S14.3: Neural control of the avian jaw and its role in consummatory behaviour
J. Martin. Wild1 & H. Philip Zeigler2
1Department of Anatomy, School of Medicine and Health Science, University of Auckland, Auckland, New Zealand, fax 64 9 3737484, e-mail jm.wild@auckland.ac.nz; 2H.P. Zeigler, Department of Psychology, Hunter College, City University of New York, New York, N.Y. 10021, USA, e-mail hpzeigler@shiva.cuny.edu
Wild, J.M. & Zeigler, H.P. 1999. Neural control of the avian jaw and its role in consummatory behaviour. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 777-783. Johannesburg: BirdLife South Africa.A sensorimotor pathway that mediates control of feeding behaviour is described as an example of a well-delineated functional circuit in the avian brain. Beak mechanoreceptors involved in specific behavioural components of the consummatory response, such as grasping, stationing and mandibulation are innervated in large part by sensory branches of the trigeminal nerve, which projects somatotopically upon the principal sensory trigeminal nucleus (PrV) in the pons. In the telencephalon, nucleus basalis (Bas) receives the input from PrV via a quintofrontal pathway that bypasses the thalamus, a distinctly non-mammalian feature. In ducks, parrots and finches Bas also receives a major sensory input from the tongue mediated via glossopharyngeal and hypoglossal nerve projections to PrV, and is connected to several regions of the overlying pallium. These regions then project upon the basal ganglia and the archistriatum, which are the origin of long descending projections that terminate either on jaw motoneurons directly or upon premotor neurons located in the lateral reticular formation of the lower brainstem. These premotor neurons then project upon jaw motoneurons in the facial and trigeminal nuclei that innervate the jaw opening and closing muscles. In the pigeon, experimental interruption of this circuit at different points produces disturbances of grasping, stationing and mandibulation, and also a reduction in responsiveness to food and peck localisation.
The avian jaw performs for birds what the hand does for humans; that is, the beak is a highly sensitive, finely tuned, sensorimotor mechanism in the form of a grasper. Its range of movements in space is made possible by the extreme flexibility of the bird's neck, which is analogous to the upper limb of the human that allows the hand to be placed in a wide cone of space. For the bird, as for the human, some of the most interesting objects in extrapersonal space are food, the bodies of others, and nest building materials. But the bird, unlike the human in this case, is almost entirely dependent on the jaws for all activities directed at these objects.
Just as these activities differ tremendously in their form between different species, so the jaws themselves differ tremendously between species, both in their gross structure (c.f. the beaks of tucan, duck, parrot and pigeon), and in the organisation of sensory and motor elements that mediate the species-specific behaviours, particularly those involved in feeding. In turn, as Stingelin (1961) pointed out over 35 years ago, the functional and morphological specialisations of beak structure are correlated with the relative size of the brain nuclei that mediate the sensorimotor mechanisms involved in feeding and other behaviours.
What has become even more clear in recent years, however, is that not only is this true for the size of the nuclei, but for their functional organisation as well, and for the neural pathways that link sensory and motor structures. That is, the functional specificity of feeding mechanisms is reflected in the detailed representation, within specific brain nuclei and pathways, of the sensory and motor components of the feeding apparatus.
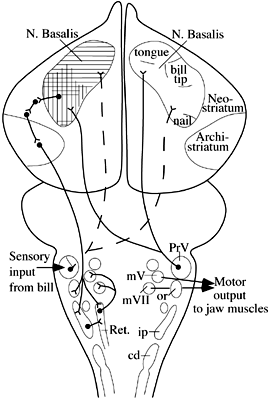
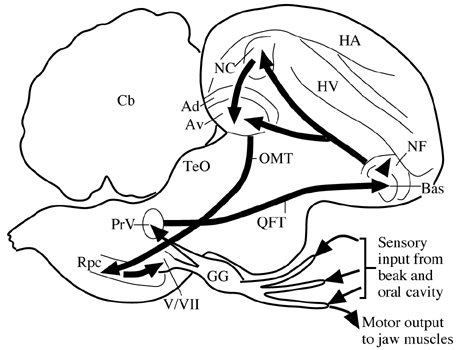
This has been demonstrated now for a variety of common species: Zweers, Dubbeldam and colleagues at Leiden have carried out extensive functional morphological and neuroanatomical investigations in the mallard duck (Zweers, 1974; Zweers et al. 1977; Dubbeldam 1984); Zeigler and colleagues and Zweers have analyzed the pigeon's peck in terms of its behavioural and neural control and its functional morphology (Zeigler 1989; Zeigler et al. 1975; 1993; Wild et al. 1985; Wild & Zeigler 1980; 1996; Zweers 1982); and Wild and colleagues have examined the functional neuroanatomy of peripheral and central structures involved in feeding in finches and parrots (Wild 1981; 1990; Wild et al. 1997). In all these cases there appears to be a common plan to the organisation of the sensorimotor pathways mediating control of the jaw, as illustrated in Fig. 1 and Fig. 2, which summarise the sensorimotor pathways involved in control of feeding in the pigeon and mallard, respectively.
