S19.2: Revealing quantitative genetic relationships in sexual
selection using molecular markers Ben C. Sheldon Department of Zoology, Uppsala University, Villavägen 9, S-752 36 Uppsala, Sweden, fax 46 18 559888, e-mail ben.sheldon@zoologi.uu.se Sheldon, B.C. 1999. Revealing quantitative genetic relationships in sexual selection using molecular markers. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1099-1114. Johannesburg: BirdLife South Africa.The relative importance of direct and indirect (genetic) benefits of mate choice has been the subject of lively debate. It is often difficult to determine the relative importance of one or other form of benefit from mate choice, because it is likely that different benefits offered by individuals are correlated. In this talk I describe an approach which enables unbiased estimates of genetic contributions made by fathers to their offspring. This is based upon using maternal half-siblings with known paternity , resulting from extra-pair copulations and detected by microsatellite markers. In a Swedish population of Collared Flycatchers Ficedula albicollis, comparisons within natural broods of half-siblings revealed that fathers contribute genes affecting nestling fledging condition, and that their genetic contribution is predicted by the size of a sexually selected character. Experimental removals of males, to increase rates of extra-pair paternity, in a different year with contrastingly different (poor) conditions for nestling growth revealed a relationship of opposite sign to that in the more favourable year. These results suggest that environmental variation may occasionally reverse the sign of genetic correlations between ornamental and fitness traits, and can therefore act to maintain genetic variation in traits normally subject to directional selection.
INTRODUCTION
The idea that, by its choice of mate, an individual may increase the fitness of its offspring dates back to Darwin. If one accepts that individuals choose their mates (something for which there is substantial evidence from birds: Gibson & Langan 1996) it is hard to explain this behaviour without postulating some accompanying benefit which can maintain choosing in contemporary populations. What sort of benefits might individuals gain from choosing a mate? It is common to divide possible benefits into two kinds: ‘direct’ and ‘indirect’. Direct benefits are generally those which can be thought of as acting directly upon a choosing individual, in the breeding attempt in which the choice is made. Examples are increased food supply on a high quality territory, increased parental care provided by the chosen individual, or reduced risk of predation. Indirect benefits are those which benefit the choosing individual only through effects on the offspring produced in the breeding attempt, and as such have usually been thought of as any benefit resulting from inheritance of genes which in some way increase the fitness of offspring. The distinction is somewhat artificial, since there are potential direct benefits which will also benefit a choosing individual via effects on its offspring, and it may be very difficult to partition offspring fitness into components that reflect the dichotomy described above. Also, few, if any, real mate choice decisions will convey benefits of exclusively one sort. Nevertheless, the terms have become widely used, and I will continue to use them here, together with the terms ‘genetic’ and ‘non-genetic’ benefits, which better reflect the distinction which this paper addresses.
The relative role of direct and indirect benefits in selecting for choice by other individuals has been a central question in the study of sexual selection for several decades (Andersson 1994). As with many debates about the relative worth of apparently polarised viewpoints, with hindsight, neither extreme is very realistic. All populations must carry at least some genetic variation for traits affecting fitness, simply due to the occurrence of new deleterious mutations. Equally, genes cannot play a very large role in determining fitness in most populations: any natural historian must be aware of the great role that chance plays in nature. However, there is plenty of ground in-between these two modified points, and where the balance lies in current populations is something that remains unresolved. In this paper, I first describe some recent issues which are relevant to the question of how much genetic variation for fitness can be found in natural populations, and to how that question might be answered. I then review some of the studies of birds which have attempted to dissect fitness variation arising from mate choice into genetic and non-genetic components. I discuss some of the problems that face anyone attempting an empirical study of these questions. I will also describe some techniques that offer possibilities for further resolution of these questions.
Natural and Unnatural Populations
The suggestion that individuals can improve the genetic quality of their offspring through mate choice is a statement, either implicitly or explicitly, about genetic variance for fitness. Fisher’s fundamental theorem of natural selection (Fisher 1958) has often been interpreted as suggesting that there should be little or no genetic variance for fitness remaining in natural populations after selection, but any realistic natural population will violate several of the assumptions required for an ideal ‘Fisherian’ population. Thus, other than the continuing input from mutation, populations are likely to retain some genetic variation for traits affecting fitness through the action of processes such as migration and numerous forms of genotype-environment interaction. Attempting to estimate the proportion of fitness due to additive genetic sources is a very difficult task, for a number of reasons. In most natural populations of animals postnatal dispersal, high mortality among juveniles, and uncertainty over parentage combine to make fitness itself very difficult to estimate. Estimating the contribution of genes to fitness is even more difficult, given that genotype-environment correlations, maternal and paternal effects may commonly affect traits such as this (Lynch & Walsh 1998). Burt (1995) reviewed studies that have attempted to estimate the additive genetic variance for fitness in natural and semi-natural populations. The standard errors of most of the estimates was such that they did not differ from zero, but Burt’s conclusion was that the standardised additive genetic variance for fitness was less than 0.3, which can be used to set an upper limit on the genetic benefits obtainable from mate choice of about 5-10% (Burt 1995). Charlesworth (1987) reached a broadly similar conclusion.
