S12.3: Seabird responses to long-term changes of prey resources off southern Africa
Robert J.M. Crawford
Sea Fisheries Research Institute, Private Bag X2, Rogge Bay, 8012, South Africa, e-mail crawford@sfri.wcape.gov.za
Crawford, R.J.M. 1999. Seabird responses to long-term changes of prey resources off southern Africa. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 688-705. Johannesburg: BirdLife South Africa.Life-history characteristics buffer seabird populations from variations in their food supply on time scales of several years or less. There are longer-term changes in food availability caused by decadal-scale regimes of prey populations and century-scale trends in the utilisation of forage resources by man and other predators. Responses during the 20th century of three southern African seabirds to such low-frequency variability are described. The three seabirds, African Penguin Spheniscus demersus, Cape Gannet Morus capensis and Cape Cormorant Phalacrocorax capensis are all endemic to the region and subsist to a large extent on sardine Sardinops sagax and anchovy Engraulis capensis. Sardine collapsed off South Africa in the 1960s and off Namibia in the 1970s, with anchovies being the dominant species off South Africa. Collapse of the sardine was followed by massive decreases in penguin colonies between Lüderitz and Table Bay, but increases in colonies north of Lüderitz and southeast of Table Bay. There were large decreases in Namibian gannet colonies, whereas those in South Africa increased. Populations of Cape Cormorants increased in both countries. In the 1980s, there was a resurgence of sardine off South Africa, where anchovy started to decrease with fluctuations. There was a large decrease in penguins at Dyer Island, southeast of Table Bay, whereas colonies between Lüderitz and Table Bay stabilised. Three new penguin colonies were established in the vicinity of Table Bay and grew rapidly. South African colonies of Cape Gannets remained stable or continued to increase, but there was poor breeding by Cape Cormorants when anchovy was scarce. The overall populations of penguins and gannets were buffered to an extent against decadal-scale changes in the food base by the ability of first-time breeders to move to colonies where food was plentiful at the time. Additionally, both species were able to switch their diet between anchovy and sardine. Cape Cormorants have not utilised sardine as effectively as anchovy, but are able to move to areas where anchovy is abundant and have potential to increase more rapidly than penguins and gannets.
INTRODUCTION
Seabirds face regional, seasonal, inter-annual and long-term changes in the availability of prey resources. They need to cope with these changes to survive and to breed. Life-history characteristics of seabirds that act to buffer populations from fluctuations in their food supply have been reviewed by Hunt et al. (1996). They include high annual survivorship, great longevity, delayed sexual maturity, and a relatively low reproductive rate.
These characteristics serve especially to shield seabird populations from shorter-term (periods of a year or less) variations in their food supply. For example, except in extreme cases of wide-scale collapse of all available prey stocks, adult seabird survival is unlikely to be affected by temporary, localised depletion of food supplies (Cairns 1987). Although such scarcity of food may cause reproductive failure, seabird populations will not directly track fluctuations in prey recruitment because breeding populations typically comprise several year classes (Hunt et al. 1996).
There are longer, decadal-scale, changes in food supply, which have been particularly evident in marine ecosystems where sardine Sardinops sagax and anchovy Engraulis spp. are dominant prey species (Lluch-Belda et al. 1989; 1992). Populations of these fish undergo ‘ regimes’ of high and low abundance. For example, off South Africa sardine collapsed in the 1960s and remained at a low level of abundance for 20 years (Crawford in press). The demographic characteristics that buffer seabirds from annual variability in food resources are less able to maintain populations in the face of these longer-term changes. In coping with long-term change, behavioural responses such as prey switching are likely to assume increased importance.
Intensive purse-seine fisheries developed off southern Africa after World War 2. Additionally, the population of Cape Fur Seals Arctocephalus pusillus, which feed on the same prey as many of the seabirds off southern Africa, grew throughout the 20th century. It is estimated that in the 1980s man and seals removed several million tons live mass more per annum from southern African seas than in 1930 (Crawford et al. 1992b). These influences on food supply have an even longer period than the regime shifts of sardine and anchovy. Very low-frequency environmental change may also influence food available to seabirds. In the north-west Atlantic a century-long increasing trend for Northern Gannets is correlated with warming surface water conditions (Montevecchi & Myers 1997).