When a pigeon pecks a seed, or a duck dabbles and strains food in water, there is a specific sequence of sensory events that is set up within the beak and oral cavity (e.g., Zweers 1982). The pigeon's peck may be broken down into four stages: grasp, stationing, transport or mandibulation, and swallowing (Zeigler et al. 1980). During this sequence, different sets of mechanoreceptors in specific locations in the beak and oral cavity are stimulated, the sensory input from which is used to modulate and guide the next phase of the feeding sequence.
In the feeding response of the mallard duck, an even more elaborate sequence of sensory events take place, during which stimulation of intraoral sensory receptors (Berkhoudt 1985) is essential for the identification, selection or rejection, processing and intraoral transport of food items (Zweers et al. 1977).
In seed husking species, such as finches and parrots, the tongue as well as the beak plays a major role in the feeding process, and in both these orders there are highly specialised lingual mechanoreceptor complexes for this purpose (Ziswiler 1965; Krulis 1978; Wild 1981;1990).
The input from mechanosensory receptors in the beak and much of the oral cavity enters the brain over the trigeminal nerve (Watanabe & Yasuda, 1970; Dubbeldam & Karten 1978; Dubbeldam 1980; Kishida et al. 1985; Wild & Zeigler 1996). Mechanoreceptors in the tongues of finches and parrots are innervated by the glossopharyngeal and hypoglossal nerves (Ziswiler 1974; Wild 1990). Taste input is mediated via the facial and glossopharyngeal nerves (Dubbeldam 1984). Although these inputs project centrally to several levels of the trigeminal column and gustatory nuclei, only the input to the principal sensory trigeminal nucleus (PrV) will be considered further because it is this nucleus that provides the input to the telencephalon, where much of the higher order processing of sensory information related to feeding takes place.
By using neural tracers that are transported transganglionically from the periphery, either from a nerve or skin, the topographic specificity of the inputs to PrV can be determined (Wild & Zeigler 1996). The projection of the mandibular branch of the trigeminal nerve terminates dorsocaudally in PrV, that from the ophthalmic nerve rostroventrally, while the projection of the maxillary nerve in the pigeon is relatively sparse, reflecting its limited intraoral distribution. Thus, each major territory in the periphery has a more or less separate representation in the principal nucleus and, presumably, there is little or no integration of information from the different territories at this level.
Wallenberg (1903) showed 95 years ago that in birds PrV projects directly to nucleus basalis (Bas) in the frontobasal telencephalon, without a thalamic relay, unlike the case for second order trigeminal projections in mammals. This projection can be seen more clearly using modern neural tracers (Dubbeldam et al. 1981; Wild et al. 1985). Neurons in each principal nucleus project to both sides via branched axons, and the specificity of the projections arising in different regions of PrV is retained in terms of their terminations in Bas (Wild & Farabaugh 1996). That is, within Bas there is a somatotopic organisation of the inputs from PrV, and hence of the inputs from the beak and tongue (Dubbeldam et al. 1981; Berkhoudt et al. 1981; Wild & Farabaugh 1996; Wild et al. 1997). As for PrV, therefore, it does not appear as though there is integration of inputs from the various regions of the feeding apparatus at the level of Bas.
