S19.1: Sexual selection and avian speciation
Marion Petrie
Evolution and Behaviour Research Group, Department of Psychology, University of Newcastle, Newcastle-upon-Tyne, NE1 7RU, UK, fax 44 191 222 5622, e-mail Marion.Petrie@ncl.ac.uk
Petrie, M. 1999. Sexual selection and avian speciation. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1089-1098. Johannesburg: BirdLife South Africa.The aim of this paper is to suggest a new mechanism whereby mate choice can drive sympatric speciation. Differences among females in their mate preferences can result in reproductive isolation if one group of females prefers a different sort of male to another group of females. In sexually selected species, where female choice is maintained by high levels of genetic variability among males, females need to assess genetic compatibility as well as the overall genetic quality of their mates. High levels of genetic variation and local risks of inbreeding as a result of polygyny both increase the need to take account of genetic compatibility when choosing a mate. It is the need to assess genetic compatibility in relation to a female’s own genotype that generates variable female preferences and can explain why not all females mate with the highest quality male. Signals that reveal genetic identity and allow the assessment of genetic compatibility will evolve since there are fecundity costs of mating with a genetically incompatible individual. Signals that reveal genetic identity to females, with which females can compare their own genotype, give rise to variable female preferences, reproductive isolation and ultimately to speciation.
INTRODUCTION
There are clear indications that sexual selection could be important in the adaptive radiation’s of birds. Firstly, closely related species appear to differ in traits such as song and plumage and it is these traits that appear to have evolved by sexual selection. Numerous bird studies have shown female preferences for song and/or plumage (see Andersson 1994; Johnstone 1995 for reviews). Secondly, there is some evidence that sexual selection is linked to an accelerated rate of speciation in birds. Bird groups that are more sexually dimorphic, and therefore show indications of the results of sexual selection, are relatively speciose (Barraclough et al. 1995, Møller & Cuervo 1998). Moreover, a link between mating system and speciation has been found, with larger numbers of species in promiscuous taxa (Mitra et al. 1996). However, the way in which sexual selection and speciation are linked is largely unknown, although there are some theoretical models that suggest an association (Lande 1981, Schluter and Price 1993, Turner and Burrows 1995). It is possible that speciation caused by ecological differentiation could result in enhanced sexual selection but, what is perhaps of more interest is the possibility that sexual selection itself can drive sympatric speciation, and the aim of this paper is to suggest a new mechanism by which this could occur.
Speciation initially requires reproductive isolation of a part of a population. Once reproductive isolation is achieved this can lead to population differentiation and divergence. Theoretically, differences in female preferences could result in reproductive isolation if one group of females in a population preferred one sort of male and another group of females preferred another sort of male. Under these circumstances reproductive isolation could be achieved (Turner & Burrows, 1995). So if we could understand why female preferences vary intraspecifically it may be possible to throw some light on the causes of reproductive isolation.
However, before we can understand variation in female preferences we need to know why female preferences exist at all. What selective forces maintain female preferences in birds? Female preferences can be maintained by direct selection where males provide resources for the female and/or her offspring. In addition to this, or alternatively in species where the male provides no paternal care, female preferences can be maintained by indirect selection where females gain genes that either improve the mating success or viability of their offspring (Kirkpatrick & Ryan 1991). The aim of this paper is to consider how variability in female preferences might arise under the different evolutionary scenarios for the maintenance of female preferences. In this way I hope to provide insights into the possible role of sexual selection in driving reproductive isolation. I concentrate on the possibility that indirect selection could maintain female preferences and variability in the sort of males that females prefer and I suggest a new model for the role of sexual selection in driving speciation.
How are female preferences maintained?
There are three basic models for the evolution and subsequent maintenance of female preferences for elaborate male morphology.
(1) Direct selection on female preferences where the female gain's material or resource based benefits from her preference (Heywood 1989, Hoelzer 1989). Andersson (1995) reviews the empirical evidence for female choice for direct benefits.
(2) Indirect selection on female preferences where females gain genetic benefits for their sons who inherit the preferred characteristic of their fathers and themselves have high mating success (Fisher 1930, Pomiankowski et al. 1991). There is very little evidence for a pure 'Fisherian' process in birds (Bakker & Pomiankowski 1995).