This paper examines responses of seabirds in southern Africa to regime-scale or longer changes in prey availability. Upwelling in the Benguela system and on the Agulhas Bank gives rise to primary and secondary production that supports the high populations of sardine and anchovy. Seabirds that feed extensively on these fish (Crawford et al. 1991) are the African Penguin Spheniscus demersus, Cape Gannet Morus capensis and Cape Cormorant P. capensis, all of which are endemic to southern African (Cooper et al. 1984).
LONG-TERM CHANGES IN THE FOOD BASE
Prey populations
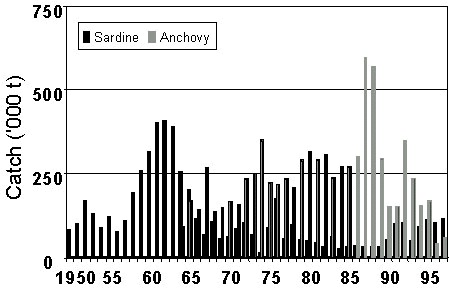
Relatively discrete populations of both sardine and anchovy are found off South Africa and Namibia, each separated by an area of intense upwelling near Lüderitz (Crawford et al. 1987). Sardine was abundant in both countries in the 1950s. It decreased off South Africa in the 1960s and off Namibia in the early 1970s. It was replaced by anchovy in South Africa (Crawford in press), and by horse mackerel Trachurus trachurus, pelagic goby Sufflogobius birarbatus and anchovy in Namibia (Crawford et al. 1985). Catches of sardine and anchovy in South Africa are shown in Fig. 1 and in Namibia in Fig. 2. It is clear that, from a fishery perspective, the replacement of sardine by anchovy was considerably more successful off South Africa than off Namibia.
A possible explanation for this is the disparate fishing policies that were pursued in the two countries. Once sardine had collapsed in South Africa, anchovy became an attractive alternative resource with which to perpetuate the fishery and attempts were made to manage it in a sustainable manner (Newman et al. 1979; Newman & Crawford 1980). When sardine collapsed off Namibia, the South African experience prompted belief that anchovy was a competitor of sardine and that this competition would be minimised by subjecting the anchovy to heavy fishing pressure (Butterworth 1983). Off South Africa, horse mackerel had been fished when sardine was abundant, and its dominant year classes depleted (Newman & Crawford 1980). Off Namibia, the fishery for horse mackerel was only commencing when the sardine collapsed and there was no fishery then for pelagic goby (Crawford et al. 1987). In both instances, it was the fish species least intensively exploited that later dominated the forage-fish community.
In South Africa, sardine began to increase again after the formation of an abundant year class in 1982/83 (Armstrong et al. 1987). Growth of the population was steady, and by 1997 the spawner biomass was estimated to be more than 750 000 tons (M. Barange, Sea Fisheries Research Institute, South Africa). Anchovy, which had provided a stable catch to birds (Berruti et al. 1993) and man in the early 1980s, began to fluctuate widely in the late 1980s with a long-term decrease (Crawford in press).
In Namibia, anchovy dominated purse-seine catches from 1978 to 1983 and then decreased. Although a large catch was also taken in 1987, this may have been of fish that moved north from South Africa (Hewitson 1988). Sardine began to increase in the early 1990s, but adverse environmental conditions brought about a decrease in 1994 and 1995 (Benefit 1997).
Global synchrony in regimes of sardine and anchovy suggest a natural cause of these decadal-scale fluctuations (Lluch-Belda et al. 1989; 1992). There also is evidence from proxy records, e.g. fish scales deposited in sediments (Soutar & Isaacs 1974; Baumgartner et al. 1992), harvests of seabird guano (Crawford & Shelton 1978) and records of pre-industrial fisheries (e.g. Alheit & Hagen 1997), that sardine, anchovy and other populations of small pelagic fish species underwent low-frequency shifts in abundance before they were commercially exploited to any great extent. Therefore, even prior to the development of commercial fisheries, it was necessary for seabirds utilising these forage fish to adjust to such long-term changes in prey populations.
