S14.2: A sexual dimorphic peptidergic system in an avian species: Development and possible function
R. Grossmann,1 S.W. Barth,1 A. Jurkevich2, W.J. Kuenzel3 & A. Köhler1
1Institute for Animal Science and Animal Behaviour, Department of Physiology, Celle, Germany, fax 49 5141 381849, e-mail Grossmann@ktf.fal.de; 2Institute of Ecology, Vilnius, Lithuania; 3University of Maryland, College Park, USA
Grossmann, R., Barth, S.W., Jurkevich, A., Kuenzel, W.J. & Koehler, A. 1999. A sexual dimorphic peptidergic system in an avian species: Development and possible function. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 770-776. Johannesburg: BirdLife South Africa.The bed nucleus of the stria terminalis (BnST) which influences reproduction and sexual behavior shows sex differences in morphology, steroid responsiveness and synthesis of neuropeptides e.g. arginine vasopressin (AVP). Arginine vasotocin (AVT), the avian homologue to AVP, is a main endocrine regulator of fluid balance in avian species and, in addition, is involved in oviposition in these species. Our recent studies clearly demonstrated that AVT secretion after osmotic stimulation is sexually dimorphic. In order to investigate whether AVT is expressed and synthetized in the BnST in a sexually dimorphic manner we have used in situ hybridization (ISH) technique and immunocytochemistry (ICC) to analyze AVT gene expressing neurons in the parvocellular (small-celled nuclei) BnST of adult male and female chickens. In cocks AVT peptide containing neurons were detected in the parvocellular BnST and high density of AVT immunoreactive material was visible in fibers within the parvocellular BnST and the lateral septal area, whereas no AVT immunoreactive neurons were detected in the corresponding regions of the hen. The ICC results correspond very well with our ISH results: neurons containing AVT transcripts were labelled in the parvocellular BnST of cocks, but no specific hybridization signals were observed above neurons in the female BnST. Even after osmotic stimulation (water deprivation for 48 h) AVT gene expression in neurons of the parvocellular BnST of hens was not upregulated. These results demonstrate for the first time that the AVT gene is expressed in the parvocellular BnST of chickens and exhibits a strong sexual dimorphism in this region. Furthermore, we conclude from this study that AVT synthesis in the BnST is regulated on the transcriptional level independent from osmotic stimuli. Thus, sex steroids might be the main regulator of AVT gene expression in the parvocellular BnST.
INTRODUCTION
Arginine vasotocin (AVT) is one of the key hormones regulating hydromineral balance in birds. Its contribution to reproductive functions promoting oviduct contraction and oviposition was also unequivocally demonstrated (Arad and Skadhauge 1984; Simon-Oppermann et al. 1988; Shimada et al. 1986). In the domestic fowl, AVT immunoreactive perikarya have originally been described in the preoptic, supraoptic and paraventricular regions of the hypothalamus (Goossens et al. 1977; Tennyson et al. 1985). With minor variations, comparable plans of distribution were reported for AVT perikarya in other bird species (quail: Viglietti-Panzica 1986; canary: Kiss et al. 1987; zebra finch: Voorhuis & de Kloet 1992). Among all investigated avian species, AVT cells in extrahypothalamic locations were observed only in the brain of songbirds, canary and zebra finch. In these species clustered AVT perikarya were located in the bed nucleus of the stria terminalis (BNST; Kiss et al. 1987; Voorhuis & de Kloet 1992) on the border between the lateral septum and the dorsal diencephalon. This cell group and parvocellular AVT neurons in the dorsal region of the hypothalamic paraventricular nucleus exhibit a clear sexual dimorphism in the canary brain. AVT cell bodies and fibers are more intense in males than in females and steroid hormones appear to influence and regulate this dimorphism. Seasonal changes in AVT immunoreactivity with maximal intensity during breeding and the decline during moult coincide with the fluctions of plasma testosterone levels (Voorhuis et al. 1991). A number of findings have described that sex-related differences in vasotocinergic circuits show an earlier phylogenetic lineage. Sexually dimorphic AVT immunoreactive cells and fibers located in extrahypothalamic regions have been demonstrated in amphibians (Zoeller & Moore 1986) and reptiles (Stoll & Voorn 1985). This review is an attempt to summarize the growing body of data concerning neurochemical sex differences in the brain vasotocinergic system and its known sexually dimorphic behavioral and physiological effects in birds. Although the presence of a sexual dimorphism in vasotocinergic elements is a common feature of the BNST, interspecific differences do occur. While neither AVT immunoreactivity nor AVT gene expressing neurons were reported in the hen, AVT neurons are present in both sexes in the canary, but they are less abundant in females than in males (Voorhuis et al. 1988).
