S17.2: Adaptations to a migratory lifestyle: An Australian perspective
Ursula Munro
Department of Environmental Sciences, University of Technology, Sydney, PO Box 123, Broadway, NSW 2007, Australia, fax 61 2 9514 4003, e-mail Ursula.Munro@uts.edu.au
Munro, U. 1999. Adaptations to a migratory lifestyle: An Australian perspective. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 956-978. Johannesburg: BirdLife South Africa.Little research has been conducted on the migration and orientation of birds that live and migrate exclusively in the Southern Hemisphere. In Australia perhaps the most conspicuous intracontinental seasonal movements are performed by the Yellow-faced Honeyeater Lichenostomus chrysops (Meliphagidae). It is widely believed that the movements of honeyeaters are mainly governed by exogenous factors, such as the flowering patterns of their food plants. This implies that the mechanisms that (a) control migration, and (b) are required for orientation may differ to those of Northern Hemisphere migrants. In order to test these assumptions, I studied (1) the daily and seasonal locomotor activity, and (2) the orientation of Yellow-faced Honeyeaters. My honeyeaters showed a broad range of distinct adaptations which are characteristic of migratory birds and are directly related to their migratory habit. It was found that (1) there exists an endogenous annual cycle of migratory restlessness and seasonally appropriate orientation based on magnetic, solar and polarised light cues; and (2) these honeyeaters share a common migration program with Northern Hemisphere migrants which is based on the magnetic inclination compass. In summary, Yellow-faced Honeyeaters possess a migratory behaviour equivalent to that of birds living in the Northern Hemisphere.
MIGRATION IN AUSTRALIA
Our knowledge on the migration and orientation of birds in Australia is very limited. Estimates of how many species are migratory range from 8% (Keast 1968) up to 15% (Rowley 1974) overall, but a considerably higher percentage is expected for cool localities (Recher et al. 1983). Until 1990 attempts to unravel the movements of Australian landbirds concentrated on banding (e.g. Lane 1972; Purchase 1985) and field observations (e.g. Hindwood 1956; Robertson 1958, 1965; Liddy 1966; Lane 1972).
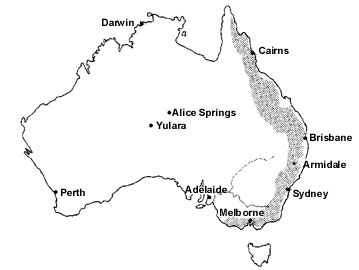
While banding studies revealed that numerous species leave their breeding areas after their young have fledged and do not return until the following breeding season, generally they did not reveal migratory pathways or overwintering areas of the birds. Consequently, many banding operations were discontinued because of low band returns and unjustifiably high costs (e.g. Purchase 1985). More detailed information on avian movements were often gained through observational studies (e.g. Hindwood 1956; Robertson 1958). In particular, field observations along the southeastern coastline and adjacent Great Dividing Range (Fig. 1) have shown that many landbirds conduct seasonal movements heading northward during autumn and winter, and return on a southward course to their breeding areas during spring (e.g. Hindwood 1956).
THE CASE OF THE YELLOW-FACED HONEYEATER
Perhaps the most conspicuous movements along the southeastern coast of Australia have been recorded in honeyeaters (Meliphagidae) (Hindwood 1956; Robertson 1958, 1965; Liddy 1966). Honeyeaters are an endemic family of the Australo-Papuan region (Longmore 1991) with only a distant relationship to any Northern Hemisphere family (Sibley & Ahlquist 1985). The mobile lifestyle of many honeyeater species, like the Yellow-faced Honeyeater or the White-naped Honeyeater Melithreptus lunatus, gave rise to numerous speculations about their movements and how these movements might be initiated (Keast 1968).
Along the southeastern coast of Australia the flowering of many food plants of honeyeaters occurs during autumn and winter (Pyke 1988; Taylor & Hopper 1988). This led to the belief that the birds' movements were a direct response to the flowering pattern of these plants, and could not be regarded as truly migratory movements (Dorst 1961). The possibility that endogenous factors could be involved in the control of such movements was denied vigorously. This is surprising in view of the fact that, for example, Yellow-faced Honeyeaters have been found up to 745 km away from their breeding grounds (Purchase 1985), and have been observed flying through or leaving areas with high nectar abundance (e.g. McFarland 1986), ignoring the rich food resource completely. It has been reported that these flights take place at above tree top height (Longmore 1991), and show a ‘marked constancy’ in direction and an ‘ever present impression of urgency’ (see Robertson 1958, 1965). From these observations it appears unlikely that the Yellow-faced Honeyeater's movements are unpredictable and solely governed by the flowering of its food plants (Keast 1968).
If the movements of the Yellow-faced Honeyeaters are a direct response to food shortages and thereby are initiated primarily exogenously, it could be argued that mechanisms that (a) control seasonal movements, and (b) are required for orientation may differ to those reported for Northern Hemisphere migrants. In a detailed laboratory study on Yellow-faced Honeyeaters, I addressed this question and focused specifically on their (1) daily and seasonal locomotor activity in order to establish whether they exhibit migratory restlessness and (2) seasonally appropriate orientation.
LOCOMOTOR ACTIVITY IN YELLOW-FACED HONEYEATERS
In migrants from the Northern Hemisphere a series of endogenous migratory programs that control the direction, time course (Gwinner & Wiltschko 1978; Beck & Wiltschko 1988) and distance of migration (Gwinner 1975, 1986, 1996) have been described. The distance of migration is controlled indirectly through the amount and duration of migratory restlessness (Gwinner 1986, 1996). Migratory restlessness is expressed in caged nocturnal migrants through a species-specific increase in the amount and duration of locomotor activity at night during their migratory seasons, which is proportional to the total distance covered during migration (Gwinner 1986). During times of migratory restlessness nocturnal migrants usually show seasonally appropriate orientation. Locomotor activity studies on day-migrating birds are scarce and are limited to Palaearctic seed-feeding species (Palmgren 1943, 1949; Dolnik & Blyumental 1967; Berthold 1978; Glück 1978).
The Yellow-faced Honeyeater, a nectar-feeder, conducts its movements exclusively during the day (Robertson & Woodall 1983). Its seasonal and daily locomotor activity patterns were studied in Armidale, Northern Tableland, NSW (30°30'S, 151°42'E), which is located on the Yellow-faced Honeyeater’s migration route (see Fig. 1). The birds were kept indoors under a simulated natural photoperiod over a period of 13 months with constant ad libitum food. Yellow-faced Honeyeaters showed no nocturnal locomotor activity, but exhibited distinct patterns of diurnal seasonal and daily locomotor activity typical for a migratory bird.
