S15.4: Energetic constraints and predation pressure during stopover
David A. Cimprich & Frank R. Moore
Department of Biological Sciences, University of Southern Mississippi, Southern Station Box 5018, Hattiesburg, Mississippi 39406-5018, USA, fax 091 601 266 5797, e-mail david.cimprich@usm.edu; e-mail Frank.Moore@usm.edu
Cimprich, D.A. & Moore, F.R. 1999. Energetic constraints and predation pressure during stopover. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 834-846. Johannesburg: BirdLife South Africa.During migratory stopover, birds must balance the conflicting demands of foraging and predator avoidance. How birds make this trade-off is likely affected by conditions imposed by migration, namely, highly variable predation risk, the necessity of large fat stores, elevated foraging demands, the need for fast travel, and information limitations. Changes in risk have been shown to alter both foraging location and intensity. Such changes may have consequences for rates of fat gain and stopover length, but current evidence is not conclusive. Results concerning the effects of fat are mixed. In one case, fat and lean birds differed in habitat use while in another, behaviour of these two groups did not differ following an encounter with a model hawk. Investigations on a variety of species using diverse habitats are needed to clarify the role of fat. The effects of elevated foraging demands and time limitations remain unexplored, though related work suggests that these factors decrease predator avoidance. Information limitations have likewise received little attention. However, preliminary work revealed no behavioural differences between adult and immature migrants, groups with different levels of experience and, hence, information. While it is clear that predation risk does affect the stopover behaviour of migrants, the nature and extent of these effects is not currently understood. Indeed, even the proximate cues migrants use to assess risk and how accurately they accomplish this task are not known. However, the available information suggests that the effects of predation risk deserve further consideration in studies of stopover.
INTRODUCTION
Conflict often arises between the need to satisfy nutritional demands and the need to avoid predation because foraging can increase an animal's exposure to predators (Lima 1986; McNamara & Houston 1987; Lima 1998). Consequently, there must be some trade-off between these opposing needs. Although a wide variety of animals face this difficulty, the problem is particularly complex for birds during migratory stopover. This complexity arises from the conditions imposed on birds by migration: (1) predation risk is variable and unpredictable during migration, (2) migrants often carry relatively large fat stores, (3) migrants experience elevated foraging demands, (4) there is pressure to travel quickly, and (5) lack of information concerning predation risks and foraging opportunities. The combination of these factors creates a complex and shifting environment within migrants must trade-off safety and foraging. To date, the behaviour of birds confronted by this situation has received little attention. Here we discuss these factors, predict their effects on the behaviour, and review relevant research.
VARIABLE PREDATION RISK
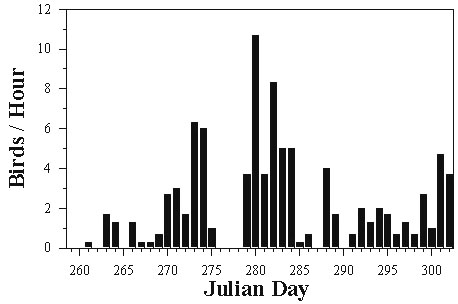
Among the primary hazards faced by migrants during stopover is the threat of predation by birds of prey (Rudebeck 1950-51; Walter 1979; Lindström 1989; Moore et al. 1990; Niles et al. 1996). Many depend to some extent upon migrating birds as a food source. An extreme example is Eleonora's Falcon Falco eleonorae which nests during fall and feeds its young on migrant birds (Walter 1979). More commonly, the migration of many predatory species coincides with that of their avian prey (Aborn 1994) and these hawks and falcons capture prey opportunistically as they travel (Allen & Peterson 1936; Rudebeck 1950-51; Hofslund 1973). Thus, risk for migrants during stopover should correlate positively with the number of migrant raptors present. This number and, likewise, the level of risk can vary greatly from day to day even during periods of peak migration (Allen et al. 1996) (Fig. 1). Because migrating hawks tend to concentrate in certain areas such as coastlines and ridges (Porter and Willis 1968, Heintzelman 1979, Evans and Lathbury 1973), spatial variation is layered over temporal variation (Fig. 2).
Alerstam and Lindström (1990) predicted that if predation is a major selective force during stopover, then birds should forage in habitats that minimise the ratio of predation rate (attack rate) to the rate of fat gain. The same idea can also be extended beyond habitat to make predictions of microhabitat use during foraging. If birds choose foraging locations based upon the ratio of attack rate to energy gain, then these ratios will change as predation risk varies and, therefore, migrants may shift location in response to changes in risk.