Given the difficulties that have been experienced in obtaining even the estimates reviewed by Burt (1995), studies of laboratory populations (e.g. of Drosophila) have been advocated by some as the only means to be able to (i) measure fitness adequately, (ii) control for confounding environmental effects and (iii) obtain sample sizes sufficient to set useful confidence intervals on such estimates. As an example, a recent study of Drosophila melanogaster (Fowler et al. 1997) concluded, by extrapolating from the fitness effects of a sample of third chromosomes (44% of the genome) competed against each other, that the standardised additive genetic variance for fitness was as high as 0.45. Fowler et al. (1997) acknowledge that, as with any study of fitness in a laboratory population, measuring genetics of fitness in the lab and extrapolating these results to natural populations is fraught with difficulties. One major difficulty that has attracted recent attention is the possibility that the intensity of selection may be reduced under the relatively benign conditions experienced by organisms in the laboratory, and that this may be particularly the case for traits strongly affecting fitness. In other words, genotype-environment interactions may be common for loci affecting fitness, and may be such that genetic variation for fitness will be most evident under harsher environments which are more likely to approach those in which a species has spent most of its evolutionary history. Experimental evidence in support of this suggestion has recently been obtained from several studies of Drosophila (e.g. Kondrashov & Houle 1994; Shabalina et al. 1997).
The realisation that genotype-environment interactions may influence measurements of fitness in laboratory populations in a systematic fashion has also cast doubt on the ability of geneticists to measure one of the fundamental parameters influencing genetic variation for fitness, at least in a way that is meaningful to natural populations. The rate at which new mutations occur in populations is fundamental to the question of genetic variation for fitness, because it forms one side of the mutation-selection account that determines how much genetic variation is present in a population: as selection removes variation, mutation replaces it. In addition to being fundamental, the rate at which mutations occur is very difficult to measure, and has been estimated only in populations of humans, bacteria, nematodes and Drosophila. Considerable differences (a factor of 100 spans the lowest and highest estimates) exist in estimates of this parameter (Mukai et al. 1972; Fernandez & Lopez-Fanjul 1996; Kibota & Lynch 1996; Keightley & Caballero 1997; Shabalina et al. 1997), and the problem of environmental-dependence of expression for mutations has been offered as an explanation for these differences (Peck & Eyre-Walker 1997).
These large-scale studies of quantitative genetics of fitness in laboratory populations have a number of important implications for the role of studies of quantitative genetic consequences of mate choice in natural populations. These are that: (1) Additive genetic variance for fitness is certainly present in populations, and probably at levels that are sufficient to cause at least moderately strong selection on choosing individuals. A 10% fitness advantage (c.f. Burt 1995) may not sound like much, but it represents sufficiently strong selection to cause an evolutionary response that would appear instantaneous over geological timescales. (2) The environment in which a study is conducted may be of critical importance: assessment of fitness consequences of mate choice should not be performed in benign, non-natural, environments. (3) Genotype-environment interactions for traits affecting fitness may be common. Although studies of natural populations can never attain the degree of control of laboratory studies, and to attempt to obtain an accurate estimate of additive genetic variance for fitness in a wild population would be ambitious to say the least, I believe that they have a very important role to play. Our aim in understanding the processes governing evolution by sexual selection should, ultimately, be to understand how sexual selection in natural populations has produced the outcomes that we see now, and what the future consequences of continued selection may be. These aims can not be realised without studying populations in the environments in which they evolved.
Empirical studies of Natural Populations
While natural populations may not be the place to attempt to measure additive genetic variance for fitness (which is a prerequisite for genetic benefits models of mate choice), one does not need to do this in order to assess whether genetic benefits explanations of mate choice apply in nature. The central assumption of genetic benefit models is that a genetic correlation exists between a preferred trait and fitness. This can, in principle, be demonstrated in nature, although there are considerable difficulties involved in doing so. Most attempts to do so would involve measuring the phenotypes of chosen individuals (e.g. ornament size of males), and correlating them with the phenotypes of their offspring (e.g. viability of offspring). This is relatively easy. What is difficult is to show that any phenotypic correlation found has arisen at least in part due to the action of genes. The difficulty is primarily caused by the following factors:
(1) An individual with a large value of a preferred trait may provide many things other than genes to its offspring. Higher quality territories and better parental care are widely accepted as being important for the fitness of offspring, and are also often suggested to be targets of mate choice in themselves.
(2) Attractive individuals may attract mates of higher quality, which may also, as a consequence of breeding with an attractive individual, invest more in a breeding attempt. A mate of higher quality may affect the fitness of offspring in numerous potential ways. One which has gained particular attention in recent years, and which is of particular relevance for birds, which lay large, but variably provisioned ova, concerns the possibility of maternal effects influencing the fitness of offspring.
