumes comprised of extensive short forehead plumes (1-2 mm in length) and bilaterally symmetrical auricular plumes behind the eyes (averaging 15 mm in length, closely resembling the auricular plumes of Parakeet, Crested and Whiskered Auklets), a horny knob at the base of the culmen (1-3 mm height), and red bill colouration (Fig. 2; Jones & Montgomerie 1992). All the ornaments exhibit considerable variation in expression among adults, but there is no evidence for sexual dimorphism of any ornament (Jones & Montgomerie 1992). Adult individuals vary from unmarked white to nearly black in underpart colouration, with evidence for increasing lightness with age (Fig. 3; Jones 1990).
Three prominent ornaments are displayed by Crested Auklets: a forehead crest, composed of 11-31 curved feathers averaging about 40 mm in length, bilaterally symmetrical white auricular plumes averaging about 30 mm in length, and brightly-coloured bill plates (Fig. 2; Jones et al. ms submitted). Ornament sexual dimorphism is absent or very slight: males’ auricular plumes and bill plates are slightly larger than females’ but the crest ornament shows no significant difference in absolute length between the sexes. Crest and auricular plume length are positively correlated within individuals. Breeding adult females and males have greater crest and plume ornament expression than non-breeding adults. Females’ crests and bill plates were more variable than males’ crests and bill plates. Subadults had smaller ornaments than adults. Based on adults remeasured with an interval of one to seven years, the size of individuals’ feather ornaments increased with age; there was also evidence for population-wide ornament variability among years. Male auricular plumes and female crests weakly correlate with indices of body condition. Crested Auklets also have a distinctive citrus-like plumage odour that may have communicative function, as several mutual courtship displays appear to involve partners ‘smelling’ one another’s nape feathers, where the odour is most pronounced (Jones 1993a).
Whiskered Auklets have the most elaborate ornaments of any auklet species (Fig. 2; Gaston & Jones 1998). The white facial plumes are divided into three distinct bilaterally symmetrical tracts: one tract extends from the base of the bill upward and backward (super-orbital plumes, ca. 31 mm length), one tract extends from the base of the bill downwards and backward (sub-orbital plumes, ca. 30 mm length), and the third tract extends backwards from below the eye (auricular plumes, ca. 33 mm length). The forehead crest, presumably homologous to the similar ornament of the Crested Auklet, is composed of ca. 12 black forward-curving feathers ca. 36 mm in length. The bill lacks accessory plates but is brightly coloured (red with an ivory-coloured tip). The ornaments show considerable variation among individuals, with no evidence of sexual dimorphism. There is evidence that ornament expression increases with age up to at least five years of age, and weak correlations with indices of body condition (I.L. Jones & F.M. Hunter, unpublished data). Whiskered Auklets have a faint citrus-like plumage odour similar to that of the Crested Auklet.
Parakeet Auklets display a bright red bill and variable white auricular plumes (ca. 35 mm length) similar to the auricular plumes of Least, Crested and Whiskered Auklets (Fig. 2; Gaston & Jones 1998). These ornaments show minimal sexual dimorphism, but ornament variation has not been intensively studied in this species.
Cassin’s Auklets have no conspicuous ornaments, but do have small crescent-shaped white marks above each eye and a grayish-white base of the lower mandible of their otherwise black bills (Fig. 2; Manuwal & Thoresen 1993).
Function of ornaments
Least Auklet
Model-presentation experiments in which intra-sexual competition was controlled suggested that Least Auklets have mating preferences for red bill colour and extensive auricular plumes, but no evidence for a preference for the bill knob ornament was found (Jones & Montgomerie 1992). No experiments on intra-sexual selected ornament function were performed, nor was it possible to assess whether ornaments correlated with dominance status by observation of natural interactions. However, this species’ variable underpart colouration correlated with dominance and experiments and observations confirmed that this plumage variability signals dominance status (Jones 1990). The most pertinent finding to this paper was the experimental confirmation that Least Auklets have a heterospecific preference for crest ornaments (an ornament not naturally expressed by this species; Jones & Hunter 1998). Like Least and Crested Auklet preferences for their own ornaments, this preference appeared to favour larger ornament size.