(3) Indirect selection on female preferences where females gain genetic benefits in the form of enhanced viability for their sons and daughters (Iwasa et al. 1991). Evidence from several intraspecific bird studies suggests that there is a link between the preferred male character and offspring viability (see Table 1).
These hypotheses are not mutually exclusive and it is possible that in some species females may gain all three potential benefits. However, in those species where males provide no obvious resources for their offspring other than seminal fluid it is clear that some form of indirect selection must be operating. Moreover those species that show the strongest sexual selection and the highest variance in mating success tend to be those species where there is the least amount of direct resource contribution, for example, Peafowl Pavo cristatus, which is a lekking species where males provide no paternal care or defend any resources. Whilst studies showing a link between a preferred male character and offspring viability (Table 1) do not preclude the possibility that females also gain from producing attractive sons it is clear that so called 'viability indicator models' are responsible for the maintenance of female preferences in at least some species of birds.
How can differences in female mate preferences be generated?
The causes of variation in female preferences have received less attention than the maintenance of female preferences and generally remain poorly understood (Jennions & Petrie 1997).
If female preferences have arisen and are maintained because females gain benefits from their mates (either direct or indirect) it is hard to see why there should be any variability among females in their choice of partner. Theoretically, all females should prefer to mate with the male that is of highest quality or provides the most resources. This would lead to there being very little variation amongst males. This lack of variability among males is a general problem for the maintenance of female choice and it is particularly acute when one also considers that any genetic fitness benefits a female might receive would also lead rapidly to a reduction in genetic variability amongst males. For a female preference for males of high genetic quality to be maintained there must be persistent genetic variation among males. Similarly, for there to be variability among females in their choice of partners there also needs to be variation among males.
How is genetic variation among males maintained?
None of the studies that have provided evidence for genetic benefits accruing to choosy females have provided any clues as to how the underlying genetic variation in fitness amongst males is maintained. Female choice for genetic benefits is directly dependent upon male genetic variation (Petrie & Lipsitch 1994). When males do not differ genetically there is little benefit for female choice, and yet there is good evidence that females can receive genetic benefits in birds (Table 1).
Any genetic variation ultimately arises from mutation and mutation could directly continually replenish the genetic variation removed by selection (Lande 1976). Mutation could be indirectly maintained in a population by other mechanisms, such as by a co-evolutionary arms race between host and parasite (Hamilton & Zuk 1982). In a sexually selected species where female choice is maintained by indirect benefits, selection is strong as female choice is acting in the same direction as viability selection, and any genetic variation should quickly go to fixation. It can be concluded that if mutation is to balance this selection there must be a relatively high level of mutation in sexually selected species.
In a recent paper, I have suggested that female preferences have arisen in order to distinguish the mutational state of male partners and that rather than choosing 'good genes' females are avoiding mating with males that have 'bad genes' or which have very high mutation rates (Petrie ms). Female choice is maintained by high levels of mutation (Petrie ms).
What are the consequences of genetic variability among males?
If genetic variation is maintained by mutation and sexual preferences have evolved to reduce the costs of these mutations then in general it can be said that sexually selected traits have evolved to indicate overall mutation level (either the number of accumulated mutations or mutation rate). A good quality male is one who can signal a low level of mutation. It follows that in sexually selected species with a relatively high mutation rates that there will be a higher incidence of deleterious mutations. As a result of a high level of mutation the chances of an individual producing offspring that are homozygous for a recessive lethal will also be higher. It is also possible that high mutation levels result in a higher incidence of major mutations such as chromosomal inversions. Therefore, it follows that evolution will favour those females that not only select males on the basis of their overall level of mutation (their genetic quality) but, that they should also monitor the genetic identity of potential partners, and only mate with those males who are genetically compatible with their own genotype. It will not only pay an individual female to mate with a male that has the lowest mutation level but also to choose one which is genetically dissimilar (e.g. to avoid recessive lethals) or genetically compatible (e.g. to avoid the harmful effects of chromosomal inversions) in order to provide her with viable offspring. In the case of a female that is heterozygous for a lethal gene, she should choose to mate with a male who is homozygous for the dominant non-lethal allele (rather than a heterozygote male). In the case of a female carrying a chromosomal inversion she should choose a male partner who also carries the same compatible inversion.