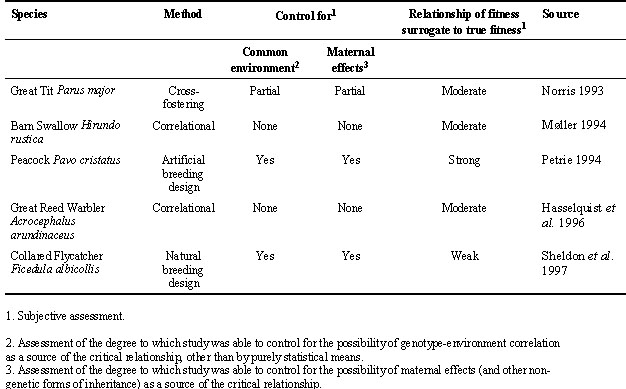
Five relatively recent studies of birds have been presented as evidence in direct support of genetic indicator models of sexual selection; they are summarised in Table 1. All five studies report a positive association between the size of a male secondary sexual character, known (or suspected to be) the target of female choice, and some component of fitness in the offspring of these males. The way in which these studies have dealt with the two major problems outlined above is also briefly described in Table 1. Møller (1994) and Hasselquist et al. (1996) rely on statistical arguments when asserting that common environment or maternal effects cannot be important in their studies. Although their arguments are reasonable, they are also less than completely satisfactory, since one must always allow for the possibility that an unmeasured environmental character varies systematically with the phenotype of attractive males. Norris (1993) attempted to control for common environment effects by cross-fostering clutches of Great Tits Parus major: while this method is frequently used in studies of avian quantitative genetics to control for common environment effects, its efficacy can be questioned, since it will only control for effects that occur after the time at which young or eggs are transferred between nests (e.g. Lynch & Walsh 1998). In addition, this design cannot control for the possibility that maternal effects influence both secondary sexual character size and viability in the great tits and could thus, at least partly, be responsible for the relationship that Norris found. Petrie (1994) took a more directly experimental approach by randomly assigning female Blue Peafowl Pavo cristatus to males in aviaries, and rearing the eggs away from the females before releasing them into semi-natural conditions (a zoological park). The random allocation of females to males, and the separation of parents from offspring as early as possible together provide very strong evidence in favour of genetic benefits models of sexual selection.
The Case of the Collared Flycatcher
My colleagues and I (Sheldon et al. 1997) took a slightly different approach in our work on Collared Flycatchers Ficedula albicollis, and the results of this work will be the subject of the remainder of this paper. We reasoned that one way to control for the possibility of different maternal effects and common environment effects was simply to make comparisons between groups of individuals which by definition experience the same maternal environment. In this way, the problem simply vanishes. Since the first application of DNA fingerprinting to wild bird populations (Burke & Bruford 1987; Wetton & Parkin 1987), demonstrations that broods of nestling birds, particularly passerines, may contain offspring with different fathers have become almost commonplace (see reviews in Westneat & Webster 1994; Petrie & Kempenaers 1998). Because nestlings sired through extra-pair copulations have the same mother and experience the same rearing environment (given that factors such as fertilisation order are not important for development of nestlings), any systematic differences between maternal half-sibling nestlings must arise through the action of genes inherited from the father. This system thus provides an unusual opportunity (a ‘natural breeding design’) to estimate paternal genetic effects on offspring (see Sheldon 1994; Weigensberg et al. 1998, for further discussion).
It must be noted here that employing a maternal half-sib design does not formally exclude the possibility that differential investment by females could occur at some stage before or after hatching, and thus confound any paternal genetic effects. Indeed, it has been suggested that artificial insemination is required in order to be certain that differential investment has not occurred (Møller & Thornhill 1998; would even this be enough though? It is logically possible that sperm might carry signals correlated with male ornamental displays that enabled females to distinguish between the sperm of attractive and unattractive males, and to provision the resulting offspring accordingly). Møller and Thornhill’s suggestion is, in fact, irrelevant in the present context, because in order for there to be selection for differential investment in one set of half-siblings rather than another requires that there are genetically-based differences in the reproductive value of those offspring (Sheldon et al. 1997). Hence, any differential investment by females in such offspring can be regarded as part of the ‘extended phenotype’ (sensu Dawkins 1982) of those offspring.
Previous work conducted on the collared flycatcher has revealed that the size of a patch of white feathers on the forehead predicts a male’s success in several components of sexual selection. Males with large white forehead patches are likely to have experienced better environmental conditions in the past, either as adults or nestlings (Gustafsson et al. 1995), are more likely to be polygynous (Gustafsson et al. 1995), produce a progeny sex ratio biased in favour of sons (Ellegren et al. 1996), are more likely to win contests over nestbox ownership (Pärt & Qvarnström 1997) and enjoy greater success in sperm competition (Sheldon & Ellegren 1998a). Furthermore, studies of interspecific discrimination suggest that this is a trait which reinforces premating isolation between the collared flycatcher and its sister species, the Pied Flycatcher F. hypoleuca, in areas of sympatry (Sætre et al. 1997). Forehead patch size thus provides a natural candidate for testing for the genetic indicator mechanism in this species.
In order to detect cases of maternal half-sibs, we screened a large number of families of collared flycatchers with three polymorphic microsatellite markers: details of methods are given in Sheldon & Ellegren (1996, 1998a) and Sheldon et al. (1997). Extra-pair paternity is relatively frequent in collared flycatchers: approximately one third of broods contained young sired by a male other than that which provided them with parental care (Sheldon & Ellegren 1998a). This population is one that exhibits unusually high natal philopatry for a trans-Saharan migrant: approximately 11% of fledged nestlings recruit locally (Lindén et al. 1992; Merilä et al. 1997). Notwithstanding this high rate of recruitment, our ability to measure fitness of offspring is limited: simple demographic analyses show that at this rate of recorded recruitment we detect only c. 40% of surviving nestlings (Pärt 1996; Sheldon & Ellegren 1998a). For this reason we used as a surrogate for fitness a measure of nestling fledging condition, body mass corrected for body size (residuals from a linear regression of mass at fledging on tarsus length), which has been shown to be consistently related to probability of recruitment in this and a number of other passerine bird species (this species: Lindén et al. 1992; Merilä et al. 1997: others: Tinbergen & Boerlijst 1990; Verboven & Visser 1998). There is no evidence from this population that this trait is negatively correlated with any component of fitness in adults (Gustafsson & Sutherland 1988; Gustafsson et al. 1995).