Seasonal Locomotor Activity
Two major peaks of enhanced activity were recorded. The first peak occurred during autumn/winter (March to July), exactly at the time when large flocks of Yellow-faced Honeyeaters head northward in the field (e.g. Hindwood 1956). A second peak in spring (September to December) (Fig. 2) coincided roughly with the time when extensive movements are recorded in the field (e.g. Hindwood 1956; Robertson 1965). Between July and August, when relatively few honeyeater movements are observed in the wild (Hindwood 1956; Robertson 1958; Liddy 1966), activity levels were reduced. During spring, the honeyeaters’ activity levels extended well beyond the time when their movements are recorded in the wild (Hindwood 1956; Robertson 1958; Liddy 1966). Similar behaviours have been observed in other migrants, and it appears likely that this behaviour is the result of the inability of caged birds to arrive in their breeding environment, and/or reproduce, which usually terminates migration (see Gwinner & Czeschlik 1978; Ketterson & Nolan 1990).
Daily Locomotor Activity
Yellow-faced Honeyeaters showed two major peaks of daily activity (Fig. 3), which corresponded to those found in other migratory and non-migratory passerine species (Berthold 1978). The first peak ranged from the early morning hours to approximately early afternoon. A second smaller peak occurred during late afternoon. During spring and autumn/winter both the morning as well as the afternoon peak were considerably higher than in months when Yellow-faced Honeyeater movements had ceased in the wild. Specifically, during the time of autumn and spring movements, locomotor activity was increased considerably in the morning and early afternoon (early peak), exactly at the time of day when extensive Yellow-faced Honeyeater movements are reported in the wild (Robertson & Woodall 1983). The size of the second activity peak (late afternoon) varies over the year but considerably less than the size of the morning peak. The afternoon peak is also present when no honeyeater movements occur in the wild (Robertson & Woodall 1983). It might be linked to increased feeding activities before roosting.
THE ORIENTATION OF YELLOW-FACED HONEYEATERS
The locomotor activity patterns of Yellow-faced Honeyeaters strongly suggest that the movements of these birds are not exclusively based on exogenous factors. However, in a next step it became necessary to confirm the birds’ migratory status by studying whether they show, like numerous nocturnal migrants, seasonally appropriate orientation during times of increased locomotor activity. Surprisingly, Northern Hemisphere diurnal migrants have failed to show seasonally appropriate migratory orientation (Glück 1982), when tested for their directional preferences during periods of enhanced locomotor activity during their migratory season (Glück 1978).
In a first step, the orientation of these honeyeaters was studied in Armidale, NSW, which is approximately 500 km south of Brisbane, Queensland. The tests took place in autumn/winter, when mainly northward movements have been reported at various locations along the east coast and adjacent ranges of New South Wales (Davey et al. 1984; Hindwood 1948, 1956; Liddy 1966), while limited recordings from East Brisbane (27°30'S, 153°00'E), Queensland, in May and June suggest a northwesterly course (Robertson 1958). Based on these and other observations (Bravery 1970; Blakers et al. 1984) it appears that the main stream of migration follows the Great Dividing Range and the east coast of Australia (Fig. 1), with a change of direction from northeast to northwest at around the end of April to early May in the area around Brisbane.
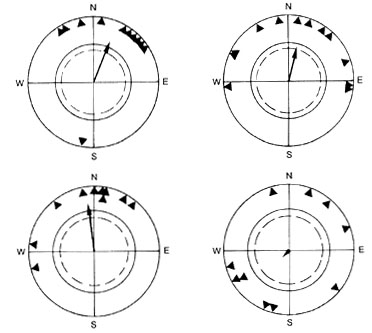
The honeyeaters oriented in a seasonally appropriate direction during March and July, exactly at the time when they also show increased locomotor activity (Munro & Munro 1998). The directional tendencies of my captive birds recorded in Armidale reflect the migration route of the species along the Australian east coast and the Great Dividing Range. The northeasterly mean direction found in March and April (Fig. 4, left) coincides well with the directions of migratory flocks of Yellow-faced Honeyeaters reported from locations in New South Wales (Hindwood 1948; Liddy 1966; Davey et al. 1984). The northwesterly direction from May onward (Fig. 4, right) is in agreement with the observation of Robertson (1958) in Brisbane, and the direction that birds should take once they have reached Queensland.
The phenomenon of birds changing direction in the course of their migration is also known from Northern Hemisphere migrants. Garden Warblers (Sylvia borin) and Pied Flycatchers (Ficedula hypoleuca) from central Europe migrate first southwest to Spain and then continue on a south/southeasterly course. Eastern European Blackcaps (Sylvia atricapilla) on the other hand, take a southeasterly route to the Balkan Peninsula and then continue their migration in a southerly direction. When birds of these species were tested for their orientation in Germany during autumn migration, they changed their directional headings exactly at the time when their conspecifics do so in the wild (Gwinner & Wiltschko 1978; Beck & Wiltschko 1988; Helbig et al. 1989). From these results it was concluded that the changes in direction were based on an endogenous component, being controlled by an innate migratory time program which also determines the amount and duration of migratory activity (Berthold 1996).
All species tested to date, however, migrate at night. For such birds, landscape features such as mountain ranges and coastlines might be generally only of minor importance. Yellow-faced Honeyeaters, in contrast, are day migrants. For these birds, landscape features are much more prominent. Therefore, one might have assumed that the eastern coastline and the adjacent mountain ranges are important cues for directing their migration. Interestingly, the birds shifted their directional tendencies even in captivity in Armidale, when such landscape cues were not available to them. This strongly suggests that the change in migratory direction is, at least in part, controlled by an endogenous program in day migrants as well as in night migrants.
It is interesting to note that during the locomotor activity recordings, a brief significant drop in locomotor activity occurred during early May, which was followed by an overall peak of activity in late May (see Fig. 2). This drop in activity occurs exactly at the time when Yellow-faced Honeyeaters change their course from north/northeast to northwest. A similar decrease in locomotor activity has been observed in Pied Flycatchers (Thalau 1994; Beck pers. comm.), when they show an equivalent directional shift during their autumn migration (Beck & Wiltschko 1988). So far little attention has been paid to these findings. However, these results could suggest that Yellow-faced Honeyeaters and possibly other species (see Gwinner & Wiltschko 1978; Beck & Wiltschko 1988; Helbig et al. 1989) rely on separate migration component programs for completing the two legs of autumn migration with each component program controlling the duration and direction specific for a separate part of their migration. Whether the same applies for spring migration is unclear. The seasonal activity levels of Yellow-faced Honeyeaters dropped slightly in early October, and could suggest such a behaviour. However, corresponding orientation data and similar observations in other species have not yet been reported.
In summary, it has been shown that Yellow-faced Honeyeaters are oriented seasonally appropriate at times when their locomotor activity is increased and they are recorded migrating in the wild (Hindwood 1956). This leaves little doubt that this activity is linked to migratory activities and can, therefore, be regarded as migratory restlessness in the same way as in many nocturnal migrants (for summary, see Berthold 1996). Locomotor activity patterns in combination with orientation results further suggest that autumn migration in these honeyeaters might be controlled by two separate component migration programs.