Like other primary sensory receiving stations in the avian telencephalon, Bas projects to surrounding or overlying areas of the pallium (i.e., neostriatum and hyperstriatum ventrale: Wild et al. 1985; Veenman & Gottschaldt 1986; Dubbeldam & Visser 1987) and it may be here that integration of inputs arising from different parts of the periphery takes place, although there is no physiological evidence bearing on this possibility in birds. In turn, these 'secondary' sensory telencephalic areas project to the basal ganglia, and directly and indirectly to the archistriatum (Wild et al. 1985; Veenman & Gottschaldt 1986; Dubbeldam & Visser 1987). Both the basal ganglia and the archistriatum are major sources of extratelencephalic projections in the avian brain. Some outputs of the ventral paleostriatum may terminate upon jaw motor nuclei (Arends & Dubbeldam, 1982), while lateral or rostrolateral parts of the avian archistriatum, which receives the multisynaptic trigeminal input, projects bilaterally throughout the brainstem and terminates extensively within the lateral reticular formation (Zeier & Karten 1971; Berkhoudt et al. 1982; Arends & Zeigler 1982; Dubbeldam et al. 1997; Wild & Farabaugh 1996). It is here that many premotor neurons for the jaw motor nuclei are located (Arends & Dubbeldam 1982; Berkhoudt et al. 1982; Bout & Dubbeldam 1994; Tellegen & Dubbeldam 1996), and these premotor neurons provide a bilateral projection to both the dorsal facial nucleus that innervates the opening muscle of the lower jaw and the trigeminal nuclei that innervate the opener of the upper jaw and the closers of the lower jaw.
The circuit described herein for the control of the pigeon's jaw has been experimentally interrupted at various points - either at the level of the peripheral sensory trigeminal nerves, or Bas, or at different points along the descending pathway from the archistriatum. Lesions of the three sensory branches of the trigeminal nerve result in a marked decline in feeding efficiency, as might be expected from the loss if input from beak and intraoral sensory receptors (Zeigler et al. 1975). The peck is initiated in normal fashion but the grasp and mandibulation components are such as to cause the bird either to lose the seed, or to expend much more effort in getting the seed from the tip of the beak to the back of the beak so that swallowing can be initiated. Such deafferentations, besides disturbing the consummatory response, also bring about a marked decline in responsiveness to food, i.e. the motivation to eat is reduced, for reasons which are not clear, and they also cause deficits in peck localisation, again for reasons that are not clear. Bas lesions cause similar deficits in the sensory control of the peck, while lesions of the descending pathway produce a complex syndrome of effects that include an impairmant in grasping, a reduction in responsiveness to food, and a disruption of food-reinforced key pecking (Levine & Zeigler 1981). Interestingly, the control of jaw opening size (gape), which is normally scaled to the size of the seed to be pecked, does not appear to be mediated by circuitry that involves the telencephalon (Jäger et al 1992). In other words, peck localisation and gape are controlled by structures at different levels of the brain.
REFERENCES:
Arends, J.J.A. & Dubbeldam, J.L. 1982. Exteroceptive and proprioceptive afferents of the trigeminal and facial motor nuclei in the mallard (Anas platyrhynchos L.). Journal of Comparative Neurology 209:313-329.
Berkhoudt, H. 1980. The morphology and distribution of cutaneous mechanoreceptors (Herbst and Grandry corpuscles) in bill and tongue of the mallard (Anas playrhynchos L.) . Netherlands Journal of Zoology 30:1-34.
Berkhoudt, H. 1985. The role of exteroceptive sense organs in avian feeding behaviour. Fortschritte der Zoologie 30:269-272.
Berkhoudt, H., Dubbeldam, J.L. & Zeilstra, S. 1981. Studies on the somatotopy of the trigeminal system in the mallard, Anas platyrhynchos L. IV Tactile representation in the nucleus basalis. Journal of Comparative Neurology 196:407-420.
Berkhoudt, H., Klein, B.G. & Zeigler, H.P. 1982. Afferents to the trigeminal and facial motor nuclei in pigeon (Columba livia L.): Central connections of jaw motoneurons. Journal of Comparative Neurology 209:301-312.
Bout, R. & Dubbeldam, J.L. The reticular premotor neurons of the jaw muscle motor nuclei in the mallard (Anas platyryynchos L.). European Journal of Morphology 32:134-137.