The first step in our analyses of the relationship of this trait in nestlings to the appearance of their fathers was simply to ask whether we could detect any paternal genetic influence on the character. As discussed above, genetic variance for a character is an absolute requirement for genetic indicator mechanisms. This was done using mixed-model ANOVA to calculate the variance components of this character: the nesting of sires within dams that results from extra-pair paternity allows one to estimate the contributions made by the combination of maternal genes, maternal effects and rearing environment versus those made by paternal genes. There were 21 families for which two males had sired offspring in the same brood. These analyses revealed that although the largest component of variance (approximately 54%) was due to the former term, there was a statistically significant effect of sire which explained about 18% of the variance in nestling fledging condition (Table 2a). This finding is in broad agreement with a previous study of this population, which estimated the genetic component of fledging condition via a partial cross-fostering experiment (Merilä 1996). Thus, although paternal genes contribute a significant amount to the variance in this character, the largest effect is due to terms reflecting the environment experienced by the nestlings (corresponding to the nestbox term in Table 2).
In this study we used only three microsatellite markers which, while giving an acceptably high probability of detecting cases where a male was not the father of the offspring that it provided parental care to, do not give sufficient resolving power to identify the fathers of such offspring with confidence in all cases (Sheldon & Ellegren 1996: those cases where identification of extra-pair sires is possible are generally those where the extra-pair sire has contributed alleles that are rare in the population). As a consequence we were able to identify both sires in broods of multiply sired-offspring in only five cases. Despite the small sample size, we found that there was a significant positive relationship between the difference in forehead patch size of the two sires of multiply-sired broods, and the difference in fledging condition of their offspring (Fig. 1a). This relationship suggests that females that engage in extra-pair copulations with males with a larger forehead patch than that of the male they are paired with will obtain genes that increase the fledging condition of their offspring, a trait known to be related to fitness in this population. Consequently, one would expect pursuit of extra-pair copulations by females to be dependent on the size of the forehead patch of the male that they are paired with. This has been shown to be the case in this population (Sheldon & Ellegren 1998a).
Experimental Creation of Half-Siblings
Although relying on natural cases of extrapair copulation to produce half-siblings can produce useful data, the rate of extra-pair paternity in the collared flycatcher (and in many other species) is such that the majority of broods for which paternity is analysed using molecular markers prove not to contain any extra-pair sired offspring. Such analyses are expensive and time-consuming, and while revealing the absence of extra-pair paternity in a particular brood is useful in some contexts (such as estimating the strength of sexual selection via this mechanism: Sheldon & Ellegren 1998a), they provide no useful data for the kinds of analysis described in the previous section. Lifjeld et al. (1997) described an experimental technique involving temporary male removal (which they employed to study how timing of copulation relates to fertilisation in the Pied Flycatcher), which has the added effect of increasing the rate of extra-pair paternity, and facilitating the identification of extra-pair sires. Since 1996 I have been employing this technique to create experimental broods of maternal half-sibs in the Gotland Collared Flycatcher population. Here, I report some results obtained from the first year of these manipulations, in 1996. Further details of these experiments can be found in Sheldon et al. (1998). These broods have also been used to study the relationship between paternity and paternal effort in male flycatchers (Sheldon & Ellegren 1998b).
Experimental Technique
The experimental technique relies on the fact that if a paired male is removed close to laying (by capture in a mist net, following attraction to a tape-recording of song and a decoy), in the majority of cases (81% of 37 male removals in 1996) a replacement male appears, which defends the nestbox when subsequent presentations of tape and decoy are made. Replacement males are relatively easy to capture, and in the majority of cases in 1996 proved to be immediate territorial neighbours (Sheldon et al. 1998). Nests at which removal occurred were chosen on the basis of the state of completion of the nest, and in 62% of cases (23/37) occurred between one and three days before the first egg was laid. Removed males were released close to the nestbox at which they were caught after 48 h in captivity: most males soon regained their nestbox, and in more than 80% of cases provided some parental care to the nestlings in that nestbox (Sheldon & Ellegren 1998b).
The removal experiments had a marked effect on the rate of extra-pair paternity. Whereas in non-experimental broods in a previous year only 33% of broods (26/79) contained extra-pair sired offspring, 94% of experimental broods (29/31) in 1996 did so (Sheldon & Ellegren 1998a,b). The difference is statistically significant (G = 38.81, df = 1, P < 0.0001). In addition, the proportion of multiply-sired broods increased significantly (23/79 [29%] versus 23/31 [74%]: G = 19.97, df = 1, P < 0.0001).
One potential worry with this technique is that it may induce correlations between fertilisation order (and hence laying order) and male phenotype that are different from those in naturally occurring cases of extra-pair paternity. There are reasons to think that this is not a problem in the current study species, because hatching is very synchronous, with the result that there is little evidence for a correlation between the brood hierarchy at hatching and at fledging (Sheldon et al. 1997). Also, because removals are random with respect to male phenotype (as far as we are aware), there should be an even distribution of offspring of sires with different phenotypes with respect to fertilisation order among the broods.
As in 1994, when we performed mixed-model ANOVA to estimate variance components for fledging condition of nestlings, we found that the largest proportion of the variance was explained by nestbox, but again a significant amount of variance in fledging condition was explained by the term sire (Table 2b). There was quite good agreement between the estimates of variance components obtained in the two different years (Table 2a and Table 2b). These results therefore lend further weight to the suggestion that genes inherited from the father are responsible for an appreciable degree of variation in fledging condition of offspring.