Lindström (1990) investigated the energy intake, habitat use, and predation risks for Bramblings Fringilla montifringilla during fall migration in Sweden. He concentrated on birds in two habitats, beech forest and rape Brassica napus fields. The attack rate by birds of prey on bramblings in the fields was higher than for those in forest. The birds also had a higher rate of food intake in the fields even in years when beech trees in the forest produced large nut crops. Using estimates of food intake in both habitats, Lindström determined that, during these beechnut years, the ratio of attack rate to energy intake was lower in the forest than the fields. As predicted, most bramblings foraged in the forest, shifting to the rape fields only in years when beechnuts were scarce or absent. These results seem to confirm predictions of Alerstam and Lindström (1990). However, information was only gathered on food intake, that is, energy input, rather than fat gain, a rate which integrates both energy input and output. It is unlikely that energy output was equal in the two habitats as Lindstrom noted that Bramblings in the forest spent a greater proportion of their time foraging than those in the rape fields. Without a comparison of fat gain in the two habitats, it is not possible to evaluate in a strict sense whether the birds behaved as predicted by the Alerstam and Lindström (1990) model.
The shifts in habitat use observed by Lindström took place from one year to the next. Migrants should show such shifts over much shorter time scales in response to changes in attack rate. These shifts would provide evidence that predation risk plays a role in habitat selection by migrants and that they have the ability to assess risk quickly, an ability assumed by the idea of habitat choice based on the ratio of attack rate to fat gain. Cimprich and Woodrey are currently conducting such an investigation using a combination of observation and experimentation. The observational component of this work consists first of counting the number of bird-eating hawks passing through the study site as an index of attack rate. While one observer counts hawks, another observes the foraging locations of migrant Blue-gray Gnatcatchers Polioptila caerulea. Preliminary data indicate that gnatcatchers forage deeper within scrub oak (Quercus geminata and Q. chapmanii) cover at times when hawk counts are large than when no hawks have been counted (Fig. 3). During the higher risk periods, the birds foraged in positions that are interior to most of the foliage of these evergreen bushes.
The experimental component of this work focuses on gnatcatcher foraging locations as well. In this case, locations before and after exposure to a model hawk are compared. Each gnatcatcher is observed for at least two minutes. Every 15 s during the first minute, we estimate the bird's depth within the vegetation. Next, we fly an Accipiter-shaped glider above and within 5 m of the focal bird. This glider is catapulted into flight using an elastic band, a technique requiring minimal observer movement. As the glider flies overhead, gnatcatchers typically ‘freeze,’ that is, become motionless. After the bird resumes movement, we again note its location every 15 seconds. In this way, we are able to compare the location before and after exposure to the model hawk. Preliminary results reveal that gnatcatchers foraged deeper within bushes following exposure to the model (Fig. 4).
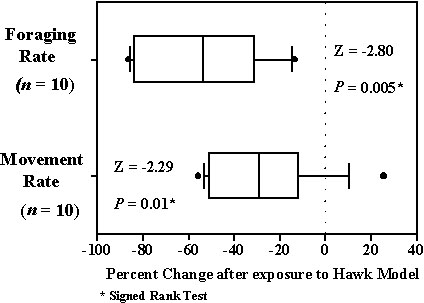
Weber et al. (1998) show that, if there is an increase in predation risk due to time invested in foraging as opposed to being vigilant, then the optimal strategy is to feed at lower intensity, become more vigilant, and extend stopover. Increases in vigilance should be reflected by decreases movement and foraging. Cimprich and Woodrey are applying the same observational and experimental approaches already described to determine if these changes take place in the behaviour of migrant Blue-gray Gnatcatchers as risk changes. Preliminary observational results show no differences in gnatcatcher movement rates between periods when no hawks were present and periods with relatively large hawk counts (median with no hawks present=28.6 moves min-1, n=17; median when >20 hawks h-1 counted=26.3 moves min-1; one-tailed Mann-Whitney U-test: Z=0.91, P=0.37). The same was true for foraging rates (median with no hawks present=5.71 foraging manoeuvres min-1, n=17; median when >20 hawks h-1 counted=4.44 manoeuvres min-1; one-tailed Mann-Whitney U-test: Z=0.74, P=0.47). However, trends toward lower values in both rates when hawk counts were high suggest that further study may reveal the predicted behavioural changes. Preliminary experimental results did show the predicted behavioural changes; gnatcatchers moved and foraged at slower rates after exposure to the flying model hawk (Fig 5).