ORIENTATION MECHANISMS OF YELLOW-FACED HONEYEATERS
Intensive research in the Northern Hemisphere has shown that migratory birds can rely on (a) magnetic and (b) stellar cues, and (c) the sun at sunset and the associated pattern of polarised light (for summary, see Able & Bingman 1987; W. Wiltschko & Wiltschko 1988; R. Wiltschko & Wiltschko 1995). In contrast, only little attention has been paid to orientation mechanisms of bird species from the Southern Hemisphere (W. Wiltschko et al. 1993a). Data on Southern Hemisphere migrants are, however, interesting for two reasons. Firstly, there exist many endemic families without close relationships to any of the Palaearctic species studied so far. Secondly, the environmental conditions of the Southern Hemisphere are different in many respects. For these reasons, the orientation behaviour of the Yellow-faced Honeyeater was analysed focusing on the importance of (1) magnetic, (2) solar, and (3) polarised light cues in the orientation system of these honeyeaters.
Magnetic cues
In order to assess the role of magnetic cues in the orientation of this diurnal migrant, the directional behaviour of Yellow-faced Honeyeaters was recorded in Emlen orientation cages (Emlen & Emlen 1966; Munro & W. Wiltschko 1992) under natural and manipulated magnetic field conditions in Armidale, NSW. During the autumn migration period, the birds were subjected to the following magnetic conditions: (1) the local geomagnetic field (56000 nanoTesla (nT), magnetic North (mN) = 360°, -62° inclination (incl.)); (2) a magnetic field with north deflected to eastsoutheast (56000 nT, mN = 120°, -62° incl.), and (3) a magnetic field with a compensated horizontal component (49500 nT, mN --, -90° incl.), i.e. a field without meaningful information.
In spring, the birds were tested under two magnetic conditions: (1) the local geomagnetic field (56000 nT, mN = 360°, -62° incl.), and (2) a magnetic field with an inverted vertical component (56000 nT, mN = 360°, +62° incl.) (for details see Munro & W. Wiltschko 1993).
During both seasons, the birds oriented seasonally appropriate in the local magnetic field, heading northward in autumn and southward in spring. During autumn, the birds showed in the local geomagnetic field a significant directional change from north initially to northwest later in the season (Fig. 5, upper diagrams). This change reflects the general pattern of movement of this species in the field, including the shift of direction from north to northwest during autumn migration (Hindwood 1956; Robertson 1958, 1965; Liddy 1966). Deflecting magnetic North to eastsoutheast resulted in a clockwise shift of the mean direction by 77° during early autumn migration, and 71° during late migration (Fig. 5, central diagrams), while no significant directional tendencies were observed in a magnetic field with a compensated horizontal component (Fig. 5, lower diagrams).
These results clearly show that Yellow-faced Honeyeaters can use the magnetic field to orient their migration like many other Palaearctic migrants (for summary, see R. Wiltschko & Wiltschko 1995). However, most other species tested so far for their magnetic orientation are nocturnal migrants, while experiments on diurnal migrants are scarce. The only other diurnal migrants whose orientation has been studied yet are the European Starling Sturnus vulgaris (Kramer 1950; R. Wiltschko 1981) and the Meadow Pipit Anthus pratensis (Orth & Wiltschko 1981; Helbig et al.1987), which seemed to be able to orient in the absence of visual cues. Responses to manipulated magnetic field conditions as in the Yellow-faced Honeyeaters have only been reported in Snow Buntings Plectrophenax nivalis, which also have a magnetic compass (Sandberg & Pettersson 1996).
All species, which have yet been tested for a magnetic compass are closely related to each other, belonging to the families Muscicapidae, Sylviidae, Emberizidae and Icteridae (W. Wiltschko & Wiltschko 1991). This is also true for the Australian Silvereye Zosterops lateralis, which is beside the Yellow-faced Honeyeater the only other Southern Hemisphere migrant, whose magnetic compass has been analysed (W. Wiltschko et al. 1993a, 1994). The Silvereye is an only recent immigrant to Australia from Asia (Ford 1989; Christidis & Schodde 1991), which belongs according to Sibley and Ahlquist (1985) to the superfamily Sylvioidea (Sibley & Ahlquist 1985; Ford 1989). The Yellow-faced Honeyeater on the other hand is a typical old endemic of Australia with only distant relationships to any Holarctic species (Sibley & Ahlquist 1985). This supports the hypothesis of the widespread use of the magnetic compass in birds (W. Wiltschko & Wiltschko 1988).
Subsequent tests on Yellow-faced Honeyeaters in a magnetic field with an inverted vertical component (i.e. with an inclination pointing down instead of upwards) resulted in the same reversal of direction observed in many other species whose magnetic compass has been analysed (for summary, see R. Wiltschko & Wiltschko 1995) (Fig. 6). From these tests it became apparent that the magnetic compass of Yellow-faced Honeyeaters functions as an 'inclination compass' as is known for numerous Northern Hemisphere migrants (W. Wiltschko & Wiltschko 1991; R. Wiltschko & Wiltschko 1995) and the Australian Silvereye (W. Wiltschko et al. 1993a). A magnetic inclination compass is not based on polarity as our technical compass, but rather on the inclination of the field lines. Therefore, this compass distinguishes between poleward and equatorward instead of north and south (for details, see R. Wiltschko & Wiltschko 1995).
These findings show that migratory birds rely on a magnetic inclination compass, irrespective whether they live in the Northern or Southern Hemisphere. These results have important implications. They show that migratory birds in general share a common directional migration program, which is based on the inclination of the magnetic field lines (see also W. Wiltschko et al. 1993a). This program guarantees that migratory birds from both hemispheres move equatorward in autumn and return on a poleward course in spring (Fig. 7). Such a program must have assisted migratory birds, which have changed hemisphere (1) when establishing breeding populations in their traditional overwintering areas, or (2) when being introduced by European settlers to establish new migratory courses (see W. Wiltschko et al. 1993a).
Solar Cues
Despite the fact that convincing evidence for the use of sun compass orientation in day-migrating birds is lacking, it has been widely accepted that the sun compass is the major orientation mechanism of day-migrating birds (e.g. Bellrose 1972; Emlen 1975). Even today this view is still expressed in university biology textbooks (e.g. Campbell 1996), despite the fact that studies on day-migrating birds such as European Starlings (W. Wiltschko 1980) and Meadow Pipits (Orth & Wiltschko 1981; Helbig et al. 1987) do not support this view. These birds did not show the typical directional deviations in response to manipulations of their internal clock that were observed in clock-shift experiments with Homing Pigeons Columba livia (Schmidt-Koenig 1958). While the directional preferences of these birds were random when tested under clock-shift conditions in fine weather, they were well oriented under overcast conditions (R. Wiltschko 1981; Helbig et al. 1987) suggesting that the birds had relied on a non-visual orientation cue. Starlings on the other hand responded to clock-shift in spring with the expected deviation (R. Wiltschko 1981). These contradictory findings allowed no definite conclusions about the role of the sun in the orientation system of day-migrating birds.