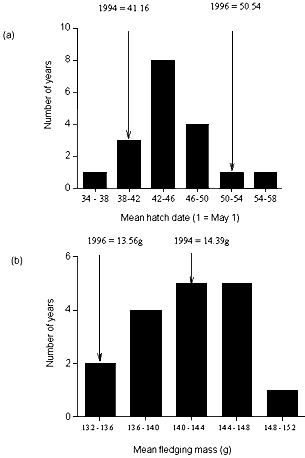
For eight broods in which we could identify both sires, and for which nestlings survived to fledge we found, once again that the difference in forehead patch size of the two sires was significantly related to the difference in fledging condition when the maternal half-sibs sired by the two males were compared (Fig. 1b). However the relationship was of opposite sign to that obtained in the previous analysis (Fig. 1a). The two relationships differ significantly (ANCOVA: F1,9 = 21.76, P = 0.001). These results therefore confirm that forehead patch size of male collared flycatchers acts as an indicator of genes influencing fledging condition (and therefore potentially the viability of offspring). However, they add a further level of complexity by suggesting that the effect of the genes inherited from a male may be dependent on the environment in which those genes are expressed. While one must be cautious in attributing between-year differences to environmental differences between those years, there were striking differences in breeding phenology and fledging success between the two years. The first year, 1994, can be characterised as a ‘warm, early’ spring, in which flycatchers bred relatively early (Fig. 2a), and in which fledging success was high (only 3% of nestlings died between hatching and fledging). In contrast, 1996 was a ‘cold, late’ spring, in which flycatchers bred late (mean hatching date was the second latest in the 18 years in which this population has been studied: Fig. 2a), and in which mortality between hatching and fledging was considerable (23%: Sheldon et al. 1998). Fledging mass showed corresponding differences. While that in 1994 was close to the long-term average, nestlings that survived to fledging in 1996 had the second lowest mean fledging mass observed over 17 years (Fig. 2b); the means differed by 0.83g between 1994 and 1996, which is a difference of approximately 0.6 standard deviations of fledging weight in a typical year. Thus, 1994 could be said to have been a fairly typical year for breeding flycatchers, whereas conditions experienced in 1996 were rather severe.
Genotype-Environment Interactions and Maintenance of Genetic Variation
The results described above are preliminary, in the sense that evidence that a specific environmental factor affects the relationship between a male trait and fitness of that males offspring is lacking. Nevertheless, they suggest some exciting possibilities for experimental tests of whether the genetic benefits to be obtained by mating with attractive males are dependent upon the environment. One potential experimental design is as follows: males are temporarily removed, as described above, in order to induce elevated rates of extra-pair paternity. After hatching, brood size is experimentally manipulated by exchanging young between experimental broods in order to manipulate rearing conditions. If the inference about the difference between the data collected in 1994 and 1996 is correct, there should exist an interaction between the experimental manipulation and the size of the father’s forehead patch on the fledging condition of that male’s offspring. This experiment indicates the potential value of the techniques described above: by restricting comparisons to maternal half-siblings, differences between nests are effectively eliminated. Similar experimental manipulations could be conducted to investigate other forms of genotype-environment interaction in relation to paternal secondary sexual character size.
If genotype-environment interactions of this form are confirmed experimentally, what implications will it have? One of the major challenges facing empirical studies of ecological genetics is to determine which mechanisms may act to maintain variation for traits subject to selection in natural populations. The white forehead patch of the collared flycatcher is a case in point. Cross-fostering experiments suggest that this trait has a moderately high heritability (approximately 0.44: Sheldon et al. 1997). In concert with demonstrable directional sexual selection on this trait (e.g. Sheldon & Ellegren 1998), the existence of quantitative genetic variation suggests that this trait should respond to selection and that selection should quite rapidly deplete genetic variation. Many mechanisms have been suggested which have the potential to maintain genetic variation in the face of selection: they can be divided into two classes. One class consists of mutation-selection balance models (Lande 1976; Turelli 1984), and their utility to explain the maintenance of variation in natural populations is suggested by the possibility that mutation rates for traits affecting fitness may have been underestimated in laboratory studies (Kondrashov & Houle 1994; Shabalina et al. 1997). The second class of mechanisms is based around the suggestion that spatially or temporally variable selection can maintain genetic variation. This was the key insight behind Hamilton & Zuk’s (1982) suggestion that parasites might be important in sexual selection: the arms race between hosts and parasites is a source of temporal variation in selection pressures on resistance genes in hosts, the possession of which is suggested to be revealed by sexual displays. Although simple haploid models suggest that fluctuating selection will not act to maintain genetic variation, models which incorporate overlapping generations suggest that this process can be an effective means to maintain variation (Chesson & Warner 1981; Ellner & Hairston 1994; Sasaki & Ellner 1997). Evidence that genotype-environment interactions for fitness-related traits may be common in nature is growing (see Coulson et al. 1998 for a recent example).