Crested Auklet
The function of this species’ ornaments has been examined extensively. (Jones and Hunter 1993) performed model-presentation experiments in which intra-sexual competition was controlled; both males and females responded to opposite sex models with more frequent sexual displays when the models had long crests compared to short crests (within the range of natural variation), suggesting that crested auklets have mating preferences that favour long crest ornaments. Similar experiments on the smaller white facial plumes of this species (auricular plumes) showed no evidence for mating preferences (Hunter, F.M. & I.L. Jones, unpublished data). Jones & Hunter (IN PRESS) also investigated the role of the crest ornament in intra-sexual selection. Based on quantifications of brief agonistic interactions at a large breeding colony, Jones & Hunter (IN PRESS) found that crest length was strongly correlated with dominance within both sexes. Across the full range of crest length, individuals with longer crests were dominant over shorter-crested individuals in agonistic interactions involving same-sex adults. In interactions involving subadults (two-year-olds) there was a similar trend towards longer crested individuals being dominant. In agonistic interactions involving different-sex and different-age individuals, adult males were dominant over adult females regardless of crest length, as were adults of both sexes over subadults. In related experiments (Jones & Hunter IN PRESS) in which crest length was manipulated, responding male auklets reacted with less frequent aggressive responses to male models with longer crests, confirming that crest length by itself signals dominance status. Taken together, these results supported the idea that the crest ornament of Crested Auklets is favoured by both intra- and inter-sexual selection. Unfortunately, no information on the inter- or intra-sexual function of other Crested Auklet ornamental traits (bill colour, bill plates, plumage odour) is available.
Cassin’s Auklet, Parakeet and Whiskered Auklets
No experiments or directed observations have been made to investigate ornament function in these species. Parakeet Auklets appear to display their ornaments during courtship at sea and on rocks near their nest sites in a manner similar to Least and Crested Auklets. Whiskered Auklet courtship on land takes place not only at night but also at well-concealed sites in caves under rocks and in cliff crevices (Williams & Byrd 1994). Much of Whiskered Auklet courtship on land may take place in nearly complete darkness in which their facial ornaments would not be visible. For this reason, it is unlikely that Whiskered Auklet ornaments have a major function on land. However, pairs of this species engage in protracted courtship on the sea during daylight hours, during which the ornaments likely function in mate choice as in Least and Crested Auklets.
Interpreting the evidence
In general, I found little conclusive evidence that auklet ornaments function as species recognition mechanisms. Most importantly, Least Auklets were found to have a preference for the most conspicuous ornament (the forehead crest) displayed by two other species (Crested and Whiskered Auklets). It is difficult to construct a plausible argument for the evolution of the crest ornament as a species recognition mechanism given that members of the most widespread and abundant auklet species (Least Auklet, possessing no crest) apparently have a mating preference for this ornament. Although the presence of a crest might help Crested Auklets to recognise one another as conspecifics, this ornament would apparently not act as a repelling signal to the smaller species. Another prediction of the species recognition hypothesis for the evolution of ornaments is that auklet species that breed together in the same colonies should have the most different ornaments. Least, Crested and Parakeet Auklets occur together at many colonies and even nest frequently in adjacent crevices (Gaston & Jones 1998). Crested Auklets differ from the others in this group in having a crest, which would not be an effective isolating mechanism from Least Auklets for the reasons explained above, but might reduce confusion with Parakeet Auklets. These three species have quite different bill shape and colour, consistent with their function as isolating mechanisms (Pierotti 1987). Each of Least, Crested and Parakeet Auklets posses similar white auricular plumes on the face, eliminating this ornament as a potential isolating mechanism. Furthermore, each of these species has the identical foot colour (but see Pierotti 1987). Whiskered Auklets, arguably the auklet species with the most elaborate ornaments, almost nowhere nest in close proximity to other auklet species or encounter other auklet species in aggregations of courting birds, suggesting that this species’ elaborate adornments do not have a major function in species isolation. The suggestion that auklet ornaments evolved as isolating or species recognition mechanisms is further weakened by the fact that auklets differ in numerous aspects of appearance in addition to ornaments. For example, Least and Crested Auklets, the two species that occur together most frequently differ by a factor of three in body size, have different overall plumage colour (black and white versus uniform dark gray), and have strikingly different vocalizations. Taken together, these observations suggest that the species recognition hypothesis forms a poor explanation for the evolution of auklet ornaments.
