S14.4: A neural system proposed to regulate the annual cycle of migratory birds
Wayne J. Kuenzel
Department of Animal and Avian Sciences University of Maryland College Park, MD 20742, USA, fax 001 301 314 9059, e-mail kuenzel@wam.umd.edu
Kuenzel, W.J. 1999. A neural system proposed to regulate the annual cycle of migratory birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 784-792. Johannesburg: BirdLife South Africa.Migratory birds which breed in the northern hemisphere and migrate south prior to the onset of persistent cold weather, show dramatic shifts in body weight dependent upon the season and energetic demands of the annual cycle. Due to the rigorous requirements to migrate, reproduce and return to the wintering grounds under stringent time constraints, birds display poikilostasis: dynamic shifts in homeostasis. Each phase of annual cycle represents an homeostatic plane which is defended, however, migratory birds can shift to another plane, dependent upon the particular phase of the annual cycle. The autonomic nervous system (ANS) comprises two antagonistic components: sympathetic and parasympathetic. The regulation of the ANS and its balance are controlled by the brain, specifically the visceral forebrain system (VSF). The VSF is proposed to function to shift the balance of ANS either towards a dominance of the sympathetic (SNS) or parasympathetic nervous system (PNS), dependent upon the behavioural and/or physiological demands placed upon a bird during a particular season. The VFS receives information from key sensory systems and projects to the central gray and nucleus tractus solitarius which are major recipient brain sites of the SNS and PSN, respectively.
INTRODUCTION
The annual cycle of migratory birds comprises several distinct behavioural and physiological (ethophysiological) events. Examples of some events include the development of obesity prior to spring and fall migration, pre-nuptial and post-nuptial moults, gonadal development and regression, territoriality, courtship and mating behaviour, incubation behaviour and care of the young. An example of some of the components of the annual cycle of birds that can be measured readily in a small, captive population of migratory birds held in an outside aviary is shown in Fig. 1 (Kuenzel & Helms 1974). Note that when one examines the annual cycle of body weight in a White-throated Sparrow, Zonotrichia albicollis, it is quite clear that there is no body weight level that is characteristic of this avian species. In fact, there are at least three peaks of body weight (spring migration, fall migration, and winter) and two periods of low body weight (pre-nuptial or breeding moult and post-nuptial or basic moult). Body weight prior to spring migration is approximately 20% higher than body weight during post-nuptial moult. This drastic change in body weight throughout the year is typical of migratory as well as other species of birds and suggests that a single setpoint of body weight and fat level about which body mass is regulated does not exist.
A second factor that is obvious from many field studies of migratory birds, as well as other living organisms, is that the major, ultimate factor driving many of the ethophysiological events that comprise an annual cycle, is reproductive success for the next generation. When one examines the vernal portion of the annual cycle of a bird such as the White-throated Sparrow, it is apparent that time is a severely limiting factor. The purpose of this paper is to introduce a physiological hypothesis involving the autonomic nervous system (ANS) that suggests how birds can achieve maximum fitness during the course of their annual cycle. In addition, a specific neural system within the brain is proposed that regulates the tone or balance of the ANS to optimise a bird
’s chances for breeding and raising young successfully each year.HYPOTHESIS: POIKILOSTASIS (SHIFTS IN HOMEOSTASIS) IS DISPLAYED BY MIGRATORY BIRDS.
