S15.5: Endocrine aspects of physiological condition, weather and habitat quality in landbird migrants during the non-breeding period
Rebecca L. Holberton1, Peter P. Marra2 & Frank R. Moore3
1Department of Biology, University of Mississippi, University, Mississippi, 38677 USA, fax 601 232 5144, e-mail byrlh@olemiss.edu; 2Department of Biological Sciences, Dartmouth College, Hanover, New Hampshire, 03755 USA, e-mail Peter.P.Marra@Dartmouth.edu; 3Department of Biological Sciences, Box 5018, University of Southern Mississippi, Hattiesburg, Mississippi, 39406-5018 USA, e-mail fmoore@whale.st.usm.edu
Holberton, R.L., Marra, P.P. & Moore, F.R. 1999. Endocrine aspects of physiological condition, weather and habitat quality in landbird migrants during the non-breeding period. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 847-866. Johannesburg: BirdLife South Africa.Plasma corticosterone levels may act as reliable indicators of energetic condition in migratory birds. Chronic high levels of corticosterone mobilise energy reserves needed when food intake cannot meet energy demand. During the non-breeding period high corticosterone levels are often negatively correlated with energetic condition. For example, wintering American Redstarts Setophaga ruticilla exhibit a non-random habitat distribution known as sexual habitat segregation whereby older males exclude individuals from preferred habitats. In spring, redstarts in less-preferred, female-biased habitats exhibited higher baseline corticosterone levels than they did in autumn and lost significant body mass over the winter period. In contrast, redstarts in preferred, male-biased habitats showed no change in corticosterone levels or body mass over the same period. Furthermore, redstarts in less-preferred habitats departed later in spring due to their inability to gain energy reserves in preparation for migration. On stopover during spring and autumn migration, birds in poor energetic condition often exhibit elevated corticosterone believed to be a consequence of poor weather and low food availability encountered en route or from poor conditions experienced during the previous stationary period. These birds often require longer stopover to replenish energy reserves causing them to arrive late at their destinations. In spring, leaner birds may suffer delayed gonadal development and reduced reproductive success, a possible consequence of maintaining high corticosterone longer into the breeding period. Measures of physiological and behavioural responses to food availability and changes in energy demand can provide researchers and conservationists with a greater understanding of how environmental factors throughout the annual cycle can influence survivorship and reproductive success in migratory birds.
INTRODUCTION
For most migratory birds the periods of migration and overwintering constitute the majority of the annual cycle. Historically, however, studies on factors influencing population dynamics have focused more on events occurring during the relatively short breeding season and less on the more extensive non-breeding period. Clearly, poor weather conditions, reduced food availability and predation pressure can result in nest failure and a loss in annual reproductive success and fitness, but these factors might also affect post-breeding activities such as post-nuptial moultand the preparation for autumnal migration. Further, poor conditions encountered during the autumnal migratory period may result in an individual arriving too late to compete well for suitable winter habitats. Individuals may thus be compromised in their ability to overwinter and/or prepare for spring migration. Events encountered en route in spring may also impact body condition and arrival time on the breeding grounds reducing the ability of migrants to obtain good territories and mates (Loria & Moore 1990; Arvidsson & Neergaard 1991; Andersson & Gustafsson 1995). Clearly, an important component of an individual’s reproductive potential during the breeding season is its probability of having survived the periods leading up to it. Therefore, there is a great need for understanding how migrants respond behaviourally and physiologically to events encountered not only during breeding but also during the non-breeding period. In this paper we describe recent and ongoing work on the effect that environmental factors such as weather and habitat quality may have on an individual’s ability to maintain energy reserves. Specifically, we review several studies on the relationships between corticosterone (the hormone most associated with behavioural and physiological responses to changes in energy demand) and challenges that some Neotropical landbird migrants may face in trying to meet their energy demand during the non-breeding period.
Corticosterone in Birds
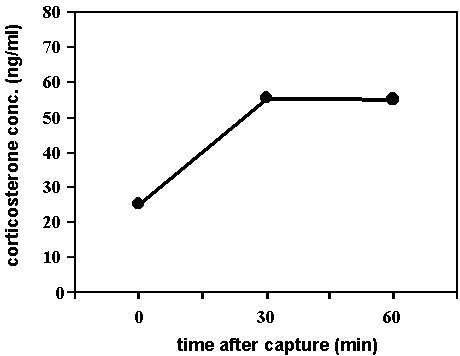
Corticosterone, the major glucocorticoid in birds, is produced by adrenocortical tissue in response to adrenocorticotropin hormone secreted by the anterior pituitary which in turn is in response to corticotropin releasing hormone secreted from the hypothalamus. The hypothalamic-hypophyseal-adrenal (HPA) axis is sensitive to a wide array of internal and external sensory information and is essential for the maintenance of homeostasis (Selye 1971; Holmes & Phillips 1976; Harvey et al. 1984; Munck et al. 1984). Many studies on a variety of bird species have now shown that corticosterone can rise rapidly in the bloodstream in response to the stress of capture and handling (e.g., songbirds: Dawson & Howe 1983; Wingfield 1994; Wingfield et al. 1992, 1994a,b, 1995a,b; Holberton et al. 1996a, shorebirds: Ramenofsky et al. 1995; O’Reilly & Wingfield 1995; Mizrahi & Holberton, unpub. manuscript, seabirds: Smith et al. 1994; Holberton et al. 1996b, doves: Wingfield et al. 1992, raptors: Dufty & Belthoff 1997). In general, plasma corticosterone concentrations obtained from individuals immediately upon capture are low and are followed by a significant rise in plasma hormone concentration with handling time with maximal levels usually observed within 30 to 60 min after capture (for example see Fig. 1). These elevated concentrations return to pre-disturbance levels soon after potentially stressful conditions abate (Wingfield 1994). The plasma profiles of acute corticosterone secretion as a function of handling stress provide several pieces of information. If taken within the first few min after capture, the initial (baseline) sample is the best estimate of the pre-disturbance plasma concentration of corticosterone. The profiles also illustrate the maximum amount of corticosterone produced by the individual as well as the rate at which the hormone is released. The protocol of taking repeated samples from individuals to produce these profiles has become the accepted method of assessing an individual’s adrenocortical responsiveness to stress (Wingfield 1994; Astheimer, et al. 1995). For most questions only two samples, baseline at the time of capture and at 30 min later, are sufficient to detect whether the HPA axis is responding with a corticosterone stress response.
Short-term, ephemeral increases in plasma corticosterone secreted in response to unpredictable events (e.g., storms) often result in the redirection of behaviours away from ongoing activities such as reproduction and territoriality and towards immediate life-saving responses such as an increase in food searching and hyperphagia (Gray et al. 1990; Wingfield & Silverin 1986; Wingfield 1988; Astheimer et al. 1992). If an individual can meet its energy demand or the perturbation passes, corticosterone levels return to baseline and normal activities may resume. In this way, the corticosterone stress response may help an individual ‘ride out’ difficult periods thus avoiding becoming seriously energetically compromised until conditions improve (Sapolsky 1987; Wingfield 1994).