Given that auklet ornaments may not have evolved principally as isolating mechanisms, the question remains as to whether there is any evidence of an association between ornament evolution and adaptive radiation in this group. With only five auklet species, comparative statistical tests are precluded, so I looked for patterns compatible with a link between ornaments and adaptive radiation. Three pieces of evidence are consistent with such a connection. First, auklet species that occur sympatrically with two or more other species are more ornamented than auklets occurring with few or no other species. Auklet species whose ranges center on the Aleutian Islands, where auklet diversity is highest, include the most ornamented species the Whiskered, Crested and Least Auklets (Fig. 1). In contrast, the two auklet species (Parakeet and Cassin’s Auklets) occurring in regions where diversity is low (the Gulf or Alaska and west coast of the North American continent south of Alaska) have the least elaborate ornaments. Thus the evolution of elaborate ornaments is presently reflected best where species diversity is highest. Secondly, the most recently split or speciated auklet sister species, Crested and Whiskered Auklet, are the two most ornamented species. These two species alone share the elaborate forehead crest and plumage odour in addition to ornaments similar to the other species. Thus the most recent products of the adaptive radiation of auklets have the most elaborate ornaments. These species occur in the area of highest auklet species diversity, so it is not clear whether co-occurrence with other species or order of appearance in the radiation is responsible for their ornament elaboration. Third, among the 23 extant species of the family Alcidae, the five auklet species represent the most conspicuous example of both adaptive radiation and evolution of multiple ornamental novelties. These pieces of circumstantial evidence suggest that adaptive radiation in this group is associated with a fertile environment for sexual selection. Large scale comparative tests of other more diverse avian adaptive radiations are required for a more complete understanding of the underlying causes of this association.
ACKNOWLEDGMENTS
I am especially grateful to Fiona M. Hunter for years of help with fieldwork and much fruitful discussion about sexual selection in auklets. I thank Anne Harfenist, Simon Gawn, Bob Sundstrom, Christine Adkins, Mark Hipfner, Ian Stevenson, Gail Fraser, Laura Cowen and Alejandra Nunez de la Mora for assistance in the field, and Vernon Byrd and Daniel Boone for logistic support and permission to conduct research on the Aleutian Island Unit of the Alaska Maritime National Wildlife Refuge. Steve Zimmerman of the National Marine Fisheries Service assisted with accommodation on St. Paul Island, Alaska. I am especially grateful to the captains and crews of the vessels R/V Tiglax, F/V American Empire, F/V Resolute, and USCGS Jarvis, and to the U.S. Fish and Wildlife Service, Empire Seafoods Inc., Arctic King Fisheries, and the U.S. Coast Guard for providing vessel transportation to Buldir Island, to the National Geographic Society Committee for Research and Exploration and NSERC Canada for providing major funding, and to Jacob Höglund and Marion Petrie for helpful comments on the manuscript.
REFERENCES
Andersson, M. 1986. Evolution of condition-dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution 40: 804-816.
Byrd, G.V. & Day, R.H. 1984. The avifauna of Buldir Island, Aleutian Islands, Alaska. Arctic 39: 109-118.
Dobzhansky, T. 1937. Genetics and the origin of species. New York; Columbia University Press.
Fisher, R.A. 1930. The genetical theory of natural selection. Oxford; Clarendon Press.
Friesen, V.L., Baker, A.J. & Piatt, J.F. 1996. Phylogenetic relationships within the Alcidae (Aves: Charadriiformes) inferred from total molecular evidence. Molecular Biology and Evolution 13: 359-367.
Gaston, A.J. & Jones, I.L. 1998. The Auks. Oxford; Oxford University Press: 349pp.
Jones, I.L. 1990. Plumage variability functions for status signaling in Least Auklets. Animal Behaviour 39: 967-975.
Jones, I.L. 1993a. Crested Auklet. In: Poole, A., Stettenheim, P. & Gill, F. (eds) The Birds of North America. Vol. 70; Washington D.C.; The Academy of Natural Sciences; Philadelphia; The American Ornithologists Union.
Jones, I.L. 1993b. Least Auklet. In: Poole, A., Stettenheim, P. & Gill, F. (eds) The Birds of North America. Vol. 69; Washington D.C.; The Academy of Natural Sciences; Philadelphia; The American Ornithologists Union.
Jones, I.L., Fraser, G. & Hunter, F.M. ms submitted. Patterns of variation in ornaments of Crested Auklets (Aethia cristatella). Journal of Avian Biology.
Jones, I.L. & Hunter, F.M. 1993. Mutual sexual selection in a monogamous seabird. Nature 362: 238-239.
Jones, I.L. & Hunter, F.M. 1998. Heterospecific mating preferences for a feather ornament in least auklets. Behavioural Ecology 9: 187-192.