CLASSICAL ENDOCRINE FUNCTIONS OF AVT IN BIRDS
Among neuroendocrine circuitries in the brain the hypothalamo-neurohypophysial system (HNS) is, as far as its classical functions are concerned, one of the best understood structures. The endocrine AVT system is represented by the HNS and consist of magnocellular neurons, the vast majority of them are located within the hypothalamic nuclei supraopticus and paraventricularis. Axons travel via the hypothalamo-neurohypophysial tract to the posterior pituitary and from there the nonapeptide hormone is released into the blood stream. The morphological and functional organization is, in general, similar in most vertebrate species with some important exceptions: in most mammalian species arginine vasopressin is the predominant osmoregulatory neurohypophysial hormone, while its analogue oxytocin is responsible for reproductive functions like contraction of the myometrium during parturition and myoepithelial contraction during milk ejection. In birds, however, AVT takes over both osmoregulation and oviposition. Since the latter function of AVT is specific for females one should expect differences between sexes in the structure and physiological regulation of the AVT system. Despite the magnocellular AVT-system vasotocinergic neurons are widely distributed in the bird brain. Parvocellular AVT immunoreactive neurons have been described in the preoptic, peri- and paraventricular regions as well as in the tuberal region of the hypothalamus (for review see Korf et al. 1988)
Functional ontogeny
For appropriate function during posthatch life the brain must attain a sufficient degree of differentiation during embryonic and fetal development in order to cope with the change from a well protected prenatal life to the environmental conditions of postnatal life. The first visible products of AVT gene expression as well as detectable amounts of immunoreactive AVT peptide in the chicken brain were revealed at Day 6 of embryonic life (E6; Milewski et al. 1989; Mühlbauer et al. 1993). These data are in agreement with immunocytochemical findings showing AVT immunoreactive neurons in the chicken hypothalamus around E8 (Tennyson et al. 1986). It is unlikely, however, that AVT serves in systemic osmoregulation at this early stage because neither axon terminals nor the neurohypophysis have developed by then (Wingstrand 1954; Von Lawzewitsch 1969). Immunoreactive AVT has not been observed before E10 in neurohypophysial tissue (Tennyson et al. 1986). In the plasma AVT has been first detected at E14 by radioimmunoassay (Mühlbauer et al. 1993).
At least from E16 onwards the chick embryo is able to respond to osmotic challenge by increased AVT secretion into the blood, although an adult-like reaction is observed only after hatching (Klempt et al. 1992; Mühlbauer et al. 1992; Xu et al. 1992). Marked changes in the electrophysiological characteristics of the hypothalamo-neurohypophysial system of the chick embryo and the newly hatched chicken were observed during the perinatal period (Grossmann & Ellendorff 1986a; b). Identified magnocellular neurons of the hypothalamic nucleus paraventricularis in D1 chickens are clearly osmosensitive (Grossmann & Ellendorff 1990; Grossmann et al. 1994). Although in chick embryos at E18 and E19 as well as in newly hatched chickens, there are no differences in AVT plasma concentrations between males and females (Kisliuk & Grossmann 1994), both sexes do react differently to acute osmotic and voleamic stimuli at D1 (Kisliuk & Grossmann 1993). Females have a higher threshold in the reaction to haemorrhage: their AVT system does not respond to moderate haemorrhage while males respond by a significant elevation of AVT plasma concentration. To a more severe haemorrhage the AVT concentrations in females increase more slowly but reach at the end the same level as in males. The reaction to an acute hyperosmotic load is also much more pronounced in male than in female chickens at D1. One possible reason of the lower activity of the AVT system in female chicks might be the preadaptation to the specific water balance conditions which takes place in laying hens. Almost every day a normal laying hen looses, with its oviposited egg, approx. 30% of its blood volume attributed to water and one may expect that in females the hydromineral regulation is more tolerant to such kind of disturbances.