When migrants change foraging location or become more vigilant as a result of increased predation risk, there is presumably some cost. A likely consequence of these behavioural changes would be lower rates of fat deposition or longer stopover as predicted by Weber et al. (1998). Fransson and Weber (1997) investigated whether predation risk could cause these effects. In this laboratory experiment, caged migrant Blackcaps Sylvia atricapilla were exposed to various objects moving overhead: a stuffed Sparrowhawk Accipiter nisus (high risk), a plastic bottle (disturbance), or nothing (low risk). The group exposed to the hawk had the greatest food intake during the first two days. Additionally, the higher level of nocturnal activity displayed by this group suggested that they would have the shortest stopover period. These results are counter to the predictions of Weber et al. (1998) and the results of Cimprich and Woodrey described previously. It is not clear how closely the behaviour of Blackcaps in this experiment reflect the behaviour of free-ranging migrants. The caged Blackcaps had no access to escape cover and the presentation of food in bowls allowed minimal search time and maximal vigilance. Free-ranging birds which must search for food and decide where to forage relative to escape cover face a situation with far more behavioural options. The authors point out that, under these conditions, the birds may have perceived changes in predation risk to be a change in the background level of mortality risk rather than a component of risk that can be affected by their behaviour (i.e. the component that these authors set out to manipulate). Perhaps these conditions account for the discrepancies between these results, the results of Cimprich and Woodrey, and the predictions of Weber et al. (1998).
Migrants could mitigate the consequences of predation risk on fat deposition and stopover length by joining foraging flocks during stopover. This could allow individuals to maintain safety without increasing vigilance (Pulliam 1973, Morse 1977, Elgar 1989). Whether migrants alter their participation flocks in response to changes in predation risk remains to be investigated.
LARGE FAT STORES
An animal's nutritional state can affect its predator avoidance behaviour such that individuals with greater motivation to forage take greater risks (McCleery 1978; Krebs 1980; Lima & Dill 1990). For example, in a study of Willow Tits Parus montanus in winter, Koivula et al. (1995) found that birds spent less time scanning and less time within protective cover when hungry than when satiated. Other food-deprived birds have been shown to extend foraging activities into habitats and times of day that are associated with greater predation risk (Lima 1988; Lima & Dill 1990). Fat could affect predator avoidance behaviour of migrants in the same ways. Migrants store fat to fuel long-distance flight and particularly large deposits are needed to cross ecological barriers (Barlein 1990; Lindström & Alerstam 1992; Lindström & Piersma 1993). However, the connection between fat stores and motivation to forage is weaker for migrants than it is for birds wintering at high latitudes where long nights, ice, or snow may prevent foraging. In the case of migrants, low fat stores often do not have the immediate and severe consequence of starvation. Thus, it is unclear whether fat will affect predator avoidance in migrants through its effect on motivation.
A second way that fat could affect the predator avoidance behaviour of migrants is by altering flight performance. It has been shown both theoretically and empirically that increases in mass such as occur when birds deposit fat decrease ability to manoeuvre, climb, and accelerate ( Hedenström 1992; Witter et al. 1994; Metcalfe & Ure 1995; Kullberg et al. 1996; Lee et al. 1996). In migrant Blackcaps, an increase in fat stores from 0% to 60% of lean body mass decreased angle of climb 32% and take-off velocity 17% (Kullberg et al. 1996). That fat impairs the ability of a bird to manoeuvre and possible to evade capture by an aerial predator provides incentive for migrants to act with increasing caution as they accumulate fat.
If fat migrants face higher predation risk relative to lean migrants, then ratios of mortality risk to fat gain of various foraging locations would also differ between these groups. Such differences may cause fat and lean migrants to segregate by foraging location. Aborn (1996) compared the habitat use of fat and lean Summer Tanagers Piranga rubra during stopover on a barrier island in spring migration. The two groups did differ. Lean birds spent more time in scrub/shrub areas while fat birds spent more time in pine forest. While Aborn did not measure predation risk in the two habitats, one of the radio-tracked birds was captured by a Cooper's Hawk Accipiter cooperii in the scrub/shrub habitat suggesting higher risk in that habitat.