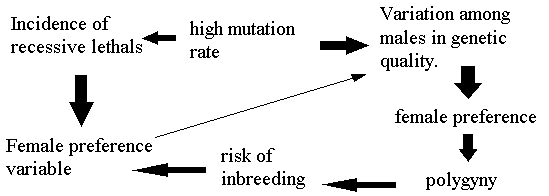
Extreme polygyny, which is a feature of sexually selected species, will also increase the tendency of individuals in a local population to have genes in common (because the effective population size is low) and this will also increase the selection pressure on females to monitor genetic identity and assess genetic compatibility when choosing a mate. Although this can mean avoiding a male that is a close relative, inbreeding does not always carry costs, because some close relatives could be genetically compatible (perhaps because they share a chromosomal inversion), and there are not necessarily fitness costs associated with mating with relatives. Selection will favour females who can precisely match their genotype with that of potential mates, and select males who are specifically compatible. It follows that the best male will not always be the same for all females, and, whilst in many cases the male with the lowest mutation overall will be the best male for most females, some females will not be genetically compatible with him. Females need to judge their own genotype and compare it with that of potential partners when choosing mates. It is the need to take account of genetic compatibility that will generate variability in female preferences in sexually selected species where female preferences are maintained by genetic benefits. This link between overall mutation level and the need to monitor genetic identity as well as genetic quality is summarised in a flow diagram depicted in Figure 1. In situations where the conditions for variation in female preferences are maintained (as a result of the need to monitor genetic compatibility), variation amongst females in their preferences for males will also contribute to the maintenance of genetic variability amongst males.
If it pays females to monitor the genetic identity of their mates and to use this to assess genetic compatibility, it follows that it will pay males to signal their genetic identity. Males have nothing to gain from mating with genetically incompatible females, and should not incur the costs of mating (for example, risk of disease) or lose valuable sperm, which could be available to inseminate females that are capable of producing viable offspring.
Is there any evidence that variability in mate preferences could arise as a result of genetic similarity being important in mate choice?
There is evidence in mammals that females are capable of monitoring genetic identity, and use this information during mate choice; female house mice prefer as mates males with dissimilar MHC genotypes (Egid & Brown, 1989; Potts et al. 1991) apparently to avoid inbreeding effects (Potts et al. 1994), human females also prefer odours of males with dissimilar MHC genotypes (Wedekind et al. 1995). However, to date there is little comparable evidence in birds. Inbreeding depression has been documented in several wild species of birds (Greenwood et al. 1978; van Noordwijk & Sharloo 1981; Gibbs and Grant 1989; Bensch et al. 1994; Keller et al. 1994; Kempenaers et al. 1996; McRae 1996) and these effects could select for a system where birds assess genetic compatibility. Sex biased dispersal (Greenwood et al. 1980) and kin recognition (Bateson 1982) might have evolved to avoid matings with close kin, but, Rowley et al. (1993) & Kempenaers & Sheldon (1996) think it is unlikely that birds can assess the genetic similarity of potential mates.
Peafowl Pavo cristatus are a highly sexually selected species where males display to females at lek sites and contribute no direct resources for reproduction (other than seminal fluid) to females or to their offspring. Previous work on this species has shown that peahens have well defined mating preferences for males with elaborate trains (Petrie et al. 1991, Petrie & Halliday 1994) and it is thought that this preference is maintained by indirect selection, since females mated to males with the most elaborate trains have faster growing offspring that are subsequently more likely to survive (Petrie 1994). It is not clear why all females in a population of peafowl do not mate with the male with the most elaborate train, or why such intense directional selection has not removed all the genetic variation among males. We have recently asked whether peahens take account of genetic similarity when choosing a mate (Petrie & Burke in prep.). We used multilocus DNA fingerprinting to assess the degree of band sharing of copulating pairs and we compared this with the degree of band sharing in pairs of birds taken at random from the same population. We found that the degree of band sharing was greater in pairs of birds taken at random. This implies that copulating pairs were less related than expected by chance and, since these data were collected from a highly inbred population, they indicate that some females are avoiding mating with close relatives (Petrie & Burke in prep).