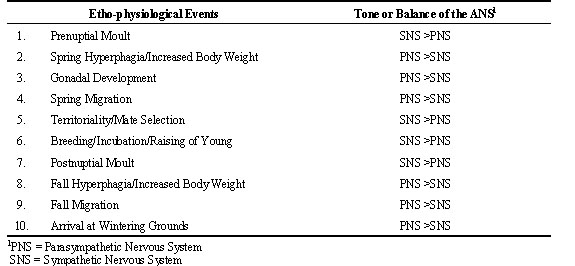
It is proposed that due to the constraints of a short breeding season, migratory birds show poikilostasis (poikilo = various; stasis = states of equilibrium or steady states). A neural system is hypothesised to effect poikilostasis by regulating the balance of the autonomic nervous system (ANS). Importantly, such deviations from homeostasis are required responses by migratory birds in order to increase their chances for survival and procreation. Table 1 lists ten typical events that occur in many species of north temperate zone, migratory birds between spring and fall seasons. The column on the right shows the tone or balance of the ANS. If the balance between the sympathetic nervous system (SNS) and parasympathetic nervous system (PSN) remained constant, as would be predicted by the well-known principle of homeostasis articulated by the French physiologist, Claude Bernard, it would be nearly impossible for avian species to complete their annual cycles in a timely manner. The statement is supported by considering the need to complete the ten listed ethophysiological events in a restricted time frame. The first listed event is prenuptial moult, a rapid moult involving feathers about the head and neck region. It is an important moult, particularly in males, for successful mate selection and breeding. Orchestrated catabolism is necessary to generate the required energy to initiate the process of co-ordinated protein synthesis to replace the hundreds of feathers in these body regions. An activation of the SNS would greatly facilitate this process and table 1 suggests that the tone of the SNS becomes temporarily greater than the PNS to insure that this process is completed during a short two-to-four week period of time. The second and third ethophysiological events listed, involving spring hyperphagia, increased body weight, and gonadal development involve different metabolic processes from the previous one for feather growth and replacement. Efficient conversion of food items consumed into body reserves and rapid lipid storage is essential in order to have sufficient calories for the demands of the migratory flight to the breeding grounds and gonadal development. If homeostasis for a single set point of body weight persisted throughout the year, birds would be unable to accumulate the needed body reserves for a successful migratory journey. Clearly, a dynamic shift in the balance of the ANS is required. For efficient anabolic processes to occur, it is suggested that the PNS dominates during this phase of the annual cycle. The result is a positive energy balance manifested by a rapid gain in body weight, fat reserves and initiation of gonadal development (Fig. 1). The PNS is hypothesised to remain dominant throughout spring migration as birds continue to feed during stopovers enroute to their final destination and have been shown to arrive on their breeding grounds with high levels of fat reserves (Fransson & Jakobsson 1998). In effect, bird migration can be compared to marathon races or long-distance runs undertaken by conditioned humans who typically display low cardiovascular rate and increased efficiency of energy utilization characteristic of a dominance of the PNS. Indirect support of the above model has been obtained in a study where the levels of corticosterone (the stress hormone) in response to acute stress in premigratory versus migratory Catbirds Dumetella carolinensis and Yellow-rumped Warblers Dendroica coronata were determined (Holberton et al. 1996). Migratory birds showed a suppressed elevation of corticosterone to acute stress compared to birds that had not come into migratory condition. The previous results would be expected if the PNS clearly dominated over the SNS during the migratory period of the annual cycle.
Anabolism displayed by birds, in preparation for and during spring migration is proposed to shift gradually to a negative energy balance as birds enter the critical breeding phase of their annual cycle. A main reason is that the gregarious nature seen by avian species during migration is replaced by aggressive behaviour as birds establish territories and males, in particular, expend considerable energy toward securing a mate. A dynamic shift toward dominance of the SNS is perhaps essential for birds to negotiate this life cycle stage which also includes feeding and caring for young.
The greatest expression of the activity of the SNS occurs during post-nuptial moult where body weight and fat deposits are at a nadir. Body reserves are catabolized and directed toward protein synthesis in order to replace body and flight feathers involved in this major moult. It is another critical phase of the life cycle when birds become photorefractory to the remaining long days of summer and gonads atrophy. Catabolism is prominent as the metabolic cost of moulting is high and it appears to be a critical organisational period for birds (Wilson 1997).
Upon completion of post-nuptial moult, as suggested in table 1, birds again display a shift toward a dominance of the PNS and increased food intake and fat deposition occur in preparation for fall migration. As can be seen in Fig. 1, the gain in body weight and fat deposition is not as great for fall migration compared to spring migration in some migratory species. From late fall through the winter period, north-temperate zone birds continue to maintain high body weight and fat reserves due to the long, cold winter nights as well as the uncertainty of food availability due to inclement weather conditions. Following winter, the annual cycle as described above repeats itself.
THE VISCERAL FOREBRAIN SYSTEM: A NEURAL SYSTEM PROPOSED TO ORCHESTRATE POIKILOSTASIS AND REGULATE THE ANNUAL CYCLE OF MIGRATORY BIRDS
The visceral forebrain system (VFS) is a neural system that has been proposed to exist in birds and perhaps regulate their annual cycles (Kuenzel and Blähser 1993; 1994; Kuenzel et al. 1998). The following is a definition of the VFS, its function in mammals, neural components described for mammals and proposed equivalent neural structures in birds.