Dubbeldam, J.L. 1980. Studies on the somatotopy of the trigeminal system in the mallard, Anas platyrhynchos L. II. Morphology of the principal sensory nucleus. Journal of Comparative Neurology 191:557-571.
Dubbeldam, J.L. 1984. Afferent connections of nervus facialis and nervus glossopharyngeus in the pigeon (Columba livia) and their role in feeding behaviour. Brain, Behaviour and. Evolution 24: 47-57.
Dubbeldam, J.L. & Karten, H.J. 1978. The trigeminal system in the pigeon (Columba livia) 1. Projections of the gasserian ganglion. Journal of Comparative Neurology 180: 661-678.
Dubbeldam, J.L. & Visser, A.M. 1987. The organisation of the nucleus basalis-neostriatum complex of the mallard (Anas platyrhynchos L.) and its connections with the archistriatum and the paleostriatal complex. Neuroscience 21:487-517.
Dubbeldam, J.L., Brauch, C.S.M. & Don, A. 1981. Studies on the somatotopy of the trigeminal system in the mallard, Anas platyrhynchos L.:III Afferents and organisation of the nucleus basalis. Journal of Compartive Neurology 196:391-405.
Dubbeldam, J.L., den Boer-Visser & Bout, R.G. 1997 Organisation and efferent connections of the archistriatum of the mallard, Anas platyrhynchos L.: An anterograde and retrograde tracing study. Journal of Comparative Neurology 388:632-657.
Jäger, R., Arends, J.J.A., Schall, U. & Zeigler, H.P. 1992. The visual forebrain and eating in pigeons (Columba livia). Brain, Behaviour and Evolution 39:153-168.
Kishida, R., Dubbeldam, J.L. & Goris, R.C. 1985. Primary sensory ganglion cells projecting to the principal trigeminal nucleus in the mallard Anas Platyrhynchos. Journal of Comparative Neurology 240:171-179.
Krulis, V. 1978. Struktur und Verteilung im Schnabel-Zungenbereich von Singvogeln, im besondern Fringillidae. Revue suisse Zoologie. 85:385-447.
Levine, R.R. & Zeigler, H.P. 1981. Extratelencephalic pathways and feeding behaviour in the pigeon (Columba livia). Brain, Behaviour and Evolution 19:56-92.
Stingelin, W. 1961. Grössenunterschiede des sensiblen Trigeminuskernes bei verschiedenen Vögeln. Revue suisse Zoologie. 69:247-251.
Tellegen, A.J. & Dubbeldam, J.L. 1996. Do craniocervical and jaw motor nuclei receive input from the same population of reticular premotor neurons? a double labeling tracing study in the mallard (Anas platyrhynchos). Neuroscience Letters 209:77-80.
Veenman, C.L. & Gottschaldt, K.M. 1986. The nucleus basalis-neostriatum complex in the goose (Anser anser L.). Advances in Anatomy, Embryology and Cell Biology 96:1-85.
Wallenberg, A. 1903. Der Ursprung des Tractus isthmostriatus (oder bulbostriatus) der Taube. Neurologie Zbl. 22:98-101.
Watanabe, T. & Yasuda, M. 1970. Comparative and topographical anatomy of the fowl. XXVI. Peripheral course of the trigeminal nerve. Japanese Journal of Veterinary Science 32:43-57.
Wild, J.M. 1981. Identification and localization of the motor nuclei and sensory projections of the glossopharyngeal, vagus and hypoglossal nerves of the cockatoo (Cacatua roseicapilla), Cacatuidae. Journal of Comparative Neurology 203:351-377.
Wild, J.M. 1990. Peripheral and central terminations of hypoglossal afferents innervating lingual tactile mechanoreceptor complexes in Fringillidae. Journal of Comparative Neurology 298:157-171.
Wild, J.M. & Zeigler , H.P. 1980. Central representation and somatotopic organisation of the jaw muscles within the facial and trigeminal nuclei of the pigeon (Columba livia). Journal of Comparative Neurology 192:175-201.