A second implication that the frequent occurrence of genotype-environment interactions of the form discussed here would have, is that selection on female choice would also be environmentally dependent. Thus, in 1996 females would apparently have done better to have extra-pair copulations with males with small forehead patches, the reverse of the situation in 1994. Models of female choice generally assume (for simplicity) that the same male is the optimal father of offspring across all environments. If this assumption is commonly violated, it may be interesting to consider the extent to which female choice strategies should show phenotypic plasticity in response to environmental variation. In the present case it may of course be that it is not possible for females to predict the environment in which offspring will be reared with sufficient accuracy to select for appropriate plasticity in choice.
Further Prospects
Using the techniques described in this paper (comparisons between maternal half-sibs, and experimental creation of broods of half-sibs), it may be possible to undertake detailed experimental investigations of the role of genotype-environment interactions in maintaining variation in natural populations. These techniques could also be used to address other questions. A number of studies (Table 1) have now demonstrated in birds that sexual signals displayed by males seem to signal genes that influence the fitness of those males’ progeny, and similar demonstrations have been made in other taxa (e.g. Moore 1994; Jia & Greenfield 1997; Welch et al. 1998). However, one of the next challenges for such studies will be to try to explain the effects observed by determining what kinds of genes are responsible for the fitness advantage enjoyed by the offspring of ornamented males. Are genes determining physiological efficiency, cognitive efficiency or the strength of immune responses the key to genetic benefits? Or does the genetic quality conferred by more ornamented males simply reflect the possession of fewer mildly deleterious mutations throughout the genome? Almost nothing is known about this question, other than a recent (and probably special) case, reported from stalk-eyed flies, where genes for a preferred trait in males are linked to genes that suppress the activity of a meiotic drive gene (Wilkinson et al. 1998), and recent evidence from Ring-necked Pheasants Phasianus colchicus that spur length is correlated with the possession of particular MHC genotypes (von Schantz et al. 1996)
As an example of how this question could be approached in wild populations I present some preliminary data concerning the sources of variation in immune responsiveness in nestling collared flycatchers. Recent interest in immune function variation in birds has been stimulated by the realisation of possible links between immune function and both sexual selection and life-history trade-offs (reviewed in Sheldon & Verhulst 1996). Variability in genes determining immune responsiveness has been seen as an extension of Hamilton & Zuk’s (1982) hypothesis relating parasites to sexual selection. Together with M. Andersson, I used the broods of half-sibs created experimentally in 1996 to test whether genes that nestlings inherited affecting their immune responsiveness were related to the phenotype of their father. We assessed one component of immune responsiveness (T-cell response) by challenging nestlings with a T-cell stimulator (PHA: see Saino et al. (1997) for further details of this technique) at the age of 10 days (fledging occurs at 13-14 days of age). Although we were able to demonstrate paternal genetic effects on some traits within nestlings in this sample (tarsus length and fledging condition: see Table 2b for the latter), there was no evidence that variation in nestling immune responsiveness at this age was influenced by genes at all (Table 3). In contrast, there was a strong effect of the nest of origin (39% of the variance in T-cell response) suggesting that cross-fostering experiments would not be an adequate means to control for common environment effects for this trait (c.f. Saino et al. 1997). Given the absence of paternal genetic effects on T-cell responsiveness, there is little reason to expect a relationship between the phenotype of males and the phenotype of their offspring in sets of half-sibs (c.f. Fig. 2): indeed, there was no relationship (r = 0.29, n = 8, P = 0.5).
A second exciting possibility for future work concerns the use of data generated by large-scale genotyping of natural populations to estimate quantitative genetic parameters relevant to sexual selection directly. Newly developed methods described by Ritland (1996) (see also Ritland & Ritland 1996; Mousseau et al. 1998), rely on using allele sharing as a measure of genetic similarity in the absence of data about relatedness of individuals, and can be used to estimate quantitative genetic parameters (e.g. genetic variances and covariances) in the absence of pedigrees. These techniques are still in their infancy, and require an appreciable degree of spatial structuring of relatives in order for them to be applicable (Ritland 1996), so that it is not clear how many avian populations will be suitable for this sort of analysis. This and other techniques discussed in this paper suggest that the application of molecular markers to wild populations of birds will continue to reveal new information about quantitative genetics of fitness-related characters and their relation to sexual selection in natural populations of birds.
ACKNOWLEDGEMENTS
Ian Owens and Marion Petrie commented constructively on the manuscript. The work described here has been funded by grants from the Swedish Natural Sciences Research Council (to BCS and to Lars Gustafsson), a Postdoctoral Research Fellowship from the Natural Environment Research Council (UK) awarded to BCS, and held at the Institute of Cell, Animal and Population Biology, Edinburgh, UK, and by research grants from the Association for the Study of Animal Behaviour and Stiftelsen för Zoologisk Forskning, Uppsala.
REFERENCES
Andersson, M. 1994. Sexual selection. Princeton: Princeton University Press: 599pp.
Burke, T. & Bruford, M. 1987. DNA fingerprinting in birds. Nature 327: 149-152.
Burt, A. 1995. The evolution of fitness. Evolution 49: 1-8.
Charlesworth, B. 1987. The heritability of fitness. In: Bradbury, J.W. & Andersson, M.B. (eds), Sexual Selection: Testing the Alternatives. Chichester: Wiley; pp.21-40.
Chesson, P.L. & Warner, R.R. 1981. Environmental variability promotes coexistence in lottery competitive systems. American Naturalist 117: 923-943.
Coulson, T.N., Albon, S.D., Pemberton, J.M., Slate, J., Guinness, F.E. & Clutton-Brock, T.H. 1998. Genotpye-environment interactions in winter survival in red deer. Journal of Animal Ecology 67: 434-445.