VFS: Definition and Function
Mammals possess a VFS, a neural system that spans the anterior portion of the forebrain to the caudal brainstem. It is proposed to function to influence cardiovascular, respiratory and gastrointestinal functions including the possibility of interfering with or overriding brainstem homeostatic mechanisms during periods of stress or emotional activity (van der Kooy et al. 1984).
VFS: Mammalian Components
Eight neural areas within the brain are included in the VFS (van der Kooy et al. 1984) and located in the following general brain regions:
cerebral cortex: medial, lateral prefrontal cortex;
sub-cortical region: central nucleus of the amygdala;
septal area: bed nucleus (n.) of the stria terminalis;
hypothalamus: paraventricular n.; posterolateral hypothalamic n.; arcuate n;
pons: parabrachial n.;
medulla oblongata (brainstem): n. tractus solitarius.
An important criterion for structures to be considered components of the VFS is that each one should have direct (monosynaptic) connections either to the n. tractus solitarius or the parabrachial n.
VFS: Avian Equivalent Components
The following components have been proposed to be avian equivalents (Kuenzel & Blähser 1993; 1994).
pallium: caudal, dorsolateral neostriatum (controversial);
sub-pallial region: dorsolateral n., posterior archistriatum (controversial);
septal area: lateral septal organ including parts of the bed n. stria terminalis and the n. accumbens;
hypothalamus: paraventricular n.; lateral hypothalamic area; infundibular and inferior hypothalamic n.;
pons: parabrachial n.;
medulla oblongata: n. tractus solitarius.
It should be noted that the first two avian neural structures listed above (the caudal, dorsolateral neostriatum and dorsolateral n. of the posterior archistriatum) are considered controversial regarding whether or not they are homologous to the medial lateral prefrontal cortex and central n. of the amygdala of mammals, respectively. The following references contain information related to the caudal, dorsolateral neostriatum (Mogensen & Divac 1982; Divac & Mogensen 1985; Divac et al. 1985; Rehkämper & Zilles 1991; Waldmann & Güntürkün 1993; Wild et al. 1990) and avian archistriatum (Zeier & Karten 1971). The major criticism pertaining to the caudal, dorsolateral neostriatum is that to date there is no evidence of a monosynaptic connection between it and either the n. tractus solitarius or the parabrachial n. (Wild et al. 1990). Similarly, although there is agreement that a portion of the n. taeniae/archistriatal complex and juxtapositioned areas contain neural elements equivalent to the mammalian amygdala, the precise neural areas and their monosyaptic connections to the n. tractus solitarius and parabrachial n. have not been completely determined. Nonetheless, it is clear that the major, basic, sub-pallial components of an avian VFS exist and the system is anatomically comparable to the proposed mammalian VFS.
EVIDENCE SUPPORTING AN AVIAN VFS AND ITS ROLE IN REGULATING THE ANNUAL CYCLE OF MIGRATORY BIRDS
The following evidence supports the concept of a VFS regulating the annual cycle of migratory birds.
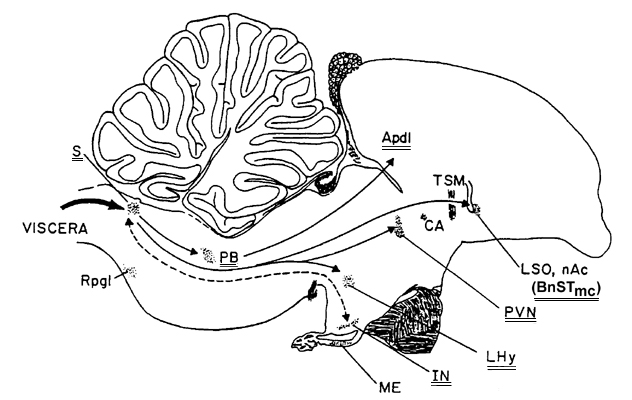
A major reason for supporting a neural system like the VFS for regulating the annual cycle of migratory birds is that a key neural structure within this system is the n. tractus solitarius (nTS). The nTS forms the 'backbone' of this neural pathway as it serves as an interface between the major portion of the ANS that is found throughout the body of a bird (hence, the ANS is considered part of the peripheral nervous system) and the brain (one of two parts of the central nervous system). As shown in Fig. 2, the nTS or S resides in the caudal brainstem or medulla oblongata. All of the proposed neural sites of the mammalian VFS have mono-synaptic projections to the nTS while five of the eight components of the avian VFS have single, neural connections to the nTS (Kuenzel & Blähser 1993).