How do peahens judge genetic compatibility?
It is not clear what mechanism peahens might use to take account of genetic distance. It seems unlikely that peahens could 'know' who their fathers are, or learn anything about them as, like other highly sexually selected species, the male does not participate in reproduction after mating. It also seems unlikely that daughters could routinely learn anything about the identity of their fathers from their mothers, since many females do not return to mate with the same male in successive years. When the same four leks were watched in two successive years only 14 out of 19 (74%) marked females mated on the same lek and only seven (37%) mated with same male (Petrie, unpublished data). Peafowl are also a species where multiple mating (Petrie et al. 1992), dump nesting (Budgey 1994), and post-hatching brood-amalgamation are common (Budgey 1994); factors that would all tend to reduce the potential for females to reliably learn who their full male sibs were. It therefore seems unlikely that peahens could learn the characteristics of their fathers or brothers. Learning the identity of close kin is only useful if females were simply trying to avoid mating with a close relative, but mating with a close relative is not always costly, and more sophisticated mechanisms whereby females actually judge genetic compatibility may have evolved.
One mechanism a peahen may use to assess genetic compatibility is to match the phenotype of a potential partner to her own phenotype (self-referent phenotype matching). It has been suggested that this type of phenotype matching could evolve in situations where other referents cannot reliably be used (Sherman 1990), as appears to be the case when peahens are choosing a genetically dissimilar mate.
Although, we do not have any direct evidence for self-referent phenotype matching by peahens, we do have some indication that peacocks do use a 'genetic' mechanism to recognise their sibs (full and half) under circumstances which cannot involve familiarity with their family members (Petrie, Krupa & Burke ms). As part of a breeding experiment designed to look at paternal effects on offspring performance (Petrie 1994) a group of first year offspring were released into Whipsnade Park in a controlled manner (in batches of one from each sire, see Petrie 1994 for details). Prior to their release the offspring were reared under controlled conditions (the eggs were removed from their parents and artificially incubated (mixing young from different sires) and all hatched offspring were subsequently placed in large mixed groups). As a result of their rearing conditions, these offspring thus had no cues to the identity of any of their family members. However, 4 years after their release they were found to have established permanent display sites close to their brothers (Petrie, Krupa & Burke ms). This unexpected result provides good indirect evidence for some sort of genetic mechanism for kin recognition since learning the characteristics of close kin clearly cannot be involved. Learning could play a part if an individual is memorising aspects of its own phenotype and then matching these with that of a relative.
What sorts of signals are involved in the assessment of genetic identity?
I have argued that it will pay females to assess genetic compatibility and that it will also pay males to advertise their genetic identity. In many highly sexually selected species where polygyny is the norm, there is a correlated lack of paternal care. Under these circumstances it is unlikely that females can use knowledge of their father's phenotype and underlying genotype when assessing the genetic compatibility of potential mates. Under these circumstances, it seems likely that females can only reliably assess the genetic compatibility of potential mates by some genetic mechanism or by self-referent phenotype matching. If self-referent phenotype matching is the important mechanism, this further suggests that signals that are important for phenotype matching must be present in both males and females and that sexually dimorphic signals are unlikely to be important in the assessment of genetic compatibility. Although this expectation comes from theoretical considerations, it does seem likely that the signalling system used for the assessment of genetic compatibility will be very different from that used to assess genetic quality. This may in part explain why sexually selected species have multiple signals, some may be involved in the assessment of genetic compatibility, they need not all advertise genetic quality.
Males and females of the same species of birds have several features in common and some aspect of their morphology could be involved, but, a likely candidate for the assessment of genetic compatibility in birds could involve some aspect of their vocalisations. Vocal signals are very complex and individuals can be identified from their calls. Females as well as males also have vocalisations. So it is possible that self-referent phenotype matching of vocalisations could be involved in the assessment of genetic similarity. In this context it may be significant that many species of birds can only be told apart by their vocalisations.