Dawkins, R. 1982. The Extended Phenotype. Oxford: Oxford University Press.
Ellegren, H., Gustafsson, L. & Sheldon, B.C. 1996. Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. Proceedings of the National Academy of Sciences of the USA 93: 11723-11728.
Ellner, S. & Hairston, N.G., Jr. 1994. Role of overlapping generations in maintaining genetic variation in a fluctuating environment. American Naturalist 143: 403-417.
Fernandez, J. & LopezFanjul, C. 1996. Spontaneous mutational variances and covariances for fitness-related traits in Drosophila melanogaster. Genetics 143: 829-837.
Fisher, R.A. 1958. The Genetical Theory of Natural Selection. Oxford: Clarendon Press.
Fowler, K., Semple, C., Barton, N.H. & Partridge, L. 1997. Genetic variation for total fitness in Drosophila melanogaster. Proceedings of the Royal Society of London, Series B 264: 191-199.
Gibson, R.M. & Langen, T.A. 1996. How do animals choose their mates? Trends in Ecology and Evolution 11: 468-470.
Gustafsson, L., Qvarnström, A. & Sheldon, B.C. 1995. Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375: 311-313.
Gustafsson, L. & Sutherland, W.J. 1988. The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature 335: 813-815.
Hamilton, W.D. & Zuk, M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218: 384-387.
Hasselqvist, D., Bensch, S. & von Schantz, T. 1996. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature 381: 229-232.
Jia, F.-Y. & Greenfield, M.D. 1997. When are good genes good? Variable outcomes of female choice in wax moths. Proceedings of the Royal Society of London, Series B 264: 1057-1063.
Keightley, P.D. & Caballero, A. 1997. Genomic mutation rates for lifetime reproductive output and lifespan in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the USA 94: 3823-3827.
Kibota, T.T. & Lynch, M. 1996. Estimate of the genomic mutation rate deleterious to overall fitness in E. coli. Nature 381: 694-696.
Kondrashov, A.S. & Houle, D. 1994. Genotype-environment interactions and the estimation of the genomic mutation rate in Drosophila melanogaster. Proceedings of the Royal Society of London, Series B 258: 221-227.
Lande, R. 1976. The maintenance of genetic variability by mutation in a ploygenic character with linked loci. Genetic Research, Cambridge 26: 221-235.
Lifjeld, J.T., Slagsvold, T. & Ellegren, H. 1997 Experimental mate switching in pied flycatchers: male copulatory access and fertilisation success. Animal Behaviour 53: 1225-1232.
Linden, M., Gustafsson, L. & Pärt, T. 1992. Selection on fledging mass in collared flycatchers and great tits. Ecology 73: 336-343.
Lynch, M. & Walsh, B. 1998. Genetics and Analysis of Quantitative Traits. Sunderland, Mass.: Sinauer: 980pp.
Merilä, J. 1996. Genetic variation in offspring condition: an experiment. Functional Ecology 10: 465-474.
Merilä, J., Sheldon, B.C. & Ellegren, H. 1997. Antagonistic natural selection revealed by molecular sex identification of nestling collared flycatchers. Molecular Ecology 6: 1167-1175.
Møller, A.P. 1994. Male ornament size as a reliable cue to enhanced offspring viability in the barn swallow. Proceedings of the National Academy of Sciences of the USA 91: 6929-6932.
Møller, A.P. & Thornhill, R. 1998. Male parental care, differential parental investment by females and sexual selection. Animal Behaviour 55: 1507-1515.
Moore, A.J. 1994. Genetic evidence for the ‘good genes’ process of sexual selection. Behavioral Ecology and Sociobiology 35: 235-241.
Mousseau, T.A., Ritland, K & Heath, D.D. 1998. A novel method for estimating heritability using molecular markers. Heredity 80: 218-224.
Mukai, T., Chigusa, S.I., Mettler, L.E. & Crow, J.F. 1972. Mutation rate and dominance of genes affecting viability in Drosophila melanogaster. Genetics 72: 335-355.
Norris, K. 1993. Heritable variation in a plumage indicator of viability in male great tits Parus major. Nature 362: 537-539.
Pärt, T. 1996. Problems with testing inbreeding avoidance: the case of the collared flycatcher. Evolution 50: 1625-1630.
Pärt, T. & Qvarnström, A. 1997. Badge size in collared flycatchers predicts outcome of male competition over territories. Animal Behaviour 54: 893-899.
Peck, J. & Eyre-Walker, A. 1997. The muddle about mutations. Nature 387: 135-136.
Petrie, M. 1994. Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371: 598-599.
Petrie, M. & Kempenaers, B. 1998. Extra-pair paternity in birds: explaining variation between species and populations. Trends in Ecology and Evolution 13: 52-58.
Ritland, K. 1996. Marker-based method for inferences about quantitative inheritance in natural populations. Evolution 50: 1062-1073
Ritland, K. & Ritland, C. 1996. Inferences about quantitative inheritance based on natural population structure in the yellow monkeyflower, Mimulus guttatus. Evolution 50: 1074-1082.
Sætre, G.P., Moum, T., Bures, S., Kral, M., Adamjan, M. & Moreno, J. 1997. A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387: 589-592.
Saino, N., Calza, S. & Møller, A.P. 1997. Immunocompetence of nestling barn swallows in relation to brood size and parental effort. Journal of Animal Ecology 66: 827-836.