A second, structural and functional reason to support the VFS for regulating the avian annual cycle is that vasoactive intestinal polypeptide (VIP) has been found in all components of the proposed avian VFS (Kuenzel & Blähser 1993; 1994; Kuenzel et al. 1997). Vasoactive intestinal polypeptide has also been shown to be associated or co-localised with neurons of the parasympathetic nervous system (Eckenstein & Baughman 1984; Luine et al. 1984) and functions as a vasodilator, consistent with parasympathetic function (Bloom & Edwards 1980). The proposed, sub-cortical (sub-pallial) equivalents of the VFS in birds are shown in Fig. 2.
A third reason is that VIP is directly correlated with photoperiodic exposure in turkeys (Mauro et al. 1992) and presumably in other avian species as well. The steady rise in VIP over the course of increased photoperiodic exposure in turkey hens and its continued rise during recrudescence of the hen's reproductive organs (similar to stages 1 through 4 of migratory birds shown in table 1), is a recognizable, physiological response that could be internally sensed or monitored by a bird. More importantly, we have shown using both immunocytochemistry and in situ hybridization histochemistry (Kuenzel & Blähser 1994; Kuenzel et al. 1997) that VIP is located in neurons in two brain areas thought to house encephalic photoreceptors: the mediobasal hypothalamus in and about the infundibular nucleus (IN) and the medial portion of the lateral septal organ (LSOm). It was previously shown in the Ring Dove Streptopelia risoria that neurons in and about the IN and LSOm immunostained with an antibody directed against opsin (Ret-P1, Silver et al. 1988), a protein found in rods or rod-like structures in the retina of vertebrates. We have also demonstrated that neurons in both the LSOm and IN show gene expression for VIP and therefore have the capability of synthesising VIP and releasing it into the cerebrospinal fluid located in the ventricular system of the brain. It is particularly relevant for the LSOm as this structure has VIP containing cerebrospinal fluid contacting neurons with bulb-like structures contacting the lateral ventricle of the rostral forebrain (Kuenzel & Blähser 1994). Of relevance is that the nTS, found in the caudal hindbrain, also contacts the cerebrospinal fluid as part of this structure, the area postrema, is a circumventricular organ that contains cerebrospinal fluid contacting neurons. Therefore the LSOm has the capacity to communicate with the nTS via the cerebrospinal fluid within the ventricular system of the brain as well as via the monosynaptic pathway that has been established between the bed n. of the stria terminalis pars magnocellularis (BnSTmc, a sub-nucleus associated with the LSOm) and the nTS.
Vasoactive intestinal polypeptide (VIP), when administered to bantam hens, has been shown to increase significantly plasma concentration of both luteinizing hormone (LH) and prolactin (Macnamee et al. 1986). It is therefore conceivable that early in the season, when VIP content of the mediobasal hypothalamus is low following the initial photostimulation of long days (Mauro et al. 1992), VIP could initially serve to stimulate LH and activate the gonadal system along with gonadotropin releasing hormone. As the season progresses and VIP hypothalamic content rises, the result would be significantly increased plasma levels of prolactin since VIP is more efficacious for releasing prolactin (Mauro et al. 1992). Peak plasma levels of prolactin have been shown to be coincident with the onset of gonadal regression (Dawson and Goldsmith 1984) and also stimulate incubation behaviour in some avian species.
Macdonald and Cohen (1973) have provided evidence that sub-pallial components of the VFS of pigeons are involved in pulmonary and cardiovascular activity and therefore directly affect autonomic nervous system function. Specifically, electrical stimulation of the posterior archistriatum, hypothalamic component of the occipital mesencephalic tract (the major tract that projects from the archistriatum to the hypothalamus), paraventricular n., lateral hypothalamic area, mediobasal hypothalamus down to the infundibular n. and near the parabrachial n. produced tachycardia, hypertension and hyperpnea (Macdonald & Cohen 1973). All of the above structures are neural areas found in the avian brain that are proposed to be equivalents of the mammalian VFS. Of interest is that all are involved in cardiovascular and respiratory function.