Competitors for food
Cape Fur Seals had been exploited to a low level of abundance by the end of the 19th century (Shaughnessy 1984), when there were fewer than 0.1 million animals (Butterworth et al. 1995), but increased through the 20th century to 1.5 million animals by 1990 (Best et al. 1997). Anchovy and sardine and other fish eaten by seabirds, such as horse mackerel and pelagic goby, are important food items for seals (David 1987). In the 1930s, seals off southern Africa ate 0.1 million tons of food annually. This rose to 0.4 million tons in 1956 and 1.1 million tons by the 1980s (Crawford et al. 1992b).
The annual fish catch from the south-east Atlantic Ocean climbed from under 0.5 million tons in 1950 to around 2.5 million tons after 1966 (Crawford et al. 1987). Since 1965, the combined annual catch of sardine, anchovy and horse mackerel has been about 1.5 million tons in most years (Crawford et al. 1987).
SEABIRD RESPONSES TO LONG-TERM CHANGES IN THE FOOD BASE
African Penguin
Responses of African Penguins to regime shifts of sardine and anchovy in the Benguela system have been described by Crawford (in press). The African Penguin breeds at 24 islands and three mainland sites between Hollams Bird Island off central Namibia and Bird Island in Algoa Bay, South Africa (Crawford et al. 1995b).
Population trends and migration
As the sardine populations off Namibia and South Africa collapsed in the 1960s and early 1970s, penguin colonies between Lüderitz and Table Bay decreased. Colonies north of Lüderitz and southeast of Table Bay increased (Crawford et al. 1990), but the overall population of African Penguins decreased by 25% (Crawford in press). The increases in the north and southeast match the contraction of the distribution of sardines to the north of Namibia and the south of South Africa (Crawford et al. 1987; Lluch-Belda et al. 1989).
At Possession Island, the penguin colony decreased by 99% between 1956 and 1986 (Fig. 3). This decrease is similar to what might be expected from adult mortality had there been no recruitment of young adults to the colony (Cordes et al. in press). The increase of the penguin colony at Dyer Island between 1956 and 1967 (Fig. 4) would have necessitated a first-year survival of 0.88 in the absence of immigration (Shelton et al. 1984), substantially higher than any measurement yet of this parameter (Crawford et al. in press). Following the collapse of sardine, and the associated contraction in its range, it is likely that many first-time breeders emigrated from colonies between Lüderitz and Table Bay to colonies such as Dyer Island, where anchovy would have provided a reliable supply of food throughout most of the year (Crawford in press).
As the Benguela system started to revert to one dominated by sardine in the 1980s, penguin colonies between Lüderitz and Table Bay stabilised. Three new colonies were established in the vicinity of Table Bay. Those at Robben Island and Boulders have increased rapidly, matching the increase in the biomass of sardine off South Africa (Fig. 5). However, the colony at Dyer Island, southeast of Table Bay, underwent a massive decrease (Fig. 4). As a result, overall numbers of African penguins decreased by a further 19% (Crawford et al. 1995b; Crawford in press).
The recent decrease at Dyer Island can be attributed to adult mortality, if minimal recruitment to the breeding colony is assumed (Crawford in press). Trends in penguins at Dyer Island follow estimated trends in biomass of anchovy off South Africa (Fig. 4). The increase in the penguin colony at Robben Island is too rapid to result from local production, and mainly attributable to immigration of first-time breeders, mostly from Dyer Island (Crawford et al. in press). Again it appears that penguins have attempted to adjust to the switch from anchovy to sardine through immigration of first-time breeders to colonies where food is available at the time.
Diet
In the 1950s, sardine was the most important prey of African Penguins contributing 30-64% by mass of the diet off South Africa (Davies 1955; 1956; Rand 1960a) and 99% by mass of the diet off Namibia (Matthews 1961). In the late 1970s and 1980s, pelagic goby and cephalopods were the main prey of penguins off Namibia (Crawford et al. 1985); anchovy were off South Africa (Wilson 1985; Randall & Randall 1986; Crawford & Dyer 1995).
Cape Gannet
The Cape Gannet breeds at six islands off southern Africa: Mercury, Ichaboe and Possession islands in Namibia, and Bird (Lambert’s Bay), Malgas and Bird (Algoa Bay) islands in South Africa (Crawford et al. 1983).