Wild, J.M. & Farabaugh, S.M. 1996. Organisation of afferent and efferent projections of the nucleus basalis prosencephali in a passerine, Taeniopygia guttata. Journal of Comparative Neurology 365:306-328.
Wild, J.M. & Zeigler, H.P. 1996. Central projections and somatotopic organisation of trigeminal primary afferents in pigeon (Columba livia). Journal of Comparative Neurology 368:136-152.
Wild, J.M., Arends, J.J.A. & Zeigler, H.P. 1984. A trigeminal sensorimotor circuit for pecking, grasping and feeding in the pigeon (Columba livia). Brain Research 300:146-151.
Wild, J.M., Arends, J.J.A. & Zeigler, H.P. 1985. Telencephalic connections of the trigeminal system in the pigeon (Columba livia): A trigeminal sensorimotor circuit. Journal of Comparative Neurology 234: 441-464.
Wild, J.M., Reinke, H. & Farabaugh, S.M. 1997. A non-thalamic pathway contributes to a whole body map in the brain of the budgerigar. Brain Research 755:137-141.
Zeigler, H.P. 1989. Neural control of the jaw and ingestive behaviour: Anatomical and neurobehavioural studies of a trigeminal sensorimotor circuit: Modulation of defined vertebrate neural circuits. Annals of the New York Acadamy of Science 563:69-86.
Zeier, H.J. & Karten, H.J. 1971. The archistriatum of the pigeon: Organisation of afferent and efferent connections. Brain Research 31:313-326.
Zeigler, H.P., Miller, M.G. & Levine, R.R. 1975. Trigeminal nerve and eating in the pigeon: neurosensory control of the consummatory response. Journal of Comparative Physiology and Psychology 89:845-858.
Zeigler, H.P., Levitt, P. & Levine, R.R. 1980. Eating in the pigeon: response topography, stereotopy and stimulus control. Journal of. Comparative Physiology and Psychology 94:783-794.
Zeigler, H.P., Jäger, R. & Palacios, A.G. 1993 Sensorimotor mechanisms and pecking in the pigeon. In: Zeigler, H.P. & Bischoff, H.-J. (eds) Vision, Brain and Behaviour in Birds. Cambridge; MIT Press: pp. 265-283.
Ziswiler, V. 1965. Zur Kenntnis des Sommenoffnens und der Struktur des hornernen Gaumens bei kornerfressenden Oscines. Journal für Ornithologie, Leipzig 106:1-48.
Zweers, G.A. 1974. Structure. movement and myography of the feeding apparatus of the mallard (Anas platyrhynchos L). A study in functional anatomy. Netherlands Journal of Zoology 24:323-467.
Zweers, G.A., Gerritsen, A.F.C. & van Kranenburg-Voogd, P.J. 1977. Mechanics of feeding of the mallard (Anas platyrhynchos L; Aves, Anseriformes). The lingual apparatus and the suction-pressure pump mechanism of straining. In: Hecht, M.K. & Szalay, F.S. (Eds): Contributions to Vertebrate Evolution. Vol. 3. Basel: Karger, S.
Zweers, G.A. 1982. Pecking of the pigeon (Columba livia). Behaviour 81:173-230.
Fig. 1. Trigeminal sensorimotor circuitry mediating feeding in the pigeon. Ad: Archistriatum dorsale; Av: Archistriatum ventrale; Bas: Nucleus basalis: Cerebellum; GG: Gasserian ganglion; HA: Hyperstriatum accessorium; HV: Hyperstriatm ventrale; NF: Neostriatum frontale; NC: Neostriatum caudale; OMT: Tractus occipitomesencephalicus; QFT: Tractus quintofrontalis; Rpc; Nucleus reticularis parvocellularis; TeO: Tectum opticum; V/VII: Jaw motor nuclei. Adapted from Wild et al. 1984, with permission.

Fig. 2. Trigeminal sensorimotor circuitry mediating feedingin the mallard. cd: Nucleus descendens nervi trigemini, pars caudalis; ip: Nucleus descendens nervi trigemini, pars interpolaris; or: Nucleus descendens nervi trigemini, pars oralis; PrV: Nucleus sensorius principalis nervi trigemini; Ret.: Nucleus reticularis parvocellularis. Adapted from Dubbeldam 1993, with permission.