In order to assess the importance of the sun in the orientation of Yellow-faced Honeyeaters, these birds were subjected to clock-shift experiments during their migration seasons. In autumn, the internal clock of these honeyeaters was shifted four hours forward (fast-shift). During the following spring the birds were subjected to a three hour fast-shift (for details see Munro & R. Wiltschko 1993). If the birds use a sun compass, it can be expected that the birds show directional deviations that are proportional to the difference of the sun azimuth between the objective time and the subjective time of day of clock-shifted birds. For the tests on Yellow-faced Honeyeaters deviations between 55° and 60° were expected for the autumn experiments, while a deviation of approximately 25° was expected for spring.
During both autumn and spring the fast-shifted birds showed initially a directional deviation in the expected direction, which was, however, considerably larger than predicted by the time-compensating sun compass model (94° for autumn, and 91° for spring) (Fig. 8). After a short two-day period of increased scatter in their directional distribution, in both seasons an orientation was observed that roughly coincided with the one found in the control tests. Thus in both seasons, the responses of the birds to the fast-shift vanished after 5 to 6 days, which indicates that any influence due to the manipulation had been lost. Since Yellow-faced Honeyeaters can use a magnetic compass for orientation (Munro & W. Wiltschko 1993) there is little doubt that these honeyeaters made use of this mechanism to find their migratory direction.
The behaviour of the fast-shifted birds does not support the traditional sun compass concept (Schmidt-Koenig 1961). The similar behaviour of the birds to the clock-shifts in both seasons was remarkable, but difficult to interpret. The similarity of responses of the honeyeaters to the fast-shifts are especially surprising when considering the fact that the fast clock-shift tests in autumn and spring differed in the size of the shift (4 versus 3 hours), and the expected directional deflection (60° versus 25°) (for further details, see Munro & R. Wiltschko 1993). These results indicate that the sun has an effect on the orientation of Yellow-faced Honeyeaters, but fail to produce clear evidence for sun compass orientation in a diurnal migrant.
Strong evidence for sun compass orientation has, however, been found in several birds. Specifically, directionally trained birds (e.g. Hoffmann 1954; St. Paul 1954, 1956; Able & Dillon 1977), Homing Pigeons (e.g. Schmidt-Koenig 1958; R. Wiltschko & Wiltschko 1981), Mallards Anas platyrhynchos (Matthews 1963, 1984), and foraging Scrub Jays Aphelocoma coerulescens (W. Wiltschko & Balda 1989) have shown that they rely on sun compass orientation. A common feature of these birds is that their orientation tasks do not involve large-scale movements, while migratory birds need to orient over long distances. The sun’s path changes with latitude and season. These changes would require a continuous adjustment of the sun compass for which an independent reference system, like the magnetic compass, would be needed (see R. Wiltschko 1981). Therefore, the sun compass might be unsuitable for the orientation of large-scale movements. It seems, however, an important orientation cue for animals with movements on a more local scale (e.g. homing, foraging) (R. Wiltschko 1995). This might explain why a sun compass has not been easily demonstrated in diurnal migrants.
Polarised Light Cues
Numerous studies on the orientation of night-migrating birds have been performed around dusk (for summary, see Able & Bingman 1987). If the e-vector axis (axis of vibration) of polarised light was manipulated during these tests (e.g. Able 1989; Helbig 1991), or, alternatively, natural skylight was depolarised (Helbig 1990), nocturnal migrants responded in a way suggesting that they used polarised light to orient. While skylight polarisation seems to be a common directional cue in the orientation system of nocturnal migrants, it is not known whether it is of any relevance to day-migrating birds. In order to study the relevance of skylight polarisation in the orientation system of a day-migrating bird, a detailed study on the orientation of Yellow-faced Honeyeaters during their autumn migration season was performed.
Yellow-faced Honeyeaters tested under a clear sky in the natural geomagnetic field showed no response to manipulations of the e-vector of incident linearly polarised light, or the depolarisation of natural skylight. Instead they maintained an orientation comparable to that of control birds (Fig. 9). This orientation was even maintained when the birds were deprived of relevant magnetic cues in a magnetic field with a compensated horizontal component (49500 nT, mN --, -90° incl.), while viewing the clear sky and sun through plexiglass, or alternatively, depolarisers (Fig. 10, upper diagrams). However, when the sun was well hidden behind clouds, honeyeaters tested under natural skylight without magnetic cues oriented in their appropriate migratory direction (Fig. 10, lower diagrams, left), whereas birds viewing the sky through depolarisers were disoriented (Fig. 10, lower diagrams, right). Similar findings have been reported for European Robins Erithacus rubecula and Blackcaps (Helbig 1990, 1991). Both species were able to orient in their appropriate migratory direction in the absence of meaningful magnetic information when natural skylight was available at dusk. Their orientation was, however, disrupted when skylight was depolarised.
Yellow-faced Honeyeaters clearly show that they can use natural polarised light for orienting their migration when all other known cues are taken away. However, this cue seems to be only of minor importance (Munro & R. Wiltschko 1995). In the presence of meaningful magnetic information and when the sun was visible, manipulations of the e-vector did not affect the birds’ behaviour significantly. This is in contrast to numerous reports that nocturnal migrants altered their directional tendencies when the e-vector axis was rotated using polarisers, in the presence of the natural magnetic field (e.g. Able 1982; Moore 1986; Moore & Phillips 1988; Helbig 1991), with the exception of results from Sandberg (1988).
However, all positive results with nocturnal migrants were obtained in tests around sunset or sunrise, i.e. at a time when natural polarisation forms a fairly simple pattern with a band of maximal polarisation running over the sky perpendicularly to the sun (Brines 1980; Brines & Gould 1982). The honeyeaters were tested for their responses two to five hours after sunrise at a time when the polarisation pattern is much more complex and very dissimilar to the parallel polarisation produced by polarisers. Under these circumstances, it is perhaps not surprising that the simple artificial pattern failed to elicit a response.
From the present results it is not certain whether Yellow-faced Honeyeaters use polarised skylight as an independent celestial compass. At the time of testing the honeyeaters were living in outdoor aviaries and thus were able to view the natural sky together with the natural geomagnetic field. Hence, they might have associated the sun and the pattern of polarisation with the direction of the geomagnetic field. When they were then deprived of magnetic cues, it is possible that they switched over to memorised information on the sun and/or polarisation to locate their migratory direction for the one hour test period. Experiments on young Savannah Sparrows Passerculus sandwichensis (Able & Able 1990) and Australian Silvereyes (W. Wiltschko et al. 1998; R. Wiltschko & Wiltschko 1999) support this view.
THE YELLOW-FACED HONEYEATER - RULE OR EXCEPTION?
In Australia, it is generally believed that the migration of many birds, such as honeyeaters, is facultative occurring mainly in response to external environmental stimuli, such as food availability (e.g. Keast 1968). However, in the case of the Yellow-faced Honeyeater, I have shown that these honeyeaters possess a broad range of distinct adaptations to their mobile lifestyle, which are also found in numerous Northern Hemisphere migrants (for summary, see Berthold 1996). Yellow-faced Honeyeaters have (1) an annual cycle of migratory restlessness, (2) seasonally appropriate orientation based on magnetic, solar and polarised light cues, and (3) a migration program based on the magnetic inclination compass, which they share with Palaearctic migrants. In summary, Yellow-faced Honeyeaters possess a migratory behaviour equivalent to that of birds living in the Northern Hemisphere. Consequently, the hypothesis that the migratory movements of Yellow-faced Honeyeaters are simply governed by the flowering pattern of their food plants (Dorst 1961) is not supported and needs to be reconsidered.