While the corticosterone stress response may help an individual through short-term periods of perturbations, chronic high levels of the hormone may be deleterious. Through corticosterone’s promotion of gluconeogenesis to obtain glucose substrates from non-carbohydrate sources, skeletal muscle serving as an energy source may be seriously catabolized (Cherel et al. 1988a; Dallman et al. 1993). Chronic high levels of corticosterone can also suppress the immune system and compromise growth, development and reproduction as well as result in neuronal cell death (for review, Sapolsky 1987; Wingfield 1994). Because high levels of corticosterone can have such severe effects it would seem that the sensitivity or responsiveness of the HPA axis could be modulated in such a way as to either reduce its sensitivity to facilitate activities that would otherwise be incompatible with high corticosterone secretion or to enhance it as a means of responding more quickly during times of stress. Indeed, several studies have now shown distinct changes in the strength of the corticosterone stress response during several stages of the annual cycle (Greenberg & Wingfield 1987; Wingfield et al. 1992; Astheimer et al. 1992, 1995; Holberton et al. 1996a; Romero et al. 1997; Marra & Holberton 1998; Holberton & Wingfield, unpub. manuscript).
Corticosterone and the Winter Period
Migratory birds may spend as much as 5 to 8 months on their Neotropical wintering grounds. Events occurring during this stationary portion of the non-breeding period may influence the physical condition of individuals during winter and impact preparation for spring migration and reproduction (Arvidsson & Neergaard 1991; Gustafsson et al. 1994; Andersson & Gustafsson 1995). While several studies have described patterns of habitat occupancy for Neotropical migrants on the wintering grounds (see Keast & Morton 1980, Hagan & Johnston 1992) few have examined the impact that these patterns may have on the survival and physical condition of individuals (but see Sherry & Holmes 1996, Marra & Holberton, in press). Because birds are not breeding, estimating the impact of winter habitat suitability on reproductive success cannot be done using the typical measures of nesting success (e.g., number of offspring). However, an assessment of how well birds may be doing in maintaining body condition in winter can provide a measure of how likely they will survive to spring and beyond.
Food availability and predation pressure are considered important causes of deteriorating body condition and lower survivorship in winter. On most Caribbean islands, bird predators in winter are relatively uncommon making food availability and its influence on energetic condition the major determinant of habitat suitability in these areas (Marra unpub. data). Most migrant birds are distributed across a wide array of habitats (e.g., Lynch 1992; Hutto 1992; Petit et al., 1992). However, it is not known if these habitats are equally suitable for survival or for the maintenance of optimum physical condition. In examining the winter distributions of migrant birds across several habitat types a distinct pattern emerges in which some habitats are biased in the number of territorial males and others in the number of territorial females (Lynch et al. 1985; Ornat & Greenberg, 1988). This non-random pattern of habitat distribution known as sexual habitat segregation is especially common in the American Redstart, Setophaga ruticilla (Sherry & Holmes 1997). In Jamaica, sexual habitat segregation is the result of behavioural dominance, i.e., the exclusion by dominant individuals, typically older males, of less dominant ones, from a particular habitat type (Marra et al. 1993, Marra, unpub. manuscript). One assumption of the behavioural dominance hypothesis is that habitats differ in suitability such that individuals in female-biased habitat, regardless of sex or age 1) survive less well or are in poorer physical condition at the end of winter or 2) have to work harder to maintain the physiological condition equivalent to that of individuals in male-biased habitats.
To assess the possible physiological consequences of sexual habitat segregation on the wintering grounds, Marra and Holberton (1998) measured circulating levels of corticosterone in redstarts occupying two different habitat types in Jamaica, W.I., each habitat characterised by distinctly different sex ratios. The first was a black mangrove, Avicennia germinans, swamp habitat containing approximately 65% male redstarts. The other habitat was second growth scrub with only 30% male redstarts. Both of these habitats appear to have similar amounts of vegetation in autumn (October) when birds first arrive but diverge dramatically over the winter period as the tropical dry season approaches in spring (March and April) (Parrish & Sherry 1994). In spring, the water in the black mangrove swamp dries up but the trees retain most of their leaves, whereas, in the second growth scrub the trees lose the majority of their leaves. In their study, redstarts were captured in both habitat types in the autumn, colour-banded and then recaptured in the spring. At each capture, a baseline and 30 min sample was taken from each individual to determine if baseline corticosterone and the strength of the stress response changed as a function of season and habitat.
The results demonstrated that in autumn, regardless of sex or age, redstarts had similar baseline corticosterone concentrations and exhibit similar plasma profiles of acute corticosterone secretion in response to handling stress across both habitat types (Fig. 2 & Fig. 3). However, concomitant with the divergence in food availability over the wintering period, redstarts in the female-biased second growth scrub exhibited significantly higher baseline corticosterone
concentrations than they had in autumn and these levels were higher than those in birds inhabiting the mangrove swamp (Fig. 2). These higher levels of corticosterone may be an indicator of the birds' relatively poor energetic condition and greater mass loss over the winter period and/or they may be needed to facilitate greater foraging effort to maintain energy reserves. Indeed, birds with high basal corticosterone also had lost mass over the winter period. In addition, the birds in the poorer habitat in spring showed a reduction in the strength of acute corticosterone secretion in response to handling stress (Fig. 3). The mechanism by which the stress response is modulated in these birds is unknown but its reduction may help protect skeletal muscle from corticosterone's gluconeogenic catabolic activity until all other resources have been depleted (Cherel et al. 1988a,b; Holberton et al. 1996a). These results clearly demonstrate an effect of habitat, most likely through food availability, on the physiological condition of redstarts at the end of the winter period. In this way, habitat quality can be an important factor influencing the timing of departure to the breeding grounds and the ability of birds to prepare for breeding in spring (Marra et al., unpub. manuscript).
Corticosterone and migration
Based on a fast growing number of studies and models developed primarily from mammals and birds, corticosterone’s effects on migratory behaviour and physiology appear to be concentration-dependent (Cherel et al. 1988a,b; Dallman et al. 1993, 1994). Independent of the small circadian changes in plasma corticosterone, moderate levels of the hormone can result in the accumulation of fat stores (Berdanier 1989; Dallman et al. 1993, 1994). Although the exact mechanism of this activity is unknown, corticosterone levels above the usually low diel range of values and below that resulting in catabolism of skeletal muscle can be lipogenic either through a possible potentiation of insulin (Dallman et al. 1993, 1994) or by increasing foraging activities (Wingfield & Silverin 1986; Gray et al. 1990; Astheimer et al. 1992) or both. Early evidence from several bird studies indicated that adrenocortical activity is higher during the migratory period (John 1966; Peczely 1976). More recently, Holberton et al. (unpub. manuscript) observed an absence of migratory fattening in captive birds who had received dexamethasone, an agonist that inhibits endogenous production of corticosterone. Clearly, corticosterone’s associations with hyperphagia and lipogenesis are extremely relevant to bird migration physiology, as first suggested by Meier and his colleagues years ago (Meier & Farner 1964; Meier et al. 1965; Dusseau & Meier 1971).