In adult chickens quantification of brain AVT mRNA content has revealed considerably more AVT transcripts in males compared to females (Barth et al. 1995). However, from these results it is unclear to what extent the magnocellular and parvocellular cell groups of the AVT system located in various brain areas contribute to this sex dimorphism. In a recent developmental study (Jurkevich et al. 1997) of the chicken parvocellular BnST we could demonstrate that AVT immunoreactive perikarya are first detectable already at E12, the earliest embryonic day of our study, in both sexes. At posthatch D1 and D2 there are clearly more AVT neurons in the male parvocellular BnST. During posthatch development the cell number and intensity of labelling in females decline rapidly, until by D70 the AVT immunoreactive elements have disappeared completely. The developmental time course of sex differences in brain AVT seems to be species-specific. While in gallinaceous birds sex dimorphism develops rather early, in the canary there is evidence that the sexually dimorphic AVT distribution in the BNST appears during the late phase of postnatal development (Voorhuis et al. 1991). In the latter species, sex differences in the vasotocinergic system are not as pronounced as in gallinaceous birds. AVT immunoreactive perikarya in the BnST could be demonstrated in the canary brain of both sexes, however, in females the intensity of staining is lower (Voorhuis et al. 1988). Studies in the quail have shown that the sexually dimorphic characteristics of the AVT system are comparable to those in the chicken. AVT immunoreactive perikarya could be observed exclusively in the parvocellular BnST of the male quail (Aste et al. 1996a) and this sex difference has been further confirmed at the level of AVT gene expression by in situ hybridization using a chicken AVT-specific cDNA probe (Aste et al. 1996b). It needs to be evaluated whether this sexual differentiation during development is accompanied by subsequent sex differences in behaviour and autonomic regulation during adulthood.
Sex dimorphism in the adult AVT system
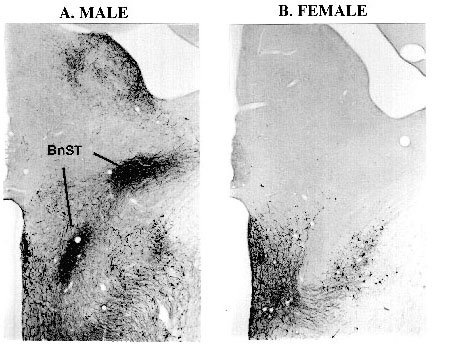
The existence of a clear sexual dimorphism has been demonstrated in the parvocellular BnST in a number of bird species (chicken: Jurkevich et al. 1996, 1997; quail: Viglietti-Panzica et al. 1992; 1994; canary: Voorhuis et al. 1988; 1991). This structure is known in the rat as an important integrative center involved in the control of sexual motivation and/or performance (Segovia & Guillamon 1993; van Furth et al. 1995) and central autonomic regulation (Moga et al. 1989). The presence of a sexual dimorphism in vasotocinergic elements is a common feature of the parvocellular BnST. While neither AVT immunoreactivity nor AVT gene expressing neurons were reported in the hen, AVT neurons are present in both sexes in the canary, but they are less abundant in females than in males (Voorhuis et al. 1988). In male chickens two densely packed parvocellular subgroups of AVT immunoreactive perikarya can be observed caudal of the anterior commissure. The dorsal cluster is located in the area of the BnST extending from the lateral ventricle to the dorsal aspect of the occipitomesencephalic tract. The ventral subgroup of AVT neurons is located ventrally to the caudal aspect of the anterior commissure in the dorsal position of the diencephalic paraventricular region (Fig. 1). Both cell clusters consist of numerous parvocellular bi- and multipolar cells surrounded by a dense plexus of beaded AVT containing fibers. The distribution pattern of AVT mRNA containing neurons in the parvocellular BnST of males is identical to the pattern of distribution of AVT immunoreactive cell bodies. In contrast, in hens immunoreactive perikarya and fiber tracts as well as AVT specific hybridization signals were completely absent in the parvocellular BnST.