Over the past 40 to 50 years, the chicken Gallus domesticus has been genetically selected into two distinct types of bird. One, the White Leghorn, has been artificially selected for increased egg production, while a second type, the broiler, has been selected for growth rate and meat production. It has been proposed that the latter shows a dominance of the parasympathetic nervous system (Kuenzel 1994). Our recent data (Kuenzel and Grossmann, unpublished) have shown that the broiler has a significantly reduced activity of the sympathetic nervous system compared to the leghorn. Clearly, if the balance of the ANS can be significantly altered in such a short period of time by genetic selection in a domestic avian species, surely poikilostasis or dynamic shifts in the balance of the ANS can occur in wild birds as a result of natural selection.
In summary, the preceding data and analysis have been presented to suggest that a VFS is present within the nervous system of migratory birds. The main function of the VFS is to regulate the balance of the ANS. Due to time constraints, poikilostasis is proposed to occur in birds resulting in times of the year when the parasympathetic nervous system dominates and other times when the sympathetic nervous system is more dominant. The benefit of having dynamic shifts in ANS balance is to give migratory birds the greatest chance of migrating to their breeding grounds to produce young in a timely manner and have the necessary time to moult their body feathers and migrate back to the wintering grounds before the onset of harsh, winter conditions.
ACKNOWLEDGMENTS
The author is indebted to Manju Masson and Michael Hamilton for preparing the final copies of the illustrations included in the manuscript. Funding has been provided in part by a competitive grant from the Maryland Agriculture Experiment Station (MAES – AASC-99-14).
REFERENCES
Dawson, A. & Goldsmith, A.R. 1984. Effects of gonadectomy on seasonal changes in LH and prolactin concentrations in male and female starlings (Sturnus vulgaris). Journal of Endocrinology 100: 213-218.
Divac, I. & Mogensen, J. 1985. The prefrontal ‘cortex’ in the pigeon: catecholamine histofluorescence. Neuroscience 15: 677-682.
Divac, I., Mogensen, J. & Björklund, A. 1985. The prefrontal ‘cortex’ in the pigeon. Biochemical evidence. Brain Research 332: 365-368.
Eckenstein, F. & Baughman, R.W. 1984. Two types of cholinergic innervation in cortex, one colocalized with vasoactive intestinal polypeptide. Nature 309: 153-155.
Fransson, T. & Jakobsson, S. 1998. Fat storage in male willow warblers in spring: do residents arrive lean or fat? The Auk 115: 759-763.
Holberton, R.L., Parrish, J.D. & Wingfield, J.C. 1996. Modulation of the adrenocortical stress response in neotropical migrants during autumn migration. The Auk 113: 558-564.
Kuenzel, W.J. 1994. Central neuroanatomical systems involved in the regulation of food intake in birds and mammals. Journal of Nutrition 124:1355S-1370S.
Kuenzel, W.J. & Helms, C.W. 1974. An annual cycle study of tan-striped and white-striped white-throated sparrows. The Auk. 91: 44-53.
Kuenzel, W.J. & Blähser, S. 1993. The visceral forebrain system in birds: its proposed anatomical components and functions. Poultry Science Review 5: 29-36.
Kuenzel, W.J. & Blähser, S. 1994. Vasoactive intestinal polypeptide (VIP)-containing neurons: distribution throughout the brain of the chick (Gallus domesticus) with focus upon the lateral septal organ. Cell & Tissue Research 275: 91-107.
Kuenzel, W.J., McCune, S.K., Talbot, R.T., Sharp, P.J. & Hill, J.H. 1997. Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus). Journal of Comparative Neurology 381: 101-118.
Kuenzel, W.J., Beck, M.M. & Teruyama, R. 1998. Neural sites and pathways regulating food intake in birds: a comparative analysis to mammalian systems. Journal of Experimental Zoology (in press).
Luine, V.N., Rostene, W., Rhodes, J. & McEwen, B.S. 1984. Activation of choline acetyl transferase by vasoactive intestinal peptide. Journal of Neurochemistry 42: 1131-1134.
Macdonald, R.L. & Cohen, D.H. 1973. Heart rate and blood pressure responses to electrical stimulation of the central nervous system in the pigeon (Columba livia). Journal of Comparative Neurology 150:109-136.
Macnamee, M.C., Sharp, P.J., Lea, R.W., Sterling, R.J. & Harvey, S. 1986. Evidence that vasoactive intestinal polypeptide is a physiological prolactin-releasing factor in the bantam hen. General Comparative Endocrinology 62: 470-478.