In many species of migrants sampled to date the profile of acute corticosterone secretion changes significantly during migration. Several studies on a variety of bird species have now shown that many migrating birds maintain relatively high baseline levels of corticosterone and often show a reduced response to handling stress (O’Reilly & Wingfield, 1995; Ramenofsky et al. 1995; Holberton et al. 1996a; Romero et al. 1997; Mizrahi & Holberton, unpub. manuscript; but see Schwabl et al. 1991; Gwinner et al. 1992). The Migration Modulation Hypothesis (MMH) was proposed by Holberton et al. (1996a) to provide an explanation for this change in the pattern of corticosterone secretion. The MMH collectively states that, as an integral part of the development of migratory condition, migrants maintain higher levels of corticosterone to facilitate hyperphagia and lipogenesis and, if able to meet their energy demand, they avoid further elevating corticosterone by reducing the expression of the stress response, a way of protecting skeletal muscle needed for flight. In support of the MMH, data collected from free-living Grey Catbirds, Dumetella carolinensis, sampled before and after the onset of migratory fattening illustrated such a change in corticosterone secretion concurrent with change in migratory status (Holberton et al. 1996a). In that study, Catbirds sampled in the field during the pre-migratory period in autumn (before post-nuptial moultwas completed or migratory fattening had begun) showed low baseline corticosterone levels that were followed by a significant increase in corticosterone with handling time. However, Catbirds sampled at the site several weeks later had completed moult and had put on significant amounts of fat. In addition, these birds had significantly higher levels of baseline corticosterone and showed a significant reduction in the corticosterone stress response concurrent with this change in migratory state (Holberton et al. 1996a).
Although the results from the Catbirds sampled in the field supported the MMH, the role that other factors such as weather, food availability or predation pressure may have had in the change in corticosterone secretion could not be definitively ruled out. To show that the profile of corticosterone secretion is an integral part of the migratory state, Holberton (accepted upon revision) held Yellow-rumped Warblers, Dendroica coronata, in captivity under controlled laboratory conditions. The birds were given food and water ad libitum and experienced two transitions from short to long day photoperiod to twice bring them into migratory condition. When the birds were held on a short, non-stimulatory daylength (10.5L:13.5D), they exhibited low baseline levels of corticosterone and a significant stress response. However, in response to an increase in daylength (15.5L:8.5D), the birds gained as much as 50% of their lean body mass in fat reserves and, concurrent with this fattening, they also showed an increase in baseline corticosterone and an absence of the stress response similar to that seen in free-living Yellow-rumped Warblers sampled on stopover during migration (Holberton et al. 1996a). These results, observed during both transitions from short to long days under controlled conditions, demonstrate that the increase in baseline corticosterone and the reduction of the stress response were concurrent with the development of the migratory state. These results do not support the idea that elevated corticosterone in migrating birds always indicates that birds are stressed or that migration, per se, is stressful. As the period of migration can comprise as much of 30% or more of the annual cycle in birds it would not seem adaptive for individuals to experience chronic physiological stress during the entire time spent migrating. This would seem particularly true with respect to the deleterious effects that chronic high levels of corticosterone can have on the individual (references above). Thus, it would seem that the levels of corticosterone observed in migrating birds sampled under optimal weather conditions or when brought into migratory condition in the laboratory may represent the intermediate, non-catabolic levels proposed by Berdanier (1989) and Dallman et al. (1993; 1994) that work to facilitate hyperphagia and fattening in migratory birds.
Variation in corticosterone secretion during migration
For the past several years, we have been sampling Neotropical migrants for the stress response at several stopover sites during spring and autumn migration in an effort to understand the role that corticosterone may play in helping a migrant meet its energy demand en route. Fig. 4 illustrates the frequent shape of the corticosterone profiles of several groups of warblers, vireos and thrushes sampled in September and October along the coastline of New England and the Gulf of Mexico in North America. These profiles fit the general pattern predicted by the MMH in that there is no apparent corticosterone stress response being expressed. This has led us to the perplexing question: if migrants reduce their corticosterone response to stress during migration how do they face the potentially higher energy demands encountered during storms, high predation pressure or when forced to forage in poor habitats en route? We addressed this question by sampling two groups of Yellow-rumped Warblers on stopover under very different environmental conditions.
As described earlier (Holberton et al. 1996a), from 10 to 17 October 1993, twelve hatching-year Yellow-rumped warblers (8 males, 4 females) were sampled for the corticosterone stress response while on stopover at Block Island, Rhode Island, U.S.A. For several days prior to and throughout the sampling period weather conditions were considered optimal for migration in autumn. Winds were light (<16 km h-1) with daytime temperatures ranging from 12 to 21 oC falling to 7 to 10 oC at night. The birds had a mean mass of 12.47 + 0.15 S.E. g and 50% of them had fat deposits filling or almost filling the furculum. The birds exhibited a mean baseline level of corticosterone of 31.14 + 2.74 S.E. ng ml-1 and showed no further increase in corticosterone with handling time.
Weather conditions were quite different, however, during a comparable sampling period at Manomet, Massachusetts, U.S.A., 16 to 18 October 1995. During the night of the 15th a cold front passed over the region bringing heavy rain and winds gusting from 56 to 72 km h-1 out of the northwest. For the next several days, the skies were clear but northwest winds continued strong with gusts up to 48 to 56 km h-1. Twenty-one of the several hundred hatching-year Yellow-rumped warblers (12 males, 6 females, 3 unknown) captured in mist nets at mid-day on the two days following the cold front were sampled for the corticosterone stress response. Most of these birds appeared to have used much of their pectoralis muscle as evidenced by the strongly protruding sternum on each bird. The mean body mass of the group was 12.39 + 0.21 S.E. g and only 10% of the birds had fat filling the furculum. These birds had significantly higher baseline levels of corticosterone (mean = 66.07 + 8.86 S.E. ng ml-1, Mann-Whitney U-test: Z = 2.51, n = 9 from Block Island and 21 from Manomet, P = 0.01) than those sampled under better conditions in the earlier study at Block Island (Fig. 5). There was no difference in corticosterone levels at 30 min after capture between the two groups (Mann-Whitney U-test: Z = 1.01, n = 11 Block Island and 21 from Manomet, P = 0.31) and the Manomet birds also failed to show a corticosterone stress response (Fig. 5). Both groups exhibited baseline levels that were apparently the maximal capacity of the adrenocortical tissue to secrete corticosterone at that time. However, the steep decline observed in the Manomet group is most likely a function of the extremely high adrenocortical activity experienced by the birds at the time of capture, which with subsequent sampling can result in exhaustion of the tissue and/or hemodilution (dilution of the plasma with extra-cellular fluid from surrounding tissues, a strategy to restore blood volume) resulting in much lower plasma concentrations in the 30 min sample. This effect would result in an observed decrease in corticosterone concentration and illustrates the inability of the adrenocortical tissue to respond further when it is at maximal output already during this time. During the non-migratory period the increase in plasma corticosterone concentrations observed in subsequent sampling at 30 min or more clearly illustrates that the adrenocortical tissue can not only increase hormone secretion in response to a stressor but it can do so overriding the ensuing hemodilution effect.