Implications on behaviour
It is reasonable to assume that the sex dimorphism in the AVT system is controlled and maintained by gonadal hormones. Although a number of findings support this assumption (Viglietti-Panzica et al. 1992; Voorhuis et al. 1988) there are some observations which cannot be explained solely on the level of androgens, e.g. the existence of both androgen and estrogen receptors in the quail parvocellular BnST (Balthazart et al. 1992) as well as a large population of aromatase-producing elements in the rat BnST (Roselli 1995). Since the BnST is a limbic structure it is reasonable to hypothesize that the observed sex difference in the AVT system is involved in specific behavioral and physiological regulation. Male social behavior may be one of the most plausible targets for such regulation. Administration of AVT stimulates mating behavior in chicken and pigeons (Kihlström & Dannige 1972), while in zebra finches aggressive behavior is enhanced (Goodson et al. 1996). An inhibitory effect of AVT on appetitive and consummatory aspects of the male sexual behavior has been described in the quail (Castagna et al. 1998). Putative effects of sexually dimorphic brain circuits on reproductive behavior may be mediated by other endocrine systems. It has been suggested that AVT may control the release and/or synthesis of gonadotropins. Recently, co-localization of AVT and GnRH-I in the chicken hypothalamus has been described (Dhondt et al. 1995). Thus, the sexual dimorphic AVT system may play a hey role in regulating either sexual behavior or gonadotropin release in birds by acting as neuromodulator and/or neurotransmitter. Further attempts are required to understand the specific contribution of AVT on these body functions.
ACKNOWLEDGEMENT
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG), the German Ministry of Agriculture and the Maryland Agricultual Experiment Station.
REFERENCES
Arad, Z. & Skadhauge, E. 1984. Plasma hormones (Arginine vasotocin, Prolactin, Aldosterone and Corticosterone) in relation to hydration state, NaCl intake and egg laying in fowls. Journal of Experimental Zoology 232: 707-714.
Aste, N., Viglietti-Panzica, C. Mühlbauer, E., Grossmann, R. & Panzica, G.C. 1996a. Sexual dimorphism of vasotocinergic structures in quail nucleus of the stria terminalis. Italian Journal of Anatomy 101: 136-137.
Aste, N., Mühlbauer, E. & Grossmann, R. 1996b. Distribution of AVT gene expressing neurons in the prosencephalon of japanese quail and chicken. Cell and Tissue Research 286: 365-373.
Balthazart, J., Foidart, A., Wilson, E.M. & Ball, G.F. 1992. Immunocytochemical localization of androgen receptors in the male songbird and quail brain. Journal of Comperative Neurology 317: 407-420.
Barth, S.W., Jurkevich, A. & Grossmann, R. 1995. Sexual dimorphism in the expression of arg-vasotocin in the chicken brain. Poultry Avian Biological Review 6: 279.
Castagna, C., Absil, P., Foidart, A. & Balthazart, J. 1998. Systemic and intracerebroventricular injections of vasotocin inhibit appetitive and consumatory components of male sexual behavior in Japanese quail. Behavioral Neuroscience (in press).
Dhondt, E., Moons, L., Pijke, K. & Vandesande, F. 1995. Immunocytochemistry of coexisting LHRH-I, beta-endorphin and vasotocin in the chicken hypothalamus. Netherlands Journal of Zoology 45: 22-24.
Goodson, J.L., Greenswood, V.R. & Adkins-Regan, E. 1996. The central control of courtship and agression in male zebra finches (Taeniopygia guttata): effect of vasotocin infusions. Society of Neurosciene, Abs. 22: 2068.
Goossens, N., Blähser, S., Oksche, A., Vandesande, F. & Dierickx, K. 1977. Immunocytochemical investigation of the hypothalamo-neurohypophysial system in birds. Cell and Tissue Research 184: 1-13.