It is not clear how females can assess genotype from phenotype. However, previous evidence from mice have shown that this ability can and has evolved in mammals (see above). There is no a piori reason to suppose that a similar ability could not have evolved in birds.
Summary of model
I suggest that to understand how sexual selection can promote speciation it is necessary to understand the origin and maintenance of variability in female preferences. When one sort of female prefers to mate with one sort of male, reproductive isolation of a part of a population and, therefore speciation can ultimately occur. If high levels of mutation help to maintain the variation in male genetic quality, in a species where females gain indirect benefits for their offspring from their choice of mate, it is relatively easy to see how variable mate preferences can arise. In a sexually selected species, with high levels of mutation and polygny, females need to assess genetic compatibility as well as overall genetic quality, because with high mutation there is an increased risk of genetic incompatibility among mating pairs. If females of a certain genotype choose to mate only with the highest quality male in the population who also happens to be genetically incompatible then these females could leave no viable offspring. It will pay females to assess overall genetic quality (generally low levels of mutation) as well as genetic compatibility. It is this need to take account of genetic compatibility which generates variability in female preferences, and thus leads to reproductive isolation. I speculate that separate specialised signals will have evolved to allow the assessment of genetic compatibility and argue that the most reliable way to do this is to use self-referent phenotype matching. Signals which allow the assessment of genetic compatibility lead to variability in female preference, reproductive isolation and ultimately to speciation.
DISCUSSION
The arguments outlined above involve several logical steps, and certain assumptions, some of which are more important than others. The assumptions of the model are as follows. (1) Females gain some genetic benefits from their mate choice, however, the model does not assume that females gain only genetic benefits nor does it specify what sort of genetic benefits females gain. (2) Mutation maintains variation in male genetic quality. However, the model does not assume that mutation is the sole cause of the maintenance of genetic variation amongst males. Nor does the model make any assumptions about why or how a high level of mutation is maintained in sexually selected species. (3) High levels of genetic variability amongst males lead to females assessing genetic compatibility when choosing a mate. I have suggested that this could happen because the local level of polygyny leads to inbreeding and/or because of the high level of deleterious mutations. It is hard to know which of these factors are likely to be more important in a sexually selected species. (4) The assessment of genetic compatibility results in females of certain specific genotypes choosing to mate with males of certain specifically compatible genotypes. The model assumes that these preferences are stable and that this effectively can lead to reproductive isolation of a part of a population. Needless to say the model does not assume that reproductive isolation could not come about in other ways, nor that it is not enhanced by other factors.
Previous models of speciation promoted by sexual selection have been developed. Lande (1981) assumed that the Fisherian process may result in rapid divergence between populations in the expression of the female preference and the male's secondary sexual characteristic (because these characters become correlated within populations) and that this process may lead to speciation. Other models of speciation as a result of Fisherian sexual selection have been proposed by Turner and Burrows (1995) and by Iwasa and Pomiankowski (1995). Both these models assume that under Fisherian selection there will eventually be viability costs associated with a female preference (when the male trait has become extreme) and that these costs lead to novel preferences being developed. Variation in female preference then leads to population differentiation. Schulter and Price (1993) have suggested that environmental differences can drive differentiation between populations as small differences between male colour and the colour of the habitat they live in can trigger changes in the female preference. To my knowledge no previous model has suggested that variable female preferences arise because of the need to take account of genetic compatibility.
I have discussed possible ways in which females may take account of genetic compatibility when choosing a mate. It may be that in some species sex differences in dispersal (Greenwood et al. 1980) could negate the need for females to have evolved mechanisms whereby females can assess genetic compatibility. It may also be the case that, in some species, learning the characteristics of kin provides a reliable means of assessing genetic distance (for example, sexual imprinting is common in many pair-bonded species). Perhaps it is only in species with polygyny and/or multiple mating that females need to judge genetic compatibility directly. In these species female preferences for genetically compatible males drives the evolution of signals in males that reveal their genetic identity.
The model described in this paper makes several empirically testable predictions and whilst space precludes my listing all those predictions that follow directly, it is my sincere hope that the role of genetic compatibility in generating variability in female preferences, reproductive isolation and speciation will nevertheless receive some attention in the future.