Arthropod sampling revealed that potential prey for the tanagers was more abundant in the scrub/shrub area than the pine forest. However, it is truly difficult to compare prey abundance in the two habitats because insect sampling was restricted to the understory. Nonetheless, the results of this study do suggest that the fat birds occupied relatively safe but food-poor habitat while lean birds foraged in the relatively dangerous habitat where food was easier to obtain.
Ydenberg and Dill (1986) developed an economic model describing how close an animal should allow a predator to approach before fleeing. They viewed the optimal flight initiation distance as a trade-off between two costs, that of fleeing (lost feeding opportunities) and that of remaining (risk of capture). As distance from the predator decreases, the cost of fleeing decreases while the cost of remaining increases. The optimal flight distance is some intermediate distance where the two costs are equal. If fat affects risk of capture, then Ydenberg's model predicts that lean birds should allow predators to approach more closely before fleeing than fat birds. Consequently, fat migrants would experience more frequent interruptions while foraging because a greater number of predators would pass within the perimeter of the area defined by their flight initiation distance. Greater interruption could lead to lower rates of fat deposition. These predictions have not been tested, but seem tractable for investigation.
If migrants become less able to escape predation as they deposit fat, then one way to maintain safety would be to become more vigilant and thereby decrease foraging intensity. Cimprich is currently testing whether migrants employ this strategy using migrant Gray Catbirds Dumetella carolinensis. These birds ‘freeze,’ that is become motionless, after detecting a flying hawk. The amount of time that they freeze can be taken as a measure of vigilance or caution with respect to predators. Catbirds with known fat stores are released into a small patch of cover constructed from cut branches. A model hawk is then flown overhead along a cable and the amount of time that each bird remains motionless is recorded. Preliminary data show no differences between fat and lean birds (one-tailed Mann-Whitney U-test: Z=0.51, n=18 fat and 18 lean birds, P=0.31). It is possible that this result may stem from the foraging and escape tactics of catbirds. Dense shrubs provide both the foraging location and escape cover for this species (Lima 1993). If escape involves little flight, then the behaviour of catbirds may be little affected by the changes in flight performance that accompany fat deposition. Behavioural effects of fat may be apparent in species that typically make long flights to escape approaching raptors.
A second factor could contribute to the apparent lack of differences in predator avoidance between fat and lean birds. Every migrant may be equally motivated to forage until their fat stores reach some ‘set point’ or optimal fat load (see Lindström & Alerstam 1992 for a discussion of optimal fat loads). If this level is high (as it might be before birds cross some large ecological barrier) then differences in predator avoidance among birds differing in fat stores would not be apparent until some reached that set point. Much more investigation is needed to determine whether predator avoidance behaviour of migrants increases smoothly as fat stores increase or remain constant only increasing after a certain quantity of fat is stored.
If predation risk did cause fat migrants to forage in safer, but less profitable locations or with decreased intensity, then these birds should exhibit lower rates of mass gain than lean migrants as a consequence. There are other reasons why fat migrants might have lower rates of gain such as physiological or flight mechanical constraints or because some fixed quantity of fat optimises migration speed (Lindström & Alerstam 1992). Regardless of whether such mechanisms are operating, differences in fat deposition rates caused by predation risk would still be detectable between migrants with little or no fat and those with intermediate amounts.
ELEVATED FORAGING DEMANDS AND TIME LIMITATIONS
These two factors could have similar effects. Available evidence supports the idea that the need to complete migration quickly plays an important role in shaping migration (Lindström 1995). Quick completion of the journey relative to conspecifics allows an individual to select from among the highest quality territories and mates (Lindström & Alerstam 1992). If migrants are selected to maximise migration speed, then individuals that maximise fat deposition rates will be favoured (Alerstam & Lindström 1990). Such fat deposition rates during stopover could only be achieved through intense foraging effort. However, this would not be true whenever migrants could quickly gather more food than their gut could process. Recent evidence that digestive tract size may decrease following and even prior to long migratory flights (e.g. Hume & Biebach 1996; Piersma & Gill 1998) raises the possibility that this situation may occur during migration.