Although there was no significant difference in the absolute body mass between the birds sampled at Block Island and Manomet (Mann-Whitney U-test: Z = 0.02, n = 12 Block Island birds and 21 Manomet birds, P = 0.98), the Block Island birds tended to have a shorter wing chord (Block Island mean = 69.6 + 1.2 S.E. mm versus Manomet mean = 72.3 + 0.7 S.E. mm, Mann-Whitney U-test: Z = 1.82, n = 12 Block Island birds and 21 Manomet birds, P = 0.07). When differences in body size were controlled (through the use of an index of energetic condition based on mass divided by wing chord3), the Manomet birds were in significantly poorer energetic condition (Mann-Whitney U-test: Z = 2.06, n = 12 Block Island birds and 21 Manomet birds, P = 0.04, Fig. 6). There were no differences in wing chord, body mass, fat scores or baseline corticosterone between the sexes in either group so data from males and females were pooled for all analyses.
The reason for the poorer condition of the birds captured at Manomet most likely lies in the weather conditions and the consequent higher energy demands under which they were sampled. The birds were among several hundred Yellow-rumped Warblers captured along the shoreline at a time of day when many birds are known to reorient to land after being blown offshore during autumnal nocturnal movements along the New England coast (Able 1977). If these birds were indeed flying back to land after having been out over the water at dawn they were most likely flying in strong crosswinds after spending many hours the previous night in flight (with much of it over water) and may have been low on the energy stores expected to have been sufficient for a much shorter overland flight.
The data presented here from warblers sampled under different weather conditions illustrate that, although the corticosterone stress response may be absent or reduced during migration, migrants encountering periods of high energy demand or critically low energy reserves are able to mobilise additional levels of corticosterone above those necessary to facilitate migratory foraging and fattening (Ramenofsky et al. 1995). Indeed, it seems likely that the higher levels of corticosterone in the Manomet birds account for the catabolism of pectoralis muscle and, hence, lower index of energetic condition, a pattern also observed in shorebirds on stopover (Ramenofsky et al. 1995). These data are congruent with the second component of the MMH in that when faced with higher energy demand migrants can mobilise additional concentrations of corticosterone resulting in the utilization of skeletal muscle as energy reserves but inhibit this response as long as they can meet their energy demand. Future studies would benefit from including measurements of protein and lipid mobilisation such as that demonstrated by Cherel et al. (1988a,b), Jenni & Jenni-Eirmann (1992), Jenni-Eirmann and Jenni (1994) and Totzke et al. (1997) in conjunction with corticosterone to fully understand energy utilisation in migrants.
In general, we have found that patterns of corticosterone secretion Neotropical migrants in spring are similar to that observed in autumn but that there is greater variation in its expression in spring. We recently surveyed our corticosterone data from over 140 Neotropical passerine migrants at stopover sites in New England and along the northern Gulf Coast. Eighty-five birds comprising 23 species were sampled during spring migration in April, 1995 and 1996 at Johnson’s Bayou, Louisiana, USA. Of the 77 birds from which we have complete stress series (baseline and sample at 30 min post capture, Fig. 7), only 50% showed elevated baseline corticosterone and the absence of a corticosterone stress response. This is in contrast to a comparable, albeit smaller, group of 55 individuals comprising 12 species sampled at Bon Secour, Alabama, U.S.A., 29 September to 1 October 1995 and at Manomet, Massachusetts 15 to 18 October 1995, in which 82% of the birds with complete stress series showed the migratory pattern of corticosterone secretion (Fig. 7) and baseline levels were significantly lower than those sampled in autumn (F1,131 = 33.33, P < 0.001)
The sources of variation in patterns of corticosterone secretion within and between migration seasons may be due to a variety of factors encountered during the stationary period as well as during migration. While events during the pre-migratory and migratory period may delay fattening and subsequent arrival at the destination, spring migrants face the additional demands of energy allocation to reproduction. During spring migration, males can have significant development of the gonads and accessory reproductive tissue such as the cloacal protuberance and produce sperm (Quay 1985, 1986; Briskie 1996). Several studies have reported evidence of copulations and the potential for pair bonding en route , clearly indicating that behavioural and physiological reproductive activities are occurring in some birds well before their arrival at the breeding grounds (Quay 1985; Moore & McDonald 1993; Briskie 1996). However, many studies on a variety of taxa have shown that elavated corticosterone levels can negatively impact breeding activities (Paolucci et al. 1990; Elsey et al. 1990; Greenberg & Wingfield 1987; Moore & Miller 1984; Wingfield & Silverin 1986; Wingfield et al. 1983; 1995a,b). As high corticosterone levels are often associated with poorer energetic condition (Schwabl et al. 1991; Gwinner et al. 1992; Wingfield et al. 1994a; Marra & Holberton, 1998; Holberton & Marra, this ms), the birds that are able to meet their energy demand during migration may begin to reduce baseline corticosterone in preparation for breeding. In contrast, those individuals encountering difficulties in spring may be unable to reduce baseline corticosterone and may retain migratory or greater levels longer into the spring period. These birds may experience longer stopovers, later arrival on the breeding grounds and may be in suboptimal condition upon their arrival which can consequently lead to reduced reproductive success (Moore & Kerlinger 1987; Loria & Moore 1990; Arvidsson & Neergaard 1991; Gustafsson et al. 1994; Andersson & Gustafsson 1995).