Since the studies on Yellow-faced Honeyeaters began, migration and orientation research has been performed on two other Australian species, the Silvereye (e.g. W. Wiltschko et al. 1993a,b, 1994; Chan 1994; Munro et al. 1997a,b) and recently the Regent Honeyeater Xanthomyza phrygia (Cooke unpublished manuscript). Both species show in the laboratory, like the Yellow-faced Honeyeater, distinct adaptations to their mobile lifestyle. All species studied so far are, however, from eastern and southeastern Australia including Tasmania. Here the environment is characterised by well defined seasons comparable to those in Europe and North America. Therefore, it does not seem surprising that birds from this region show adaptations to their mobile lifestyle equivalent to that of birds living in a similar climate in the Northern Hemisphere (e.g. Berthold 1985, 1988). However, these results should not be generalised for Australian birds. Australia is a huge continent with a vast variety of very different environmental regions. The semiarid and arid interior of Australia is characterised by its unpredictable environments (Archer & Clayton 1984). Species such as Black Certhionyx niger and Pied Honeyeater Certhionyx variegatus and chats Epthianura spp., which inhabit these unpredictable environments may rely more heavily on external environmental factors by showing movements that are more irregular in timing and direction (see Keast 1958, 1968; Ford 1978). Laboratory studies of the seasonal activity patterns and orientation behaviour of other Australian species from habitats with different degrees of environmental predictability are urgently required for the understanding of the factors that influence different forms of mobile behaviour.
ACKNOWLEDGMENTS
This work was supported by my husband John A. Munro; Hugh Ford, Stuart Cairns and the University of New England, Armidale, NSW, Australia; and Roswitha and Wolfgang Wiltschko and the J.W. Goethe-Universität, Frankfurt am Main, Germany.
REFERENCES
Able, K.P. 1982. Skylight polarization patterns at dusk influence migratory orientation in birds. Nature 299: 550-551.
Able, K.P. 1989. Skylight polarization patterns and the orientation of migratory birds. Journal of Experimental Biology 141: 241-256.
Able, K.P. & Able, M.A. 1990. Ontogeny of migratory orientation in the Savannah Sparrow, Passerculus sandwichensis: mechanisms at sunset. Animal Behaviour 39: 1189-1198.
Able, K.P. & Bingman, V.P. 1987. The development of orientation and navigation behavior in birds. Quarterly Review of Biology 62: 1-29.
Able, K.P. & Dillon, J.D. 1977. Sun compass orientation in a nocturnal migrant, the White-throated Sparrow. Condor 79: 393-395.
Archer, M. & Clayton, G. 1984. Vertebrate zoogeography and evolution in Australasia. Animals in space & time. Carlisle; Hesperian Press: 1203pp.
Beck, W. & Wiltschko, W. 1988. Magnetic factors control the migratory direction of Pied Flycatchers (Ficedula hypoleuca PALLAS). In: H. Ouellet (ed.) Acta XIX Congressus Internationalis Ornithologici. Ottawa; University of Ottawa Press: 1955-1962.
Bellrose, F.C. 1972. Possible steps in the evolutionary development of bird navigation. In: Galler, S.R., Schmidt-Koenig, K., Jacobs, G.J. & Bellville, R.E. (eds) Animal orientation and navigation. NASA SP-262; Washington, DC; U.S. Government Printing Office: 223-258.
Berthold, P. 1978. Die quantitative Erfassung der Zugunruhe bei Tagziehern: Eine Pilotstudie an Ammern (Emberiza). Journal für Ornithologie 119: 334-336.
Berthold, P. 1985. Migration: mechanisms and adaptive significance. In: Rankin, M.A. (ed.) Contributions in marine science. Supplement Vol. 27; Port Aransas; University of Texas at Austin: 526-543.
Berthold, P. 1988. The control of migration in European warblers. In: H. Ouellet (ed.) Acta XIX Congressus Internationalis Ornithologici. Ottawa; University of Ottawa Press: 215-249.
Berthold, P. 1996. Control of bird migration. London; Chapman & Hall: 355pp.
Blakers, M., Davies, S.J.J.F. & Reilly, P.N. 1984. The atlas of Australian birds. Melbourne; Royal Australasian Ornithologists Union and Melbourne University Press: 738pp.
Bravery, J.A. 1970. The birds of Atherton Shire, Queensland. Emu 70: 49-63.
Brines, M.L. 1980. Dynamic patterns of skylight polarization as clock and compass. Journal of Theoretical Biology 86: 507-512.
Brines, M.L. & Gould, J.L. 1982. Skylight polarization patterns and animal orientation. Journal of Experimental Biology 96: 69-91.
Campbell, N.A. 1996. Biology. Fourth Edition. Menlo Park; Benjamin/Cummings Publishing Company: 1206 [64]pp.
Chan, K. 1994. Nocturnal activity of caged resident and migrant Silvereyes (Zosteropidae: Aves). Ethology 96: 313-321.
Christidis, L. & Schodde, R. 1991. Relationships of Australo-Papuan songbirds - protein evidence. Ibis 133: 277-285.
Davey, C., Pendergast, H. & Taylor, I. 1984. Honeyeater migration through the Canberra region. A project proposal. Canberra Bird Notes 9: 142-146.
Dolnik, R.V. & Blyumental, T.I. 1967. Autumnal premigratory and migratory periods in the Chaffinch (Fringilla coelebs coelebs) and some other temperate-zone passerine birds. Condor 69: 435-468.
Dorst, J. 1961. The migration of birds. Toronto; Heinemann: 476pp.
Emlen, S.T. 1975. Migration: Orientation and navigation. In: Farner, D.S. & King, J.R. (eds) Avian biology. Vol. V; New York; Academic Press: 129-219.
Emlen, S.T. & Emlen, J.T. 1966. A technique for recording migratory orientation of captive birds. Auk 83: 361-367.
Ford, H.A. 1978. The Black Honeyeater: nomad or migrant? South Australian Ornithologist 27: 263-269.
Ford, H.A. 1989. Ecology of birds: an Australian perspective. Chipping Norton; Surrey Beatty.
Glück, E. 1978. Aktivitätsuntersuchungen an Tagziehern (Carduelis carduelis). Journal für Ornithologie 119: 336-338.
Glück, E. 1982. Locomotor activity of day-migrating finches. In: Papi, F. & Wallraff, H.G. (eds) Avian navigation. Berlin; Springer-Verlag: 90-95.
Gwinner, E. 1975. Circadian and circannual rhythms in birds. In: Farner, D.S. & King, J.R. (eds) Avian biology. Vol. V; New York; Academic Press: 221-285.