Ydenberg and Dill's (1986) economic model predicts that both the foraging demands and time limitations faced by migrants should affect optimal flight initiation distances. Both factors increase the cost of lost feeding opportunities (i.e. the cost of fleeing). If the risk of capture remains constant and the cost of fleeing increases, then optimal flight initiation distance will decrease, that is, migrants should allow predators to approach more closely than nonmigrants. Among migrants, those subject to greater foraging demands or time limitations will allow closer approach by predators.
While these predictions have not been directly addressed, relevant work exists. Decreases in flight initiation distance represent a shift in the foraging/safety trade-off toward foraging and away from predator avoidance. Moore (1994) examined a different aspect of this trade-off by testing whether Yellow-rumped Warblers Dendroica coronata in migratory disposition resume foraging sooner after exposure to a model predator than those not in migratory disposition. As predicted, the birds in a migratory state resumed foraging sooner. Similarly, Metcalfe and Furness (1984) observed that adult Ruddy Turnstones Arenaria interpres were more vigilant during the nonbreeding season than during a period of pre-migratory fattening. These results of these two experiments suggest that birds experience an elevated risk of predation during migration as a result of their behaviour.
INFORMATION LIMITATIONS
Upon arrival at a stopover site, migrants can have little information concerning foraging opportunities and predation risks. After arrival, monitoring and exploration may be necessary to maintain current information. During fall migration, young birds making their first migration would particularly lack information. They would encounter habitats and predators new to their experience. It is unlikely that adults of many landbird species visit the same stopover sites from year to year, but they could at least rely on past experience for some relevant information.
Numerous predictions can be made for the effects of information limitations. Several of these concern changes in behaviour over the course of stopover at a single site. Migrants would acquire increasingly more accurate information the longer they remained at a single site. Thus, over the course of stopover behavioural changes would be predicted that would reflect increasing information gathered by an individual. If relatively older, experienced migrants were better able to assess predation risk and foraging opportunities than younger, inexperienced birds, then differences between age groups would also be predicted. Such differences should be most pronounced during fall migration when differences in experience between these two age groups would be greatest.
Information deficiencies then could result in changes in behaviour over the course of a single stopover as well as differences better adult and immature migrants. Such changes and differences could manifest themselves in many ways including foraging location, flight initiation distance, and foraging intensity. However, as already discussed, fat deposition during stopover could also have effects on these same behaviours. Thus, effects of information alone would be difficult to detect without a carefully-designed study. Behavioural differences between age groups, particularly in foraging location, could result from age-specific social dominance. Again, the effects of information alone would be difficult to detect because socially dominant adults may affect the foraging locations of subordinate immatures during stopover (e.g. Woodrey 1995).
The only work addressing the effects of information limitations on predator avoidance during stopover is currently being conducted by Cimprich. Using the same protocol already described for determining the effect of fat on predator avoidance, the objective of this investigation is to determine whether differences exist between adult and immature catbirds during fall migration. Catbirds are released singly into a small patch of cover. A model hawk is flown overhead after release and the amount of time that the birds remains immobile is recorded. Preliminary data reveal no difference between adults and immatures (Mann-Whitney U-test: Z=1.70, n=18 adult and 18 immature birds, P=0.09).
Stopover, in many cases, is brief. For example, during both spring and fall migration along the northern coast of the Gulf of Mexico, most passerines stopover for less than one day (e.g. Moore & Kerlinger 1987; Woodrey & Moore 1997). Such short stopovers may not allow birds to gain enough information during stopover to have an impact on behaviour. Birds stopping for brief periods may use simple rules of thumb for predator avoidance rather than relying upon their ability to assess changing predation risk. However, the observation that Blue-gray Gnatcatchers change foraging location and possibly foraging intensity in response to changes in the local abundance of migrating hawks provides evidence against this. Alternatively, migrants could take advantage of information possessed by other individuals by foraging in a group. For example, migrants might forage in the company of residents or immatures might prefer to forage with an adult.
CONCLUSIONS
Information on how predation risk influences the stopover behaviour of migrants is currently sparse. However, it is already clear that safety from predation does affect behaviour in at least some ways. Increasing risk can cause migrants to alter foraging location and intensity. Thus, antipredator behaviour during remains flexible during migration, but further study is necessary to clarify the extent and nature of this flexibility and its consequences on social behaviour, time budgets, fat deposition, stopover length, and, ultimately, on migration speed.