Sasaki, A. & Ellner, S. 1997. Quantitative genetic variance maintained by fluctuating selection with overlapping generations: variance components and covariances. Evolution 51: 682-696.
Shabalina, S.A., Yampolsky, L.Y. & Kondrashov, A.S. 1997. Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proceedings of the National Academy of Sciences of the USA 94: 13034-13039.
Sheldon, B.C. 1994. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proceedings of the Royal Society of London, Series B 257: 25-30.
Sheldon, B.C., Davidson, P. & Lindgren, G. 1998. Mate replacement in experimentally widowed collared flycatchers: determinants and outcomes. Behavioral Ecology and Sociobiology, submitted.
Sheldon, B.C. & Ellegren, H. 1996. Offspring sex and paternity in the collared flycatcher. Proceedings of the Royal Society of London, Series B 263: 1017-1021.
Sheldon, B.C. & Ellegren, H. 1998a. Sexual selection resulting from extra-pair paternity in collared flycatchers. Animal Behaviour, in press.
Sheldon, B.C. & Ellegren, H. 1998b. Paternal effort related to experimentally manipulated paternity of male collared flycatchers. Proceedings of the Royal Society of London, Series B, in press.
Sheldon, B.C., Merilä, J., Lindgren, G. & Ellegren, H. 1998. Gender and environmental sensitivity in nestling collared flycatchers. Ecology, in press.
Sheldon, B.C., Merilä, J., Qvarnström, A., Gustafsson, L. & Ellegren, H. 1997. Paternal genetic contribution to offspring condition predicted by size of male sexual ornament. Proceedings of the Royal Society of London, Series B 264, 297-302.
Sheldon, B.C. & Verhulst, S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution 11: 317-321.
Tinbergen, J.M. & Boerlijst, M.C. 1990. Nestling weight and survival in individual great tits. Journal of Animal Ecology 59, 1113-1127.
Turelli, M. 1984. Heritable genetic variation via mutation-selection balance: Lerch’s zeta meets the abdominal bristle. Theoretical Population Biology 25: 138-193.
Verboven, N. & Visser, M.E. 1998. Seasonal variation in local recruitment of great tits: the importance of being early. Oikos, in press.
von Schantz, T., Wittzell, H., Goransson, G., Grahn, M. & Persson, K. 1996. MHC genotype and male ornamentation: Genetic evidence for the Hamilton-Zuk model. Proceedings of the Royal Society of London, Series B 263: 265-271.
Weigensberg, I., Carrière, Y. & Roff, D.A. 1998. Effects of male genetic contribution and paternal investment to egg and hatchling size in the cricket, Gryllus firmus. Journal of Evolutionary Biology 11: 135-146.
Welch, A.M., Semlitsch, R.D. & Gerhardt, H.C. 1998. Call duration as an indicator of genetic quality in male gray tree frogs. Science 280: 1928-1930.
Westneat, D.F. & Webster, M.S. 1994. Molecular analyses of kinship in birds: interesting questions and useful techniques. In: Schierwater, B., Streit, B., Wagner, G.P. & DeSalle, R. (eds) Molecular Ecology and Evolution: Approaches and Applications. Basel; Birkhäuser: pp. 91-126.
Wetton, J.H., Carter, R.E, Parkin, D.T. & Walters, D. 1987. Demographic study of a wild house sparrow population by DNA fingerprinting. Nature 327: 147-149.
Wilkinson, G.S., Presgraves, D.C. & Crymes, L. 1998. Male eye span in stalk-eyed flies indicates genetic quality by meiotic drive suppression. Nature 391: 276-279.
Table 1. Summary of published studies reporting a relationship between male trait and fitness in offspring consistent with genetic indicator models of sexual selection.

Table 2. Sources of variance in fledging condition of maternal half-sibling collared flycatchers.
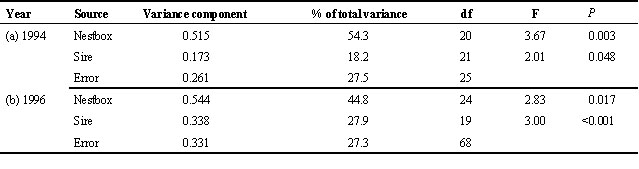
Table 3. Sources of variance in T-cell responsiveness of maternal half-sibling collared flycatchers.

Fig 1. Relationship between difference in forehead patch size of sires of maternal half-sib collared flycatchers, and the difference in fledging condition of those nestlings. (a) 1994: rS = 0.900, P = 0.037; (b) 1996: rS = -0.691, P = 0.058.
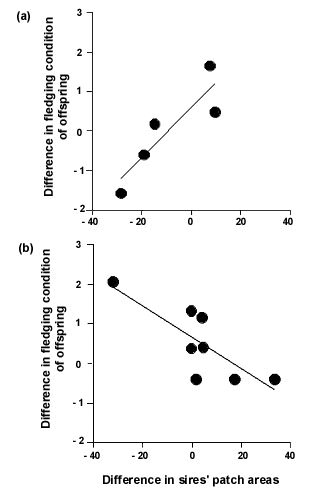
Fig 2. Distribution of (a) mean annual hatching dates in the Gotland population of collared flycatchers from 1980-1997 (n =18) and (b) mean fledging mass from 1981-1997 (n = 17). The relative positions of the two years (1994 and 1996) in which contrasting results regarding the relationship between male forehead patch size and fledging condition of their offspring were obtained are indicated.