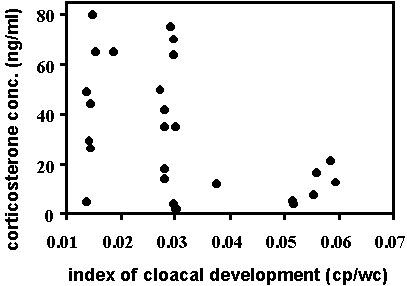
Moore and Holberton have begun investigating the relationships between energetic condition, gonadal development and the corticosterone stress response in several species of Neotropical migrants along the northern coast of the Gulf of Mexico in spring. Preliminary analyses of Kentucky (Oporonis formosus , n = 4) and Hooded (Wilsonia citrina, n = 13) Warblers (pooled for their similar size and breeding range) captured in 7 to 16 April 1995 and 1996 at Johnson’s Bayou, Louisiana, show a typical migratory profile of corticosterone secretion (Fig. 8). The variation in baseline corticosterone in this group was significantly negatively correlated with the variation in body mass (Fig. 9, r = 0.61, P < 0.05, rcrit = 0.482, n = 17) and fat score (not shown, Kendall’s Coefficient of Rank Correlation, tau = -0.36, Z = -2.04, n = 17, P = 0.04) suggesting that birds in better condition were beginning to reduce baseline corticosterone. Because the development of the cloacal protuberance in males is under direct control of testosterone by the gonads and its rate of development is proportional to the increase in androgen in the bloodstream (Wingfield 1984) it can be used as an indirect measure of gonadal activity in male birds. We measured the height of the cloacal protuberance (to nearest 0.5 mm) above the body wall at its caudal end in the males in the group to ask if males in better physiological condition were able to begin gonadal development sooner than those in poorer condition. Within this group of males, body mass was positively correlated with the development of the cloacal protuberance (not shown, Kendall’s Coefficient of Rank Correlation, tau = 0.62, Z = 2.14, n = 8, P = 0.03,) and those individuals who had greater developed protuberances had lower plasma concentrations of baseline corticosterone (r = 0.78, rcrit = 0.71, n = 8, P = 0.05, Fig. 10). Although the sample sizes are small for this group, the same pattern emerges in a larger pooled group of male wood warblers. When sizes differences among individuals between and within species are controlled for (by dividing the height of the cloacal protuberance by wing chord, to nearest 0.5 mm, for each bird), we found that those individuals with a comparatively more developed protuberance were more likely to have lower baseline corticosterone levels on stopover during our sampling period (F1,33 = 4.33, n = 33, P = 0.046, Fig. 11), a pattern that supports our hypotheses that gonadal development may be inhibited by elevated corticosterone during spring migration.
Summary
For migratory birds, the period of the non-breeding season comprises the majority of the annual cycle. Despite this fact, most studies have focused on how breeding season events influence fitness. This is probably because quantifying the physical condition of non-breeding individuals and understanding the causes of variation in physiological condition and its subsequent effect on survivorship and fitness may be more problematic in migratory birds. However, determining how factors such as weather, habitat quality and competition for resources during this formidable period can influence physical condition and survival is critical to understanding population dynamics in migrants. In this paper we have presented several studies that illustrate the contribution that an integration between endocrinology and behavioural ecology can make to our understanding of how events during the non-breeding can affect migrants.
Profiles of the corticosterone stress response change with changing demands of migration and overwintering. During migration, migrating birds can maintain elevated corticosterone levels to facilitate hyperphagia and fattening. The corticosterone stress response appears to be reduced which may result in the protection of skeletal muscle needed for flight. However, migrants are able to mobilise corticosterone above these already elevated levels in response to ‘emergency’ situations such as during storms. As illustrated by redstarts forced to forage in less-preferred habitats in winter, poor conditions encountered before initiating migration may impact the preparation for migration. Storms or other perturbations along the migratory route may increase energy demand and reduce opportunities to replenish energy reserves thus delaying arrival at the wintering or breeding grounds. In spring, poor conditions over the wintering period and during migration may also delay gonadal development possibly further compromising reproductive success. Clearly, the sources of variation in the endocrine and behavioural responses of migrants to stress is a rich area needing further study.
All birds face challenges to survivorship and fitness throughout the annual cycle. Events during the non-breeding period may be as important in influencing population dynamics as factors affecting reproductive success on the breeding grounds through their impact on physiological condition of individuals. There are many factors related to health in birds such as parasite load, immunocompetence and exposure to many man-made chemicals in the environment. These, as well as plasma glucocorticoids and metabolites of fat and muscle utilisation, may provide a rich area of study when combined with behavioural and ecological aspects of migrant bird ecology.
ACKNOWLEDGEMENTS
The authors are grateful for travel assistance provided by the American Ornithologists’ Union to attend the XXII International Ornithological Congress. RLH was also funded by the UM Faculty Development Fund. We wish to thank the numerous field assistants at Johnson’s Bayou and Bon Secour for their co-operation with sampling at those sites and the Grey Estates for allowing us to work on their property. We also greatly appreciate the co-operation and assistance of Mr. Trevor Lloyd-Evans and his extremely capable field assistants at the Manomet Bird Observatory in Manomet, MA. Much of the data presented here were collected on Block Island, RI in collaboration with Dr. Jeffrey Parrish, Megan Whitman and Scott Comings and in co-operation with Mrs. F. David Lapham and The Nature Conservancy who kindly let us use their land. RLH was funded by the UM Faculty Development Fund and the UM Small Grants for Research Fund. PPM was supported by NSF, Sigma Xi Grants in Aid of Research, and the Frank M. Chapman Memorial Fund. Yvette Strong of the Jamaican NRCA and the Petroleum Corporation of Jamaica allowed us to conduct research on their property. Richard Holmes and Thomas Sherry provided expert advice throughout the study. All activities were approved by the institutions’ Animal Care Committee (Dartmouth College and University of Mississippi) and all birds were captured, banded and sampled under state and federal permits issued to RLH, FRM and PPM.
REFERENCES
Able, K.P. 1977. The orientation of passerine nocturnal migrants following offshore drift. Auk 94: 320-330.
Andersson, M.S. & Gustafsson, L. 1995. Glycosylated haemoglobin: a new measure of condition in birds. Proceedings of the Royal Society of London B 260: 299-303.
Arvidsson, B.L. & Neergaard, R. 1991. Mate choice in the willow warbler - a field experiment. behavioural Ecology and Sociobiology 29: 225-229.
Astheimer, L.B., Buttemer, W.A. & Wingfield, J.C. 1992. Interactions of corticosterone with feeding, activity and metabolism in passerine birds. Ornis Scandinavica 23: 355-365.
Astheimer, L.B., Buttemer, W.A. & Wingfield, J.C. 1995. Seasonal and acute changes in adrenocortical responsiveness in an Arctic-breeding bird. Hormones & behaviour 29: 442-457.
Berdanier, C.D. 1989. Role of glucocorticoids in the regulation of lipogenesis. Federation of American Societies for Experimental Biology 3: 2179-2183.
Briskie, J.V. 1996. Lack of sperm storage by female migrants and the significance of copulations en route. Condor 98: 414-417.
Cherel, Y., Robin, J.P., Walch, O., Karmann, H. Netchitailo, P. & le Maho, Y. 1988a. Fasting in the King Penguin. I. Hormonal and metabolic changes during breeding. American Journal of Physiology 23: R170-177.
Cherel, Y., Leloup, J. & le Maho, Y. 1988b. Fasting in the King Penguin. II. Hormonal and metabolic changes during molt. American Journal of Physiology 23:R178-184
Dallman, M.F., Strack, A.M., Akana, S.F., Bradbury, M.J., Hanson, E.S., Scribner, K.A. & Smith, M. 1993. Feast or famine: critical role of glucocorticoids with insulin in daily energy flow. Frontiers in Neuroendocrinology 14: 303-347.