Current evidence does not indicate any effects of fat or age on predator avoidance behaviour during stopover. However, this preliminary evidence is far from conclusive. This topic warrants further investigation. A particular focus of any further investigation into the effects of fat should be to compare a variety of species which forage in different ecological settings. Because escape tactics vary according to setting, effects may be apparent only in certain habitats. Investigations on the effects of age should focus on fall migration when differences in experience between adult and immature birds is likely to be the greatest.
That migrants respond to changing risk implies some mechanism for assessing risk. How this potentially complex task is accomplished and to what degree of accuracy is unknown. Birds have innate abilities to recognise a variety of predators (Curio 1993), but assessing risk requires more than mere predator recognition. The threat posed by a predator must be placed in an environmental context: the distance to the predator, the behaviour of the predator, and the availability and location of escape cover, the behaviour of other potential prey. An ability to accurately monitor predation risk would allow birds to continually adjust their behaviour to maintain the highest possible fat deposition rate without sacrificing safety. However, the degree to which it is necessary to obtain accurate information regarding predation risk during stopover is not known. Theoretical models reveal that accurate information is not always needed to maintain low mortality risk (Bouskila & Blumstein 1992). Often simple ‘rules of thumb’ suffice. The short duration and shifting risks associated with stopover would seem to favour such rules over the acquisition of more accurate information. If such rules minimise the cost of assessing risk, then they will overestimate risk magnifying the response of migrants to predators (Bouskila & Blumstein 1992).
The extent to which migrants rely on accurate information regarding predation risk should also depend on the actual level of risk. If risk is low, then selection for the gathering of accurate information would also be low (Bouskila & Blumstein 1992). The opposite is true if risk is high. If an individual can expect to pass through at least some areas of high risk during its migration, then this could be sufficient to favour accurate information. A strategy in which migrants set behaviour according to the average risk over migration is unlikely. In simulations, such averaging strategies performed very poorly (Bouskila & Blumstein 1992). The limited information currently available suggests that mortality during migration can be high (e.g. Lindström 1989), a condition favouring accurate information. However, further studies of the predation risks faced by migrants are needed to clarify this issue.
Research on predation during stopover is already demonstrating that risk affects the behaviour of migrants. However, the exact nature and extent of these effects remains uncertain. Much research is needed to clarify how safety interacts with time minimisation as a selective force during migration. Already, it is clear that the potential impact of predation should be considered in future studies of the behaviour of migrants during stopover.
REFERENCES
Aborn, D.A. 1994. Correlation between raptor and songbird numbers at a migratory stopover site. Wilson Bulletin 106: 150-154.
Aborn, D.A. 1996. Habitat selection, movement, and activity budgets of neotropical landbird migrants following trans-gulf migration. PhD Thesis, University of Southern Mississippi, Hattiesburg, U.S.A.
Alerstam, T. & Lindstöm, Å. 1990. Optimal bird migration: the relative importance of time, energy, and safety. In: Gwinner, E.(ed) Bird Migration; Springer-Verlag; Berlin: 331-351.
Allen, P.E., Goodrich, L.J. & Bildstein, K.L. 1996. Within- and among-year effects of cold fronts on migrating raptors at Hawk Mountain, Pennsylvania, 1934-1991. Auk 113: 329-338.
Allen, R.P. & Peterson, R.T. 1936. The hawk migration at Cape May Point, New Jersey. Auk 53: 393-404.
Barlein, F. 1990. Nutrition and food selection in migratory birds. In: Gwinner, E.(ed) Bird Migration; Springer-Verlag; Berlin: 198-213.
Bouskila, A. & Blumstein, D.T. 1992. Rules of thumb for predation hazard assessment: predictions from a dynamic model. American Naturalist 139: 161-176.
Curio, E. 1993. Proximate and developmental aspects of antipredator behaviour. Advances in the Study of Animal Behaviour 22: 135-238.
Elgar, M.A. 1989. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biological Review 64: 13-33.
Evans, P.R. & Lathbury, G.W. 1973. Raptor migration across the Straits of Gibraltar. Ibis 115: 572-585.
Fransson, T. & Weber, T.P. 1997. Migratory fuelling in blackcaps (Sylvia atricapilla) under perceived risk of predation. Behavioural Ecology and Sociobiology 41: 75-80.
Hedenström, A. 1992. Flight performance in relation to fuel load in birds. Journal of Theoretical Biology 158: 535-537.
Heintzelman, D.S. 1979. A guide to hawk watching in North America. University Park; Pennsylvania State University Press: 284pp.