REFERENCES
Andersson, M. 1994. Sexual selection. Princeton: Princeton University Press: 599pp.
Bateson, P. 1982. Preferences for cousins in Japanese quail. Nature 295: 236-237.
Barraclough, T.G., Harvery, P.H. & Nee, S. 1995. Sexual selection and taxonomic diversity in passerine birds. Proceedings of the Royal Society of London, Series B 259: 211-215.
Bakker, T.C.M. & Pomiankowski, A. 1995. The genetic basis of female mate preferences. Journal of Evolutionary Biology 8:129-171.
Bensch, S., Hasselquist, D. & von Schantz, T. 1994. Genetic similarity between parents predicts hatching failure: non-incestuous inbreeding in the great reed warbler? Evolution 48: 317-326.
Budgey, H.V. 1994. Parental strategies of the feral Indian peahen Pavo cristatus. Ph.D. thesis, The Open University.
Egid, K. & Brown, J.L. 1989. The major histocompatibility complex and female mating preferences in mice. Animal Behaviour 38, 548-550.
Fisher, R. A. 1930 The Genetical Theory of Natural Selection. Oxford : Clarendon Press.
Gibbs, H.L. & Grant, P.R. 1989. Inbreeding in Darwin’s medium ground finches (Geospiza fortis). Evolution 43: 1273-1284.
Greenwood, P.J. 1980. Mating systems, philopatry & dispersal in birds and mammals. Animal Behaviour 28: 1140-1162.
Greenwood, P.J., Harvery, P.H. & Perrins, C.M. 1978. Inbreeding and dispersal in the great tit. Nature 271: 52-54.
Hamilton, W.D. & Zuk, M. 1982. Heritable fitness and bright birds: a role for parasites? Science 218:384-387.
Hasselqvist, D., Bensch, S. & von Schantz, T. 1996. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature 381:229-232.
Heywood, J.S. 1989. Sexual Selection by the Handicap Mechanism. Evolution 43: 1387-1397.
Hoelzer, G.A. 1989. The good parent process of sexual selection. Animal Behaviour 38: 1067-1078.
Iwasa, Y., Pomiankowski, A. & Nee, S. 1991. The Evolution of Costly Mate Preferences II. The 'Handicap' Principle. Evolution 45: 1431-1442.
Iwasa, Y. & Pomiankowski, A. 1995. Continual change in mate preferences. Nature 377: 420-422.
Jennions, M.D. & Petrie, M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biological Reviews of the Cambridge Philosophical Society 72: 283-327.
Johnstone, R.A. 1995. Sexual selection, honest advertising and the handicap principle: reviewing the evidence. Biological Reviews of the Cambridge Philosophical Society 70:1-65.
Keller, L.F., Arcese, P., Smith, J.N.M., Hochachka, W.M., Stearns, S.C. & Bruford, M. 1994. Selection against inbred song sparrows during a natural population bottleneck. Nature 372: 356-357.
Kempenaers, B. & Sheldon, B.C. 1996. Why do male birds not discriminate between their own and extra-pair offspring? Animal Behaviour 51: 1165-1173.
Kempenaers, B., Verheyen, G. R., Broeck, M. V. d., Burke, T., Broeckhoven, C.V. & Dhondt, A.A. 1992. Extra-pair paternity results from female preference for high-quality males in the blue tit. Nature 357: 494-496.
Kempenaers, B., Adriaensen, F., van Noordwijk, A.J. & Dhondt, A.A. 1996. Genetic similarity, inbreeding and hatching failure in blue tits: are unhatched eggs infertile? Proceedings of the Royal Society of London, Series B 263: 179-185.
Kirkpatrick, M. & Ryan, M.J. 1991. The paradox of the lek and the evolution of mating preferences. Nature 350: 33-38.
Lande, R. 1976. The maintenance of genetic variability by mutation in a polygeneic character with linked loci. Genetic Research, Cambridge 26:221-235.
Lande, R. 1981. Models of speciation by sexual selection on polygenic characters. Proceedings National Academy Sciences USA 78: 3721-3725.
McRae, S. 1996. Family values: costs and benefits of communal nesting in the moorhen. Animal Behaviour 52: 225-245.