Mauro, L.J., Elde, R.P., Youngren, O.M., Phillips, R.E. & El Halawani, M.E. 1989. Alterations in hypothalamic vasoactive intestinal peptide-like immunoreactivity are associated with reproduction and prolactin release in the female turkey. Endocrinology 125: 1795-1804.
Mauro, L.J., Youngren, O.M., Proudman, J.A., Phillips, R.E. & El Halawani, M.E. 1992. Effects of reproductive status, ovariectomy, and photoperiod on vasoactive intestinal peptide in the female turkey hypothalamus. General and Comparative Endocrinology 87: 481-493.
Mauro, L.J., Youngren, O.M., Proudman, J.A., Phillips, R.E. & El Halawani, M.E. 1992. Effects of reproductive status, ovariectomy, and photoperiod on vasoactive intestinal peptide in the female turkey hypothalamus. General and Comparative Endocrinology 87: 481-493.
Mogensen, J. & Divac, I. 1982. The prefronal ‘cortex’ in the pigeon. Behavioral evidence. Brain Behavior Evolution 21:60-66.
Proudman, J.A. & Opel, H. 1983. Stimulation of prolactin and growth hormone secretion from turkey pituitary cells. Poultry Science 62: 1484-1485.
Rehkämper, G. & Zilles, K. 1991. Parallel evolution in mammalian and avian brains: Comparative cytoarchitectonic and cytochemical analysis. Cell Tissue Research. 263: 3-28.
Silver, R.P., Witkovsky, P., Horvath, P., Alones, V., Barnstable, C.J. & Lehman, M.N. 1988. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Research 253: 189-198.
van der Kooy, D., Koda, L.Y., McGinty, J.F., Gerfen, C.R. & Bloom, F.E. 1984. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. Journal of Comparative Neurology 224:1-24.
Waldmann, C. & Güntürkün, O. 1993. The dopaminergic innervation of the pigeon caudolateral forebrain: immunocytochemical evidence for a ‘prefrontal cortex’ in birds? Brain Research 600: 225-234.
Wild, J.M., Arends, J.J.A. & Zeigler, H.P. 1990. Projections of the parabrachial nucleus in the pigeon (Columba livia). Journal of Comparative Neurology 293: 499-523.
Wilson, F.E. 1997. Photoperiodism in American tree sparrows: role of the thyroid gland. In: Harvey, S. & Etches, R.J. (eds) Perspectives in Avian Endocrinology. Bristol; Journal of Endocrinology Ltd.: 159-169.
Zeier, H. & Karten, H.J. 1971. The archistriatum of the pigeon: Organization of afferent and efferent connections. Brain Research 31: 313-326.
Table 1. Annual Cycle Events Where Shifts in the Balance of the Autonomic Nervous System (ANS) are Proposed to Occur

Fig. 1. Annual cycle of male White-throated Sparrows Zonotrichia albicollis. The arrow marks the starting point of the experiment. CLP = cloacal protuberance (with permission (Kuenzel and Helms 1974)).
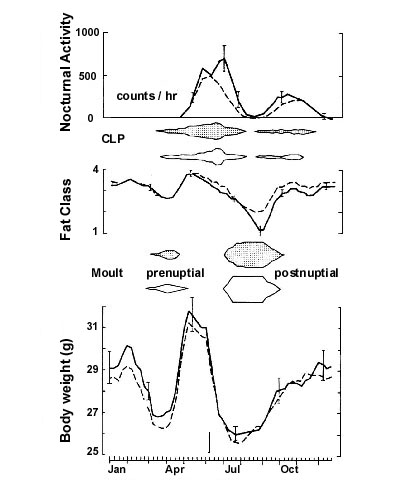
Fig. 2. Sagittal view of an avian brain showing the proposed components of the visceral forebrain system indicated by double lines under abbreviations in the schematic diagram. Apdl = posterior caudolateral archistriatum, BnSTmc = bed nucleus of the stria terminalis, pars magnocellularis, CA = anterior commissure, IN = infundibular nucleus (arcuate nucleus), LHy = lateral hypothalamic area, LSO = lateral septal organ, ME = median eminence, nAc = nucleus accumbens, PB = parabrachial nucleus, PVN = paraventricular nucleus, Rpgl = nucleus reticularis paragigantocellularis lateralis, S = nucleus tractus solitarius, TSM = septomesencephalic tract.