Hofslund, P.B. 1973. Do hawks feed during migration? Raptor Research 7: 13-14.
Hume I.D. & Biebach, H. 1996. Digestive tract function in the long-distance migratory garden warbler (Sylvia borin). Journal of Comparative Physiology 166: 388-395.
Koivula, K., Rytkönen, S. & Orell, M. 1995. Hunger-dependency of hiding behaviour after a predator attack in dominant and subordinate willow tits. Ardea 83: 397-404.
Krebs, J.R. 1980. Optimal foraging, predation risk and territory defence. Ardea 68: 83-90.
Kullberg, C., Fransson, T. & Jakobsson, S. 1996. Impaired predator evasion in fat blackcaps (Sylvia atricapilla). Proceedings of the Royal Society of London, Series B Biological Science 263: 1671-1675.
Lee, S.J., Witter, M.S., Cuthill, I.C. & Goldsmith, A.R. 1996. Reduction in escape performance as a cost of reproduction in gravid starlings (Sturnus vulgaris). Proceedings of the Royal Society of London, Series B Biological Science 263: 619-623.
Lima, S.L. 1986. Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecology 67: 377-385.
Lima, S.L. 1988. Initiation and termination of daily feeding in dark-eyed juncos: influences of predation risk and energy reserves. Oikos 53: 3-11.
Lima, S.L. 1993. Ecological and evolutionary perspectives on escape from predatory attack: a survey of North American birds. Wilson Bulletin 105: 147.
Lima, S.L. 1998. Stress and decision making under the risk of predation: recent developments from behavioural, reproductive, and ecological perspectives. Advances in the study of Behaviour 27: 215-290.
Lima, S.L. & Dill, L.M. 1990. Behavioural decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology 68: 619-640.
Lindström, Å. 1989. Finch flock size and risk of hawk predation at a migratory stopover site. Auk 106: 225-232.
Lindström, Å. 1990. The role of predation risk in stopover habitat selection in migrating bramblings. Behavioural Ecology 1: 102-106.
Lindström, Å. 1995. Stopover ecology of migrating birds: some unsolved questions. Israel Journal of Zoology 41: 1-17.
Lindström, Å. & Alerstam, T. 1992. Optimal fat load in migrating birds: a test of the time minimization hypothesis. American Naturalist 140: 477-491.
Lindström, Å. & Piersma, T. 1993. Mass changes in migrating birds: the evidence for fat and protein storage re-examined. Ibis 135: 70-78.
McCleery, R.H. 1978. Optimal behaviour sequences and decision making. In: Krebs, J.R. & Davies, N.B.(eds) Behavioural Ecology. An Evolutionary Approach; Oxford; Blackwell Scientific Publications: 91-121.
McNamara, J.M. & Houston, A.I. 1987. Starvation and predation as factors limiting population size. Ecology 68: 1515-1519.
Metcalfe, N.B. & Furness, R.W. 1984. Changing priorities: the effect of pre-migratory fattening on the trade-off between foraging and vigilance. Behavioural Ecology and Sociobiology 15: 203-206.
Metcalfe, N.B. & Ure, S.E. 1995. Diurnal variation in flight performance and hence potential predation risk in small birds. Proceedings of the Royal Society of London, Series B Biological Science 261: 395-400.
Moore, F.R. 1994. Resumption of feeding under risk of predation: effect of migratory condition. Animal Behaviour 48: 975-977.
Moore, F.R. & Kerlinger, P. 1987. Stopover and fat deposition by North American wood-warblers (Parulinae) following spring migration over the Gulf of Mexico. Oecologia 74: 47-54.
Moore, F.R., Kerlinger, P. & Simons, T.R. 1990. Stopover on a gulf coast barrier island by spring trans-gulf migrants. Wilson Bulletin 102: 487-500.
Morse, D.H. 1977. Feeding Behaviour and Predator avoidance in Heterospecific groups. BioScience 27: 332-339.
Niles, L.J., Burger, J. & Clark, K.E. 1996. The influence of weather, geography, and habitat on migrating raptors on Cape May peninsula. Condor 98: 382-394.
Piersma, T. & Gill, R.E. 1998. Guts don't fly: small digestive organs in obese bar-tailed godwits. Auk 115: 196-203.