Grossmann, R. & Ellendorff, F. 1986a. Functional development of the prenatal brain I. Recording of extracellular action potentials from magnocellular system on the 18-day-old chicken embryo. Experimental Brain Research 62: 635-641.
Grossmann, R. & Ellendorff, F. 1986b. Functional development of the prenatal brain II. Ontogeny of the hypothalamo-neurohyopohysial axis in the pre- and perinatal chicken brain. Experimental Brain Research 62: 642-647.
Grossmann, R. &. Ellendorff, F. 1990.Neural control of posterior pituitary. In: Research Trends in Fetal Physiology, (Eds. K.G. Rosen & H. Lagercrantz), Chalmers University of Technology, Göteborg, Sweden, pp. 59-62.
Grossmann, R., Xu, B., Mühlbauer, E. & Udovic, B. 1994. Functional ontogeny of the hypothalamo-neurohypophysical system in the chicken. In: Integrative and Cellular Aspects of Autonomic Functions, eds. K. Pleschka & R. Gerstberger, John Libbey Eurotext Ltd., Montrouge/France, 429-438.
Jurkevich, A., Barth, S.W., Aste, N., Panzica, G.C. & Grossmann, R. 1996. Intracerebral sex differences in the vasotocin system in birds: Possible implication on behavioural and autonomic functions. Hormones and Behaviour 30: 673-681.
Jurkevich, A., Barth, S.W., & Grossmann R. 1997. Sexual dimorphism of arg-vasotocin gene expressing neurons in the telencephalon and dorsal diencephalon of the domestic fowl. An immunocytochemical and in situ hybridization study. Cell and Tissue Research 287: 69-77.
Kihlström, J.E. & Dannige, I. 1972. Neurohypophysial hormones and sexual behavior in males of the domestic fowl (Gallus domesticus) and the pigeon (Columbia livia Gmel.) General and Comperative Endocrinology 18: 115-120.
Kiss, J.Z., Voorhuis, T.A.M., Van Eekelen, J.A.M., De Kloet, E.R. & De Wied, D. 1987. Organization of vasotocin-immunoreactive cells and fibers in the canary brain. Journal of Comperative Neurology 263: 347-364.
Kisliuk, S.M & Grossmann, R. 1993. Sex difference in the arginine vasotocin response to hypovolaemia in the chicken. 4th European Symposium on Poultry Welfare, Edinburgh (Savory CJ & Hughes, BO. eds.) 300-301.
Kisliuk, S.M. & Grossmann, R. 1994. Modulation of arginine vasotocin secretion in chicks by dehydration during embryogenesis. Journal of Endocrinology 142: 153-160.
Klempt, M., Ellendorff, F. & Grossmann, F. 1992. Functional maturation of arginine vasotocin secretory responses to osmotic stimulation in the chick embryo and the newborn chicken. Journal of Endocrinology 133: 269-274.
Korf, H.W., Panzica, G.C., Viglietti-Panzica, C. & Oksche, A. 1988. Pattern of peptidergic neurons in the avian brain: clusters - local circuitries - Projections. Basic and Applied Histochemistry 32: 55-75.
Milewski, N., Ivell, R., Grossmann, R. & Ellendorff F. 1989. Embryonal development of arginine vasotocin/mesotocin gene expression in the chicken brain. Journal of Neuroendocrinology 1: 473-484.
Moga, M.M., Saper, C.B. & Gray T.S. 1989. Bed nucleus of the stria terminalis: Cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. Journal of Comperative Neurology 283: 315-332.
Mühlbauer, E., Hamman, D., Xu, B., Ivell, R., Ellendorff, F. & Grossmann, R. 1992. Arginine vasotocin gene expression during osmotic challenge in the chicken. Journal of Neuroendocrinology 4: 347-351.
Mühlbauer, E., Hamann, D., Xu, B., Ivell, R., Ellendorff, F. & Grossmann, R. 1993: Arg-Vasotocin gene expression and hormone synthesis during ontogeny of the chicken embryo and the newborn chicken. Journal of Neuroendocrinology 5: 281-288.