Gwinner, E. 1986. Circannual rhythms. Heidelberg; Springer-Verlag: 154pp.
Gwinner, E. 1996. Circadian and circannual programmes in avian migration. Journal of Experimental Biology 199: 39-48.
Gwinner, E. & Czeschlik, D. 1978. On the significance of spring migratory restlessness of caged birds. Oikos 30: 364-372.
Gwinner, E. & Wiltschko, W. 1978. Endogenously controlled changes in migratory direction of the Garden Warbler, Sylvia borin. Journal of Comparative Physiology 125: 267-273.
Helbig, A.J. 1990. Depolarization of natural skylight disrupts orientation of an avian nocturnal migrant. Experientia 46: 755-758.
Helbig, A.J. 1991. Dusk orientation of migratory European Robins, Erithacus rubecula: the role of sun-related directional information. Animal Behaviour 41: 313-322.
Helbig, A., Orth, G., Laske, V. & Wiltschko, W. 1987. Migratory orientation and activity of the Meadow Pipit (Anthus pratensis): a comparative observational and experimental field study. Behaviour 103: 276-293.
Helbig, A.J., Berthold, P. & Wiltschko, W. 1989. Migratory orientation of Blackcaps (Sylvia atricapilla): population-specific shifts of direction during the autumn. Ethology 82: 307-315.
Hindwood, K.A. 1948. Migration of two species of honeyeaters. Emu 47: 391-393.
Hindwood, K.A. 1956. The migration of the White-naped and Yellow-faced Honeyeaters. Emu 56: 421-425.
Hoffmann, K. 1954: Versuche zu der im Richtungsfinden der Vögel enthaltenen Zeitschätzung. Zeitschrift für Tierpsychologie 11: 453-475.
Keast, A. 1958. The relationship between seasonal movements and the development of geographic variation in the Australian chats Epthianura Gould and Ashbyia North (Passeres: Muscicapidae, Malurinae). Australian Journal of Zoology 6: 53-68.
Keast, A. 1968. Seasonal movement in the Australian honeyeaters (Meliphagidae) and their ecological significance. Emu 67: 159-209.
Ketterson, E.D. & Nolan, V. jr. 1990. Site attachment and site fidelity in migratory birds: experimental evidence from the field and analogies from neurobiology. In: Gwinner, E. (ed.) Bird migration. Physiology and ecophysiology. Berlin; Springer-Verlag: 117-129.
Kramer, G. 1950. Weitere Analyse der Faktoren, welche die Zugaktivität des gekäfigten Vogels orientieren. Naturwissenschaften 37: 377-378.
Lane, S.G. 1972. A review of the co-operative Silvereye project. Australian Bird Bander 10: 3-6.
Liddy, J. 1966. Autumnal migration of the Yellow-faced Honeyeater. Emu 66: 87-104.
Longmore, W. 1991. Honeyeaters and their allies in Australia. North Ryde; Collins Angus & Robertson: 426pp.
Matthews, G.V.T. 1963. The astronomical bases of ‘nonsense’ orientation. In: Sybley, C. (ed.) Acta XIII Congressus Internationalis Ornithologici. Vol. 1; American Ornithologists’ Union: 415-429.
Matthews, G.V.T. 1984. 'Nonsense' orientation in Mallards; a resume and an investigation of the mechanism of a sun-compass. Wildfowl 35: 81-92.
McFarland, D.C. 1986. The organization of a honeyeater community in an unpredictable environment. Australian Journal of Ecology 11: 107-120.
Moore, F.R. 1986. Sunrise, skylight polarization, and the early morning orientation of night-migrating warblers. Condor 88: 493-498.
Moore, F.R. & Phillips, J.B. 1988. Sunset, skylight polarization and the migratory orientation of Yellow-rumped Warblers, Dendroica coronata. Animal Behaviour 36: 1170-1778.
Munro, U. & Munro, J.A. 1998. Migratory restlessness in the Yellow-faced Honeyeater, Lichenostomus chrysops (Meliphagidae), an Australian diurnal migrant. Ibis 140: 599-604.
Munro, U. & Wiltschko, R. 1993. Clock-shift experiments with migratory Yellow-faced Honeyeaters, Lichenostomus chrysops (Meliphagidae), an Australian day-migrating bird. Journal of Experimental Biology 181: 233-244.
Munro, U. & Wiltschko, R. 1995. The role of skylight polarization in the orientation of a day-migrating bird species. Journal of Comparative Physiology A 177: 357-362.
Munro, U. & Wiltschko, W. 1992. Orientation studies on Yellow-faced Honeyeaters Lichenostomus chrysops (Meliphagidae) during autumn migration. Emu 92: 181-184.
Munro, U. & Wiltschko, W. 1993. Magnetic compass orientation in the Yellow-faced Honeyeater, Lichenostomus chrysops, a day migrating bird from Australia. Behavioral Ecology and Sociobiology 32: 141-145.
Munro, U., Wiltschko, W. & Ford, H.A. 1993. Changes in the migratory direction of Yellow-faced Honeyeaters, Lichenostomus chrysops (Meliphagidae), during autumn migration. Emu 93: 59-62.
Munro, U., Munro, J.A., Phillips, J.B., Wiltschko, R. & Wiltschko, W. 1997a. Evidence for a magnetite-based navigational 'map' in birds. Naturwissenschaften 84: 26-28.
Munro, U., Munro, J.A., Phillips, J.B. & Wiltschko, W. 1997b. Effect of wavelength of light and pulse magnetisation on different magnetoreception systems in a migratory bird. Australian Journal of Zoology 45: 189-198.
Orth, G. & Wiltschko, W. 1981. Die Orientierung von Wiesenpiepern (Anthus pratensis L.). Verhandlungen der Deutschen Zoologischen Gesellschaft 1981: 252.
Palmgren, P. 1943. Zur Tagesrhythmik der Finkenvögel. Ornis Fennica 20: 99-103.
Palmgren, P. 1949. On the diurnal rhythm of activity and rest in birds. Ibis 91: 561-576.
Purchase, D. 1985. Bird-banding and the migration of Yellow-faced and White-naped Honeyeaters through the Australian Capital Territory. Corella 9: 59-62.
Pyke, G.H. 1988. Yearly variation in seasonal patterns of honeyeater abundance, flower density and nectar production in heathland near Sydney. Autralian Journal of Ecology 13: 1-10.
Recher, H.F., Gowing, G., Kavanagh, R., Shields, J. & Rohan-Jones, W. 1983. Birds, resources and time in a tableland forest. In: Purdie, R.W. & Noble, I.R. (eds) Proceedings of the ecological society of Australia. Vol. 12; Canberra; Ecological Society of Australia: 101-123.
Robertson, J.S. 1958. Yellow-faced Honeyeater migration. Emu 58: 370-374.
Robertson, J.S. 1965. Migration of Yellow-faced Honeyeaters. Australian Bird Bander 3: 33-34.
Robertson, J.S. & Woodall, P.F. 1983. The status and movements of honeyeaters at Wellington Point, south-east Queensland. Sunbird 13: 1-14.