Dallman, M.F., Akana, S.F., Levin, N., Walker, C-D. Bradbury, M.J. Suemaru, S. & Scribner, K.S. 1994. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. In: de Kloet, E.R., Asmitia, E.C. & Landfield, P.W. (Eds) Brain corticosteroid receptors: studies on the mechanism, function, and neurotoxicity of corticosteroid action. New York; Annals of the New York Academy of Sciences: 22-32.
Dawson, A. & Howe, P.D. 1983. Plasma corticosterone in wild starlings, Sturnus vulgaris, immediately following capture and in relation to body weight during the annual cycle. General and Comparative Endocrinology 51:303-308.
Dufty, A.M., Jr. & Belthoff, J.R. 1997. Corticosterone and the stress response in young Western Screech Owls: effects of captivity, gender and activity period. Physiological Zoology 70: 143-149.
Dusseau, J.W. & Meier, A.H. 1971. Diurnal and seasonal variations of plasma adrenal steroid hormone in the White-throated sparrow, Zonotrichia albicollis. General and Comparative Endocrinology 16: 399-408.
Elsey, R.M., Joanen, T., McNease, L. & Lance, V. 1990. Stress and plasma corticosterone levels in the American alligator: relationships with stocking density and nesting success. Comparative Biochemistry and Physiology 95A: 55-63.
Gray, J.M., Yarian, D. & Ramenofsky, M. 1990. Corticosterone, foraging behaviour, and metabolism in Dark-eyed Juncos, Junco hyemalis. General and Comparative Endocrinology 79:375-384.
Greenberg, N. & Wingfield, J.C. 1987. Stress and reproduction: reciprocal relationships. In: Norris, D.O. & Jones, R.E. (eds) Hormones and reproduction in fishes, amphibians, and reptiles. New York; Plenum Press: 461-503.
Gustafsson, L., Nordling, D., Andersson, M.S., Sheldon, B.C. & Gvarnstrom, A. 1994. Infectious diseases, reproductive effort and the cost of reproduction in birds. Phil. Transactions of The Royal Society of London B 346:323-331.
Gwinner, E., Zeman, M., Schwabl-Benzinger, I., Jenni-Eiermann, S., Jenni, L. & Schwabl, H. 1992. Corticosterone levels of passerine birds during migratory flight. Naturwissenschaften 79: 276-278.
Hagan, J.M., III & Johnston, D.W. 1992. Ecology and conservation of Neotropical migrant landbirds. Smithsonian Institution Press, Washington, D.C.
Harvey, S., Phillips, J.G., Rees, A. & Hall, T.R. 1984. Stress and adrenal function. Journal of Experimental Zoology 232:633-646.
Holberton, R.L. accepted upon revision. Changes in corticosterone secretion in response to long-day photoperiod:experimental support for the Migration Modulation Hypothesis. General and Comparative Endocrinology.
Holberton, R.L., Parrish, J.D. & Wingfield, J.C. 1996a. Modulation of the adrenocortical stress response in Neotropical migrants during autumn migration. Auk 113: 558-564.
Holberton, R.L., Helmuth, B. & Wingfield, J.C. 1996b. The corticosterone stress response in Gentoo, Pygoscelis papua, and King, Aptenodytes patagonicus, penguins during the non-fasting period. Condor 98: 850-854.
Holmes, W.N. & Phillips, J.G. 1976. The Adrenal Cortex in Birds. In: Chester-Jones, I. & Henderson, I. (eds) General and comparative endocrinology of the adrenal cortex. New York; Academic Press: 293-420.
Hutto, R.L. 1992. Habitat distributions of migratory landbird species in western Mexico. In: Hagan III, J.M. & Johnston, D.W. (eds.) Ecology and conservation of Neotropical migrant landbirds. Washington; Smithsonian Institution Press: 221-239.
Jenni, L. & Jenni-Eiermann, S. 1992. Metabolic patterns of feeding, overnight fasted and flying night migrants during autumn migration. Ornis Scandinavica 23: 251-259.
Jenni-Eiermann, S. & Jenni, L. 1994. Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the Garden warbler. Auk 111: 888-899.
John, T.M. 1966. A histochemical study of adrenal corticoids in the pre and post-migratory phases in the migratory wagtails (Motacilla alba and Motacilla flavo). Pavo 4: 9-14.
Keast, A. & Morton, E.S. 1980. Migrants in the Neotropics: ecology, behaviour, distribution and conservation. Smithsonian Institution, Washington D.C.
Loria, L. & Moore, F.R. 1990. Energy demands of migration on red-eyed vireos, Vireo olivaceus. behavioural Ecology 1:24-35.
Lynch, J.F. 1992. Distribution of overwintering Nearctic migrants in the Yucatan Peninsula, II: use of native and human-modified vegetation. In: Hagan III, J.M. & Johnston, D.W. (eds.) Ecology and conservation of Neotropical migrant landbirds. Washington; Smithsonian Institution Press: 178-196.
Lynch, J.F., Morton, E.S. & van der Voort, M.E. 1985. Habitat segregation between the sexes of wintering Hooded Warblers (Wilsonia citrina). Auk 102: 714-721.
Marra, P.P. & Holberton, R.L. 1998. Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia, in press.
Marra, P.P., Sherry, T.W. & Holmes, R.T. 1993. Territorial exclusion by a long-distance migrant warbler in Jamaica: a removal experiment with American redstarts, Setophaga ruticilla. Auk 110: 565-572.
Meier, A.H. & Farner, D. S. 1964. A possible endocrine basis for premigratory fattening in the White-crowned sparrow, Zonotrichia leucophrys gambelii. General and Comparative Endocrinology 4: 584-595.
Meier, A.H., Farner, D.S. & King, J.R. 1965. A possible endocrine basis for migratory behaviour in the White-crowned sparrow, Zonotrichia leucophrys gambelii. Animal Behaviour 13: 453-465.
Moore, F.L. & Miller, L.J. 1984. Stress-induced inhibition of sexual behaviour: corticosterone inhibits courtship behaviours of a male amphibian, Taricha granulosa. Hormones & behaviour 18: 400-410.
Moore, F.R. & Kerlinger, P. 1987. Stopover and fat deposition by North American wood-warblers (Parulinae) following spring migration over the Gulf of Mexico. Oecologia 74:47-54.
Moore, F.R. & McDonald, M.V. 1993. On the possibility that intercontinental landbird migrants copulate en route. Auk 110: 157-160.
Munck, A., Guyre, P.M. & Holbrook, N.J. 1984. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinology Review 5: 25-44.
O’Reilly, K.M. & Wingfield, J.C. 1995. Spring and autumn migration in Arctic shorebirds: same distance, different strategies. American Zoologist 35: 222-233.
Ornat, A.L. & Greenberg, R. 1988. Sexual segregation by habitat in migratory warblers in Quintana Roo, Mexico. Auk 107: 539-543.
Parrish, J.D. & Sherry, T.W. 1994. Sexual habitat segregation by American redstarts wintering in Jamaica: importance of resource seasonality. Auk 111:38-49.