Mitra, S., Landel, H. & Pruett-Jones, S. 1996 Species richness covaries with mating system in birds. Auk 113:544-551.
Møller, A.P. 1994. Male ornament size is a reliable cue to enhanced offspring viability in the barn swallow. Proceedings of the National Academy of the Sciences of the USA 91: 6929-6932.
Møller, A.P. & Cuervo, J.J. 1998 Speciation and feather ornamentation in birds. Evolution 52:859-869.
Norris, K. 1993. Heritable variation in a plumage indicator of viability in male great tits Parus major. Nature 362: 537-539.
Petrie, M. 1994. Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371: 598-599.
Petrie, M. & Lipsitch, M. 1994. Avian polygyny is most likely in populations with high variability in heritable male fitness. Proceedings of the Royal Society of London, Series B 256, 275-280.
Petrie, M. ms. Female preferences have evolved to avoid 'bad' genes.
Petrie, M. Halliday, T.R. & Sanders, C. 1991 Peahens prefer peacocks with elaborate trains. Animal Behaviour 41: 323-331.
Petrie, M., Hall, M., Halliday, T., Budgey , H. & Pierpoint, C. 1992 Multiple mating in a lekking bird: why do peahens mate with more than one male and with the same male more than once? Behavioral Ecology and Sociobiology 31: 349-358.
Petrie, M. & Halliday, T. 1994. Experimental and natural changes in the peacock's (Pavo cristatus) train can affect mating success. Behavioral Ecology and Sociobiology 35: 213-217.
Petrie, M. & Burke, T. (ms) Peahens assess genetic similarity when choosing mates.
Petrie, M. Krupa, A. & Burke, T. (submitted) Peacock Leks consist of relatives: evidence for kin recognition. Nature.
Potts, W.K., Manning, C.J. & Wakeland, E.K. 1991. Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 352: 619-621.
Potts, W.K., Manning, C.J. & Wakeland, E.K. 1994. The role of infectious disease, inbreeding, and mating preferences in maintaining MHC genetic diversity: an experimental test. Philosophical Transactions of the Royal Society of London, Series B. 346: 369-378.
Pomiankowski, A., Iwasa, Y. & Nee, S. 1991. The Evolution of Costly Mate Preferences I. Fisher and Biased Mutation. Evolution 45: 1422-1430.
Rowley, I., Russell, R. & Brooker, M. 1993. Inbreeding in birds. In: Thornhill, N.W. (ed.) The Natural History of Inbreeding and Outbreeding: Theoretical and Empirical perspectives: 304-328. Chicago: University of Chicago Press.
Sheldon, B.C., Merila, J., Qvarnstrom, A., Gustafsson, L. & Ellegren, H. 1997. Paternal genetic contribution to offspring condition predicted by size of male sexual ornament. Proceedings of the Royal Society of London, Series B 264: 297-302.
Sherman, P.W. 1990. Multiple Mating and Kin Recognition by Self-Inspection. Ethology and Sociobiology 12: 377-386.
Schluter, D. & Price, T. 1993. Honesty, perception and population divergence in sexually selected traits. Proceedings of the Royal Society of London, Series B 253: 117-122.
Turner, G.F. & Burrows, M.T. 1995. A model of sympatric speciation by sexual selection. Proceedings of the Royal Society of London, Series B 260: 287-292.
van Noordwijk, A.J. & Sharloo, W. 1981. Inbreeding in an island population of the great tit. Evolution 35: 674-688.
von Schantz, T., Göransson, G., Andersson, G., Fröberg, I., Grahn, M., Helgée, A. & Witzell, H. 1994. Female choice selects for a viability-based male trait in pheasants. Nature 337:166-169.
Wedekind, C., Seebeck, T., Bettens, F. & Paepke, A.J. 1995. MHC-dependent mate preferences in humans. Proceedings of the Royal Society of London, Series B 261: 245-249.
Table 1. Studies which have found empirical support for a link between the degree of enhancement of a sexually selected trait and offspring viability in birds.

Fig. 1. Flow diagram to illustrate main relationships between mutation, variation in male genetic quality and variability in female preferences.