Rowley, I. 1974. Bird life. Sydney; Collins: 284pp.
Saint Paul, U. von 1954. Nachweis der Sonnenorientierung bei nächtlich ziehenden Vögeln. Behaviour 66: 1-7.
Saint Paul, U. von 1956. Compass directional training of Western Meadow Larks (Sturnella neglecta). Auk 73: 203-210.
Sandberg, R. 1988. Skylight polarization does not affect the migratory orientation of European Robins. Condor 90: 264-270.
Sandberg, R. & Pettersson, J. 1996. Magnetic orientation of Snow Buntings (Plectrophenax nivalis), a species breeding in the high artic: passage migration through temperate-zone areas. Journal of Environmental Biology 199: 1899-1905.
Schmidt-Koenig, K. 1958. Experimentelle Einflußnahme auf die 24-Stunden-Periodik bei Brieftauben und deren Auswirkungen unter besonderer Berücksichtigung des Heimfindevermögens. Zeitschrift für Tierpsychologie 15: 301-331.
Schmidt-Koenig K. 1961. Die Sonne als Kompaß im Heimorientierungssystem der Brieftaube. Zeitschrift für Tierpsychologie 18: 221-244.
Sibley, C.G. & Ahlquist, J.E. 1985. The phylogeny and classification of the Australo-Papuan passerine birds. Emu 85: 1-14.
Taylor, A. & Hopper, S. 1988. The banksia atlas. Australian flora and fauna series, No 8. Bureau of Flora and Fauna Canberra, and Department of Conservation and Land Management, WA. Canberra; Australian Government Publishing Service: 258pp.
Thalau, H.-P. 1994. Verhaltensphysiologische und neurobiologische Untersuchungen zur Bedeutung des Pinealhormons Melatonin für die Magnetfeldorientierung und das endogene Zugzeitprogramm von Zugvögeln (Passeriformes). PhD thesis, J.W. Goethe-Universität, Frankfurt am Main, Germany.
Wiltschko, R. 1981. Die Sonnenorientierung der Vögel. II. Entwicklung des Sonnenkompaß und sein Stellenwert im Orientierungssystem. Journal für Ornithologie 122: 1-22.
Wiltschko, R. 1995. Kompaßsysteme in der Orientierung von Vögeln. Information Processing in Animals. Vol. 9; Mainz; Akademie der Wissenschaften und der Literatur: 116pp.
Wiltschko, R. & Wiltschko, W. 1981. The development of sun compass orientation in young Homing Pigeons. Behavioral Ecology and Sociobiology 9: 135-141.
Wiltschko, R. & Wiltschko, W. 1995. Magnetic orientation in animals. Berlin; Springer-Verlag: 297pp.
Wiltschko, R. & Wiltschko, W. 1999. Celestial and magnetic cues in experimental conflict. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 988-1004. Johannesburg: BirdLife South Africa.
Wiltschko, W. 1980. The relative importance and integration of different directional cues during ontogeny. In: Nöhring, I.R. (ed.) Acta XVII Congressus Internationalis Ornithologici. Vol. 1; Berlin; Verlag Deutsche Ornithologen-Gesellschaft: 561-565.
Wiltschko, W. & Balda, R.P. 1989: Sun compass orientation in seed-caching Scrub Jays (Aphelocoma coerulescens). Journal of Comparative Physiology A 164: 717-721.
Wiltschko, W. & Wiltschko, R. 1988. Magnetic orientation in birds. In: Johnston, R.F. (ed.) Current ornithology. Vol. 5; New York; Plenum Press: 67-121.
Wiltschko, W. & Wiltschko, R. 1991. Magnetic orientation and celestial cues in migratory orientation. In: Berthold, P. (ed.) Orientation in birds. Basel; Birkhäuser Verlag: 16-37.
Wiltschko, W., Munro, U., Ford, H. & Wiltschko, R. 1993a. Magnetic inclination compass: a basis for the migratory orientation of birds in the Northern and Southern Hemisphere. Experientia 49: 167-170.
Wiltschko, W., Munro, U., Ford, H. & Wiltschko, R. 1993b. Red light disrupts magnetic orientation of migratory birds. Nature 364: 525-527.
Wiltschko, W., Munro, U., Beason, R.C., Ford, H. & Wiltschko, R. 1994. A magnetic pulse leads to a temporary deflection in the orientation of migratory birds. Experientia 50: 697-700.
Wiltschko, W., Wiltschko, R., Munro, U. & Ford, H. 1998. Magnetic versus celestial cues: cue-conflict experiments with migrating Silvereyes at dusk. Journal of Comparative Physiology A 182: 521-529.
Fig. 1. Australia. Most avian movements in Australia have been recorded along the southeastern and eastern coastline and adjacent Great Dividing Range (shaded area).

Fig. 2. Seasonal pattern of locomotor activity of nine Yellow-faced Honeyeaters in half-monthly intervals between January 1990 to January 1991. The hopping activity is given as the percentage of total activity divided by day length (hours) over the year. Standard errors are shown by error bars (after Munro & Munro 1998).
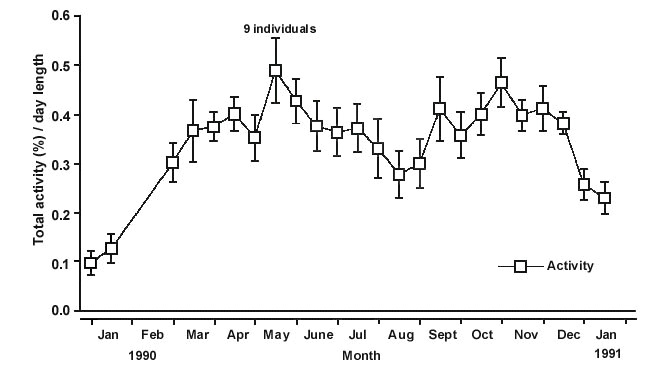
Fig. 3. Daily activity (total number of hops per hour) of nine Yellow-faced Honeyeaters during half-monthly intervals: (A) early January and late March, (B) late April and early and late May, (C) early July, late August and early September, (D) late September, early October and early November, and (E) late December and late January. Bars indicate standard errors (after Munro & Munro 1998).