Paolucci, M. Esposito, V. Di Fiore, V.M.M. & Botte, V. 1990. Effects of short postcapture confinement on plasma reproductive hormone and corticosterone profiles in Rana esculeta during the sexual cycle. Bollettino di Zoologia 57: 253-259.
Peczely, P. 1976. Etude circannuelle de la fonction corticosurrenalienne chex les especes de passereaux migrant et non migrants. General and Comparative Endocrinology 30: 1-11.
Petit, D.R., Petit, L.J. & Smith, K. G. 1997. Habitat associations of migratory birds overwintering in Belize, Central America. In: Hagan III, J.M. & Johnston, D.W. (Eds) Ecology and conservation of Neotropical migrant landbirds. Washington, D.C., Smithsonian Institution Press: 247-256
Quay, W.B. 1985. Cloacal sperm in spring migrants: occurrence and interpretation. Condor 87: 273-280.
Quay, W.B. 1986. Cloacal protuberance and cloacal sperm in passerine birds:comparative study of quantitative relations. Condor 88: 160-168.
Ramenofsky, M., Piersma, T. & Jukema, J. 1995. Plasma corticosterone in Bar-tailed Godwits at a major stop-over site during spring migration. Condor 97: 580-584.
Romero, L.M., Ramenofsky, M. & Wingfield, J.C. 1997. Season and migration alters the corticosterone response to capture and handling in an Arctic migrant, the White-crowned Sparrow, Zonotrichia leucophrys gambelii.. Comparative Biochemistry and Physiology Vol. 116C: 171-177.
Sapolsky, R. 1987. Stress, social status, and reproductive physiology in free-living baboons. In: Crews, D. (ed) Psychobiology of reproductive behaviour: an evolutionary perspective. Englewood Cliffs, New Jersey; Prentice Hall: 291-322.
Schwabl, H., Bairlein, F. & Gwinner, E. 1991. Basal and stress-induced corticosterone levels of Garden Warbler, Sylvia borin, during migration. Journal of Comparative Physiology B 161: 576-580.
Selye, H. 1971. Hormones and resistance. Springer, Berlin.
Sherry, T.W. & Holmes, R.T. 1996. Winter habitat quality, population limitation, and conservation of Neotropical-Nearctic migrant birds. Ecology 77: 36-48.
Sherry, T.W. & Holmes, R.T. 1997. American Redstart (Setophaga ruticilla). In: Birds of North America, No. 277. Poole, A. & Gill, F. (Eds) The Academy of Natural Sciences, Philadelphia, PA and The American Ornithologists' Union, Washington:1-32.
Siegel, H.S. 1980. Physiological stress in birds. BioScience 30:529-534.
Smith, G.T., Wingfield, J.C. & Veit, R.R. 1994. Adrenocortical response to stress in the Common Diving Petrel, Pelecanoides urinatrix. Physiological Zoology 67:526-537.
Totzke, U., Hubinger, A. & Bairlein, F. 1997. A role for pancreatic hormones in the regulation of autumnal fat deposition of the Garden Warbler, Sylvia borin. General & Comparative Endocrinology 107: 166-171.
Wingfield, J.C. 1984. Environmental and endocrine control of reproduction in the Song Sparrow, Melospiza melodia. I. Temporal organization of the breeding cycle. General & Comparative Endocrinology 56: 406-416.
Wingfield, J.C. 1988. Changes in reproductive function of free-living birds in direct response to environmental perturbations. In: Stetson, J.H. (ed) Processing of environmental information in vertebrates. Berlin; Springer-Verlag: 121-148.
Wingfield, J.C. 1994. Modulation of the adrenocortical response to stress in birds. In: Davey, K., Peter, R. & Tobe, S. (eds) Perspectives in comparative endocrinology. Ottawa; National Research Council of Canada: 520-528.
Wingfield, J.C. & Silverin, B. 1986. Effects of corticosterone on territorial behaviour of free-living male song sparrows, Melospiza melodia. Hormones & behaviour 20: 405-417.
Wingfield, J.C., Moore, M.C. & Farner, D.S. 1983. Endocrine responses to inclement weather in naturally breeding populations of White-crowned Sparrows, Zonotrichia leucophrys pugetensis. Auk 100: 56-62.
Wingfield, J.C., Vleck, C.M. & Moore, M.C. 1992. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran desert. Journal of Experimental Zoology 164: 419-428.
Wingfield, J.C., Deviche, P., Sharbough, S., Astheimer, L.B., Holberton, R.L., Suydam, R. & Hunt, K. 1994a. Seasonal changes in the adrenocortical responses to stress in redpolls, Acanthis flammea, in Alaska. Journal of Experimental Zoology 270: 372-380.
Wingfield, J.C., Suydam, R. & Hunt, K. 1994b. The adrenocortical response to stress in snow buntings, Plectrophenax nivalis, and Lapland Longspurs, Calcarius lapponicus, at Barrow, Alaska. Comparative Biochemistry & Physiology 108: 299-306.
Wingfield, J.C., O’Reilly, K.M. & Astheimer, L.B. 1995a. Modulation of the adrenocortical responses to acute stress in Arctic birds: a possible ecological basis. American Zoologist 35: 285-294.
Wingfield, J.C., Kubokawa, K., Ishida, K., Ishii, S. & Wada, M. 1995b. The adrenocortical response to stress in male bush warblers, Cettia diphone:, a comparison of breeding populations in Honshu and Hokkaido, Japan Zoological Science 12: 615-621.
Fig. 1. A diagram depicting the corticosterone stress profile produced by taking an initial (baseline) sample (at time 0 on the X-axis) followed by additional samples 30 and 60 min after capture. Such a profile can provide a ‘best estimate’ of the pre-disturbance plasma concentration of corticosterone, the maximal value expressed over a given period of time, and the rate of increase in corticosterone secretion in the plasma.

Fig. 2. A diagram depicting the plasma baseline levels of corticosterone in American Redstarts (Setophaga ruticilla) in male- and female-biased habitat types in autumn (October) and six months later in spring (late March to early April) on the non-breeding grounds in Jamaica, WI. (after Marra & Holberton 1998).
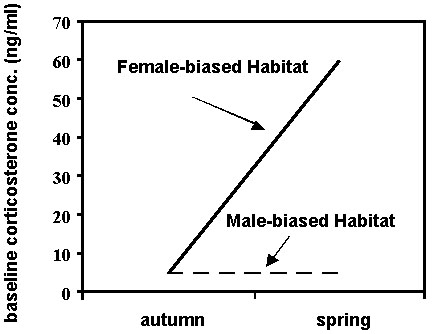
Fig. 3. A diagram depicting the stress response of corticosterone secretion at the time of capture (Time 0 = baseline) and 30 min after capture in American Redstarts (Setophaga ruticilla) in female- and male-biased habitat types in autumn (October) and six months later in spring (late March to early April) on the non-breeding grounds in Jamaica, WI. (after Marra & Holberton 1998).