Jones, I.L. & Hunter, F.M. IN PRESS. Experimental evidence for mutual inter- and intra-sexual selection favouring a Crested Auklet ornament. Animal Behaviour.
Jones, I.L. & Montgomerie, R.D. 1992. Least auklet ornaments: Do they function as quality indicators? Behavioural Ecology and Sociobiology 30: 43-52.
Kirkpatrick, M. 1982. Sexual selection and the evolution of female choice. Evolution 36: 1-12.
Lande, R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34: 292-305.
Lande, R. 1981. Models of speciation by sexual selection on polygenic traits. Proceedings of the National Academy of Sciences USA 78: 3721-3725.
Manuwal, D.A. & Thoresen, A.C. 1993. Cassin's Auklet. In: Poole, A., Stettenheim, P. & Gill, F. (eds) The Birds of North America, Vol. 50; Washington D.C.; The Academy of Natural Sciences; Philadelphia; The American Ornithologists Union.
Mayr, E. 1963. Animal species and evolution. , Cambridge, Massachusetts; Harvard University Press.
Moum, T., Johansen, S., Erikstad, K.E. & Piatt, J.F. 1994. Phylogeny and evolution of the auks (subfamily Alcinae) based on mitochondrial DNA sequences. Proceedings of the National Academy of Sciences USA 91: 7912-7916.
Pierotti, R. 1987. Isolating mechanism in seabirds. Evolution 41: 559-570.
Pomiankowski, A.N. 1988. The evolution of female mate preferences for male genetic quality. Oxford Surveys in Evolutionary Biology 5: 136-184.
Ryan, M.J. (ed.). 1990. Sexual selection, sensory systems and sensory exploitation. (Vol. 7). Oxford; Oxford University Press.
Ryan, M.J. & Rand, A.S. 1993. Sexual selection and signal evolution: the ghost of biases past. Proceedings of the Royal Society of London Series B 340: 187-195.
Strauch, J.G., Jr. 1985. The phylogeny of the Alcidae. Auk 102: 520-539.
Thoresen, A.C. 1964. Breeding behavior of the Cassin’s Auklet. Condor 66: 456-476.
Williams, J.C. & Byrd, G.V. 1994. Whiskered Auklet. In: Poole, A., Stettenheim, P. & Gill, F. (eds) The Birds of North America. Vol. 76; Washington D.C.; The Academy of Natural Sciences; Philadelphia; The American Ornithologists Union.
Table 1. Types of ornaments displayed by auklet species.

Fig. 1. Breeding ranges of the auklets in the North Pacific and Bering and Okhotsk Seas.
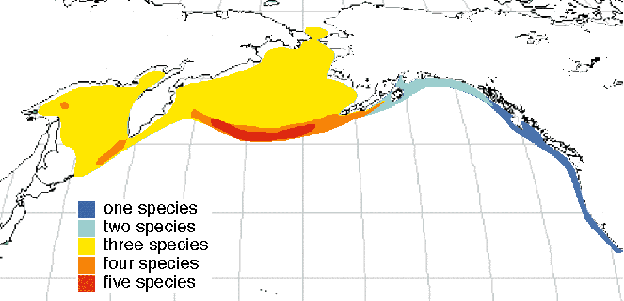
Fig. 2. Facial ornaments of the auklets (Alcidae; Aethiini). (a) Cassin’s Auklet, (b) Parakeet Auklet, (c) Least Auklet, (d) Crested Auklet, (e) Whiskered Auklet.
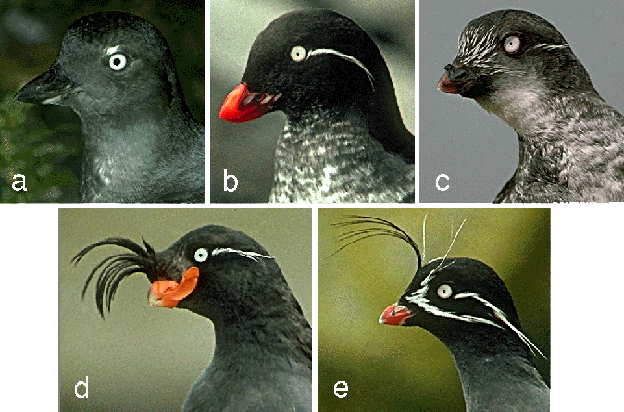
Fig. 3. Two Least Auklets at a breeding colony, illustrating underpart plumage variation within this species.
Note: these figures are on the world wide web and downloadable at:
http://www.mun.ca/acwern/IOCfigs.html