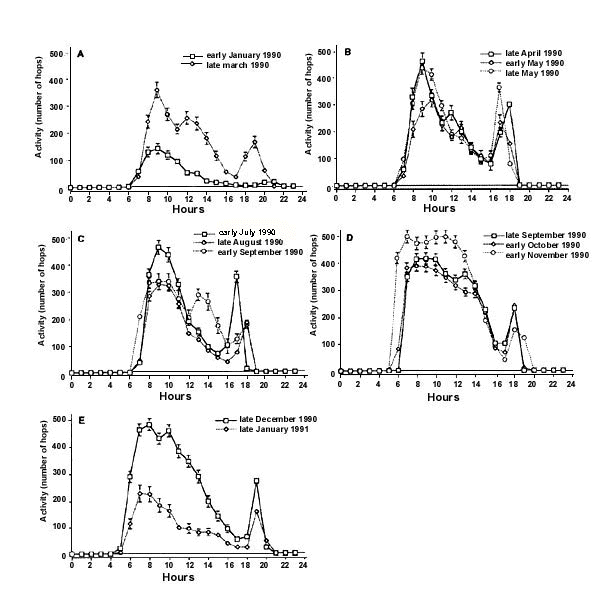
Fig. 4. Orientation behaviour of Yellow-faced Honeyeaters in outdoor tests. (Left) March-April 1990 (early migration period). (Right) May-July 1990 (late migration period). The headings of the birds are represented by solid dots at the periphery of a unit circle = 1. Mean vectors are represented by arrows. The inner circle (broken line) indicates the 5% and the outer circle (unbroken line) the 1% significance level of the Rayleigh test (after Munro et al. 1993).
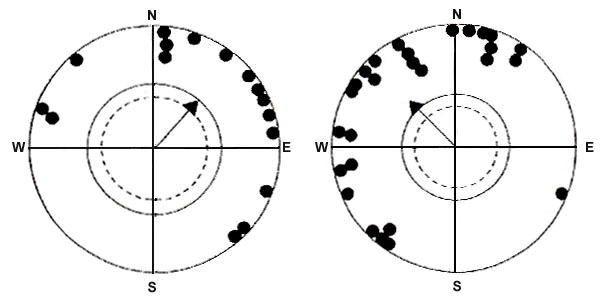
Fig. 5. Autumn orientation of Yellow-faced Honeyeaters in indoor tests, in the first (left diagrams) and second part of migration (right diagrams). Upper diagrams (left and right): tests in the local geomagnetic field; central diagrams (left and right): tests in a magnetic field with north shifted by 120° clockwise to eastsoutheast; lower diagrams (left and right): tests in a magnetic field with a compensated horizontal component. For symbols see Fig. 4 (after Munro & W. Wiltschko 1993).
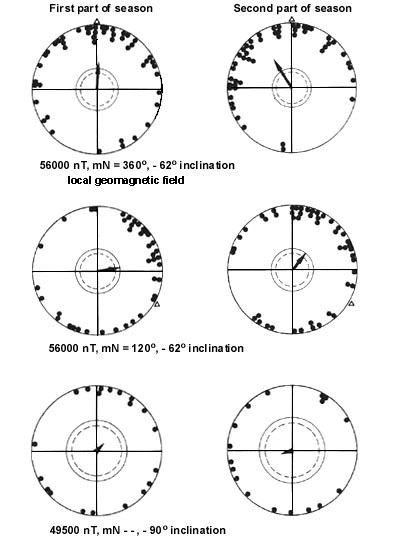
Fig. 6. Orientation of Yellow-faced Honeyeaters during spring in (left) the local geomagnetic field, and (right) a magnetic field with an inversed vertical component, pointing down instead of up. For symbols see Fig. 4 (after Munro & W. Wiltschko 1993).
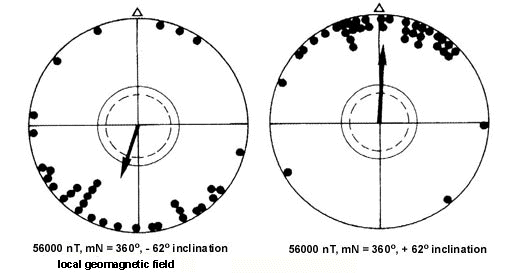
Fig. 7. The common migration program of Northern and Southern Hemisphere migrants. In autumn the birds migrate equatorward. N, S = geographic north and south; He = earth magnetic field vector; g = gravity vector; g = smaller angle between field lines and gravity vector; e, p = equatorward, poleward (after W. Wiltschko et al. 1993a).
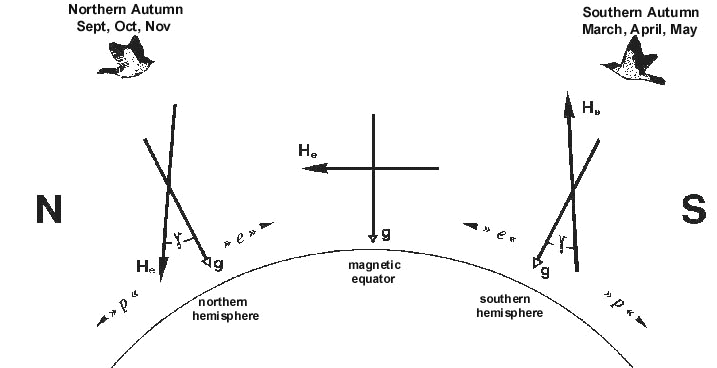
Fig. 8. Deviations from the direction of the controls of (a) four-hour fast-shifted birds during autumn, and (b) three-hour fast-shifted birds during spring (after Munro & R. Wiltschko 1993).
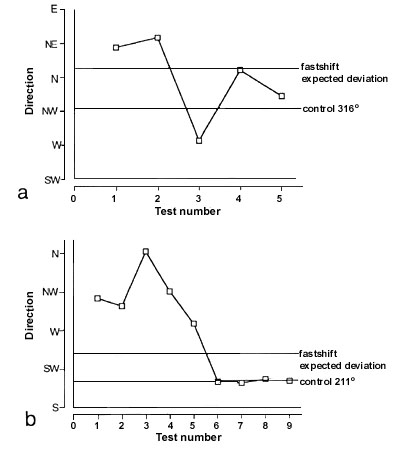
Fig. 9. Autumn orientation of Yellow-faced Honeyeaters tested in the local geomagnetic field during fine weather viewing the sky through: (upper diagrams): (left) clear plexiglass, (right) depolarizers, or (bottom diagrams) polarizers. Alignments of e-vector axes: (bottom diagrams): (left) east-west, (center) northeast-southwest, and (right) northwest-southeast. For symbols see Fig. 4 (after Munro & R. Wiltschko 1995).
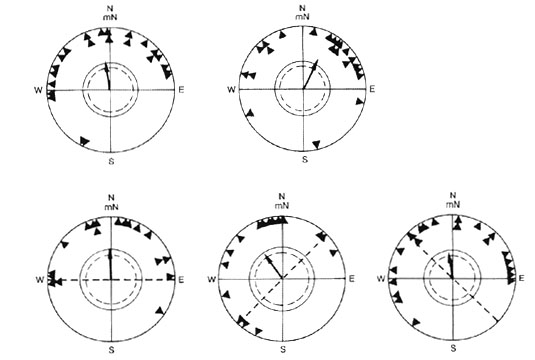
Fig. 10. Autumn orientation of Yellow-faced Honeyeaters tested in a magnetic field with a compensated horizontal component. Orientation under a clear sky (upper diagrams); orientation with the sky partly obscured by clouds with the sun fully covered by clouds (bottom diagrams). The cages were covered with: clear plexiglass (left), or depolarizers (right). For symbols see Fig. 4 (after Munro & R. Wiltschko 1995).