Porter, R. & Willis, I. 1968. The autumn migration of soaring birds at the Bosphorus. Ibis 110: 520-536.
Pulliam, H.R. 1973. On the advantages of flocking. Journal of Theoretical Biology 38: 419-422.
Rudebeck, G. 1950-51. The choice of prey and modes of hunting of predatory birds with special reference to their selective effect. Oikos 2: 65-88, 3: 200-231.
Walter, H. 1979. Eleonora's Falcon. Chicago; University of Chicago Press: 410pp.
Weber, T.P., Ens, B.J. & Houston, A.I. 1998 Optimal avian migration: a dynamic model of nutrient stores and site use. Evolutionary Ecology 12: 377-401.
Witter, M.S., Cuthill, I.C. & Bonser R.H.C. 1994. Experimental investigations of mass-dependent predation risk in the European starling, Sturnus vulgaris. Animal Behaviour 48: 201-222.
Woodrey, M.S. 1995. Stopover behaviour and age-specific ecology of neotropical passerine migrant landbirds during autumn along the northern coast of the Gulf of Mexico. PhD Thesis, University of Southern Mississippi, Hattiesburg, U.S.A.
Woodrey, M.S. & Moore, F.R. 1997. Age-related differences in the stopover of fall landbird migrants on the coast of Alabama. Auk 114: 695-707.
Ydenberg, R.C. & Dill, L.M. 1986. The economics of fleeing from predators. Advances in the study of behaviour 16: 229-249.
Fig. 1. Daily variation in the number of Sharp-shinned Hawks Accipiter striatus counted at Fort Morgan, Alabama, U.S.A. during fall migration, 1996. Numbers are based on 6 hours of counting beginning at local sunrise.

Fig. 2. Spatial variation in number of Sharp-shinned Hawks counted in the eastern U.S.A. on 9 October, 1996. Data from Hawk Migration Association of North America, Hawk Migration Studies vol. 23(1).
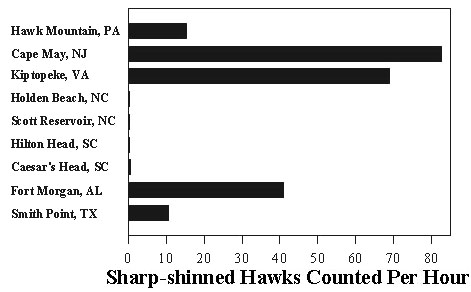
Fig. 3. Comparison of Blue-gray Gnatcatcher foraging locations within scrub oak bushes at low and high risk times. Level of risk based on hourly counts of bird-eating hawks passing through the study site at altitude of <40 m: low risk=0 hawks counted hr-1, high risk>20 hawks counted hr-1. Line in box indicates median. Box encloses 25th to 75th percentiles while whiskers enclose 10th to 90th percentiles. Dots indicate outliers.
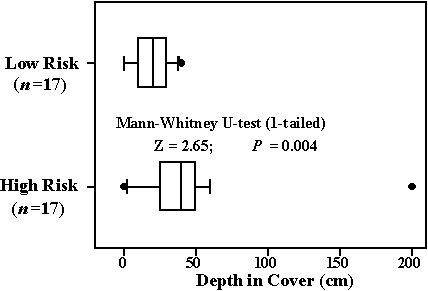
Fig. 4. Change in foraging locations of Blue-gray Gnatcatchers within scrub oak bushes after exposure to flying model hawk. Positive values indicate that birds moved deeper into the bushes. Line in box indicates median. Box encloses 25th to 75th percentiles while whiskers enclose 10th to 90th percentiles. Dots indicate outliers.
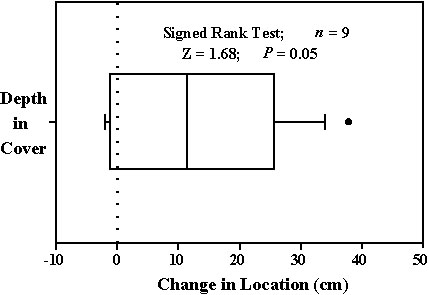
Fig. 5. Changes in foraging rate (foraging manoeuvres min-1) and movement rate (moves min-1) of Blue-gray Gnatcatchers after exposure to flying model hawk. Negative values indicate that rates decreased. Line in box indicates median. Box encloses 25th to 75th percentiles while whiskers enclose 10th to 90th percentiles. Dots indicate outliers.