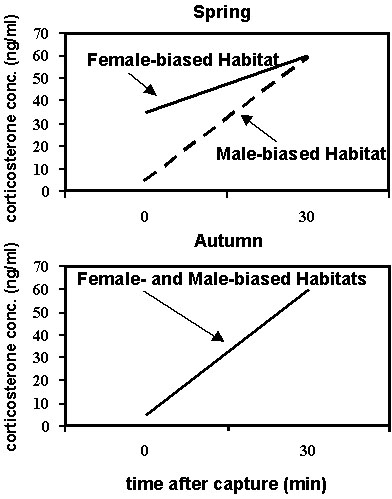
Fig. 4. Corticosterone profiles (baseline and 30 min, means only for clarity) of several groups of Neotropical migrants sampled in autumn on stopover in New England (solid squares: Catharus thrush spp., n = 6; open squares: Blackpoll warbler, Dendroica striata, n = 7; solid circles: Yellow-rumped warbler, D. coronata , n = 21; open circles: other Dendroica spp., n = 11) and Alabama, U.S.A. (open diamonds: White-eyed vireo, Vireo griseus, n = 9).
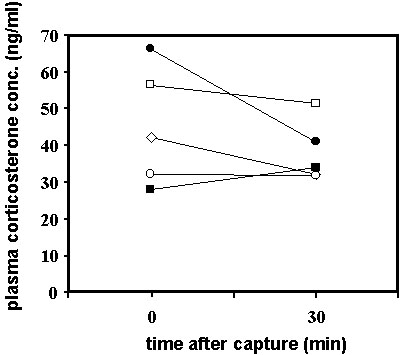
Fig. 5. Corticosterone profiles Yellow-rumped Warblers sampled during autumn migration at Manomet, Massachusetts (solid circles, n = 21) and at Block Island, Rhode Island, U.S.A. (diamonds, n = 12). The baseline corticosterone values of the Manomet warblers sampled after a storm were significantly higher than those of the Rhode Island birds sampled under optimal weather conditions. There was no difference between the two groups in the corticosterone concentrations 30 min after capture.
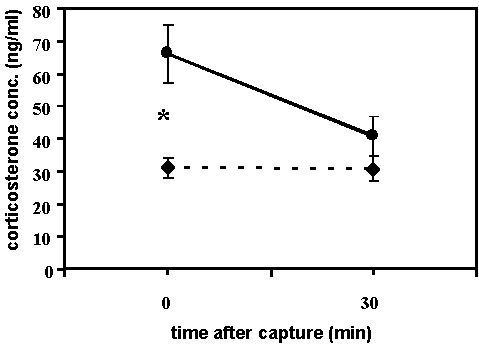
Fig. 6. Block Island warblers had a significantly higher index of energetic condition (body mass divided by wing chord3) than the Manomet birds that had experienced higher baseline corticosterone levels after the storm.
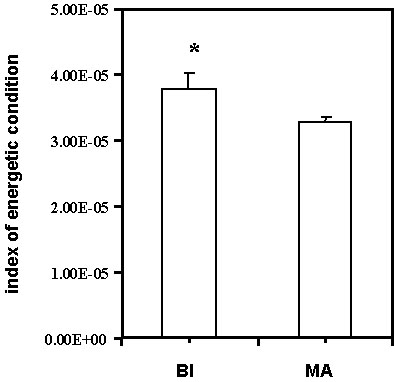
Fig. 7. Corticosterone stress profiles of Neotropical landbird migrants sampled in spring (Johnson’s Bayou, Louisiana, U.S.A., diamonds, n = 77) and in autumn (New England and Alabama, U.S.A., solid circles, n = 55). Spring birds had, on average, lower baseline corticosterone levels at the spring stopover site possibly indicating that some individuals may be able to decrease corticosterone during migration in preparation for breeding if conditions permit.
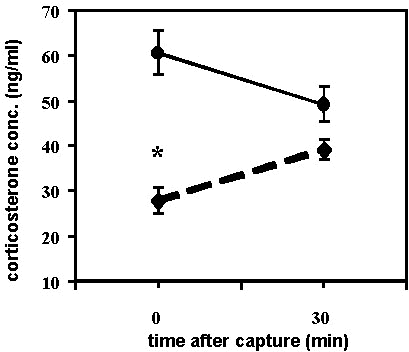
Fig. 8. The corticosterone stress profile of Kentucky (n = 4) and Hooded (n = 13) Warblers sampled on stopover in spring at Johnson’s Bayou, Louisiana, U.S.A. The profile (sexes pooled) shows baseline levels above that observed during the non-migratory season in most passerines as well as an absence of the stress response.
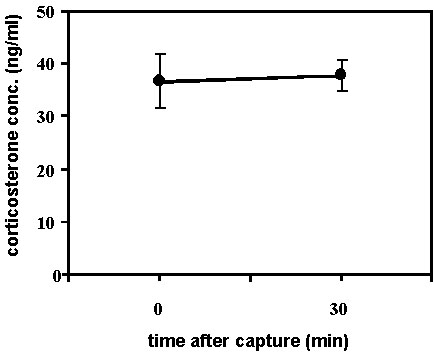
Fig. 9. The leaner birds among the group of Kentucky and Hooded Warblers (n = 17, sexes pooled) sampled in spring were more likely to have higher baseline corticosterone levels than those with greater body mass.
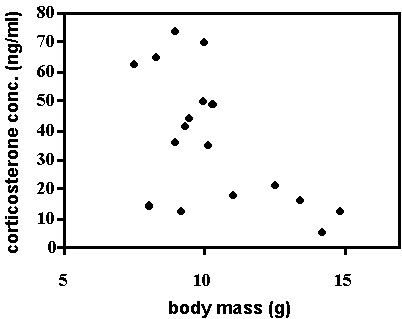
Fig. 10. Male Kentucky and Hooded Warblers (n = 8) sampled in spring showed a negative correlation between baseline corticosterone and the development of the cloacal protuberance, an indirect measure of gonadal development.
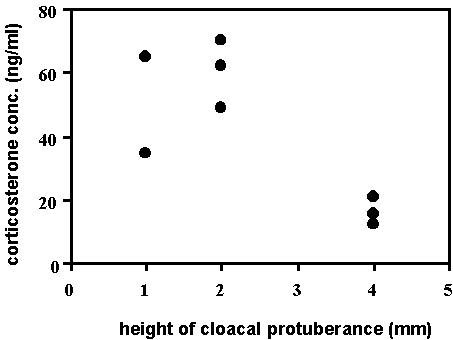
Fig. 11. An index of cloacal development (height of the cloacal protuberance divided by wing chord to control for body size) was negatively correlated with baseline corticosterone in male Wood Warblers (Parulinae spp., n = 33) sampled in spring on stopover in Johnson’s Bayou, Louisiana, U.S.A. This relationship suggests that elevated corticosterone levels may inhibit gonadal development during migration, a possible consequence of poor conditions encountered during migration.