Roselli, C.E. 1995. Subcellular localization and kinetic properties of aromatase activity in rat brain. Journal of Steroid Biochemistry and Molecular Biology 52: 469-477.
Segovia, S. & Guillamon, A. 1993. Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors. Brain Research Review 18: 51-74.
Shimada, K., Neldon, H., & Koike T.I. 1986. Arginine vasotocin (AVT) release in relation to uterine contractility in the hen. General and Comperative Endocrinology 64: 362-367.
Simon-Oppermann, C., Simon, E. & Gray, D. 1988. Central and systemic antidiuretic hormone and angiotensin II in salt and fluid balance of birds as compared to mammals. Comperative Biochemistry and Physiology 90A: 789-803.
Stoll, C.J. & Voorn, P. 1985. The distribution of hypothalamic and extrahypothalamic vasotocinergic cells and fibers in the brain of a lizard, Gekko gekko: Presence of a sex difference. Journal of Comperative Neurology 239: 193-204.
Tennyson, V.M., Nilaver, G., Hou-Yu, A., Valiquette, G. & Zimmermann, EA. 1986. Immunocytochemical study of the development of vasotocin/mesotocin in the hypothalamo-hypophysial system of the chick embryo. Cell and Tissue Research 243: 15-31.
Tennyson, V.M., Hou-Yu, A., Nilaver, G., & Zimmerman, E.A. 1985. Immunocytochemical studies of vasotocin and mesotocin in the hypothalamo-hypophysial system of the chicken. Cell and Tissue Research 239: 279-291.
Van Furth, W.R., Wolterink, G. & Van Ree, J.M. 1995. Regulation of masculine sexual behaviour: Involvement of brain opioids and dopamine. Brain Research Review 21: 162-184.
Viglietti-Panzica, C., Anselmetti, G.C., Balthazart, J., Aste, N. & Panzica, G.C. 1992. Vasotocinergic innervation of the septal region in the Japanese quail: Sexual differences and the influence of testosterone. Cell and Tissue Research 267: 261-265.
Viglietti-Panzica, C., Aste, N., Balthazart, J. and Panzica, G.C. 1994. Vasotocinergic innervation of sexually dimorphic medial preoptic nucleus of the male Japanese quail: Influence of testosterone. Brain Research 657: 171-184.
Viglietti-Panzica, C. 1986. Immunohistochemical study of the distribution of vasotocin reacting neurons in avian diencephalon. Journal für Hirnforschung 27: 559-566.
Von Lawzewitsch, I. 1969. Development of the neuroseretory hypothalamic-hypophysial system of the chick embryo as evidenced in total preparation. Acta Anatomica 72: 83-93.
Voorhuis, T.A.M. & De Kloet, E.R. 1992. Immunoreactive vasotocin in the zebra finch brain (Taeniopigia guttata). Developmental Brain Research 69: 1-10.
Voorhuis, T.A.M., Kiss, J.Z., De Kloet, E.R. & De Wied, D. 1988. Testosterone-sensitive vasotocin-immunoreactive cells and fibers in the canary brain. Brain Research 442: 139-146.
Voorhuis, T.A.M., De Kloet E.R. & De Wied D. 1991. Effect of vasotocin analog on singing behavior in the canary. Hormones and Behaviour 25: 549-559.
Wingstrand, K.G. 1954. Neurosecretion and antidiurectiv activity in chick embryos with remarks on the subcommissural organ. Arkiv för Zoologi 6: 41-67.
Xu, B., Ellendorff, F. & Grossmann, R. 1992. Neurohypophysial hormones are synthesized very early during embryonic life in the chicken. Acta Endocrinologica 126: Suppl. 4, 128.
Zoeller, R.T. & Moore, F. 1986. Arginine vasotocin (AVT) immunoreactivity in hypothalamic and extrahypothalamic areas of the amphibian brain. Neuroendocrinology 42: 120-123.
Fig. 1. Microphotograph illustrating the distribution o AVT immunoreactive perikarya and fibers in the brain of male (A) and female (B) chicken. AVT immunoreactive parvocellular neurons and fibers are located in the cockerel but not in the hen BnST.