Population trends
Cape Gannets decreased at all three Namibian colonies during and after the collapse of the Namibian sardine population (Crawford & Shelton 1981). The most severe relative decrease was at Possession Island (Fig. 6), the southernmost Namibian colony, and the least severe relative decrease at Mercury Island, the most northern colony. This corresponds with the contraction of the range of the Namibian sardine to the north as it decreased (Crawford et al. 1997). At Possession Island, the decrease between 1967 and 1978 was 12% per annum, approximating adult mortality (Nelson 1978, Crawford & Shannon unpublished information), and hence suggesting poor recruitment of new breeders to the colony then. At Ichaboe Island, the decrease 1978 and 1996 (10% per annum) was also similar to adult mortality and suggests minimal recruitment of new breeders to this colony after 1978.
In contrast to the decreases at the three Namibian islands, areas occupied by Cape Gannets increased between 1956 and 1996 at all three South African islands where the species breeds (Randall & Ross 1979, Crawford & Dyer 1995). At Lambert’s Bay and Malgas Island, most of the increase came after 1978 (Fig. 7 & Fig. 8). In Algoa Bay, there was steady growth of the gannet colony from 1956 (Randall & Ross 1979).
The overall area occupied by Cape Gannets at their six breeding localities decreased during the period of decreasing and low sardine abundance, and increased after the mid 1980s as sardine in the Benguela system began to increase again.
Migration
Klages (1994) noted little interchange of Cape Gannets between Bird Island in Algoa Bay and other colonies during 1978 to 1994. By contrast, off western southern Africa breeding by 59 Cape Gannets at an island other than their natal island was shown between 1978 and 1997. At least 27 other gannets that were seen at non-natal islands between three and nine times, over periods that sometimes exceeded six years, probably also transferred colonies (Crawford & Shannon unpublished information). Sixteen of the 86 demonstrated or supposed interchanges were birds that emigrated from Namibian colonies to Lambert’s Bay or Malgas Island; 41 moved from Lambert’s Bay to Malgas Island and 29 moved in the reverse direction from Malgas Island to Lambert’s Bay. All these movements were of birds that were banded as chicks. Two of them were observed breeding at both Lambert’s Bay and Malgas Island, but generally birds were faithful to their breeding locality once they initiated breeding.
Assuming an adult survival of 0.93 per annum, a mean survival during the first two years of life of 0.71 per annum, a mean age at first breeding of four years and a mean production of 0.74 chicks per breeding pair (Nelson 1978; Crawford et al. 1983; Crawford and Shannon unpublished information), colonies of Cape Gannets could expand at a rate of 9% per annum in the absence of immigration. The modelled increase at Lambert’s Bay between 1978 and 1987 (10% per annum) is greater. It seems likely that there was immigration to Lambert’s Bay from other colonies, probably the Namibian islands. At this time there appears to have been minimal recruitment of new breeders to Ichaboe Island. At Malgas Island, there was a sharp increase in the breeding population between 1985 and 1986 (Fig. 8). This again suggests immigration, although in 1985 Malgas Island became part of West Coast National Park and scraping of guano was not subsequently permitted (Crawford & Cochrane 1990). Availability of guano for nest construction may have encouraged more birds to breed in 1986.
Nelson (1978) considered there was large-scale interchange between widely separated colonies of North Atlantic Gannets Morus bassana, with some regional increases, e.g. that off south-west Britain, too rapid to explain in the absence of immigration. The Cape Gannet apparently shares with its North Atlantic counterpart the potential for first-time breeders to settle at new colonies. As with the African Penguin, this ability is likely to play an important role in ameliorating the impact on the overall population of a prolonged regional food shortage, such as that off Namibia following the collapse of the sardine there. It is worth noting that Cape Gannets may move from one colony to another when nesting habitat is severely reduced. Cape Gannets may have colonized Halifax and Possession islands in Namibia in the mid 19th century, after displacement from Ichaboe Island caused by harvesting of guano (Crawford et al. 1983).
In the 1950s and 1960s, recoveries of banded Cape Gannets were regularly reported from Angola north to the Gulf of Guinea, and as far west as Nigeria, but more recently no recoveries have been reported from this region although large numbers of birds have been banded (Oatley 1988). Political changes in what were previously west African colonial states almost certainly resulted in a diminished reporting rate, but are unlikely to be entirely responsible for the lack of recent records from the north as some reports of other banded birds from west Africa are still received (Oatley 1988). The recoveries from Angola northwards were mainly of first-year birds. The decrease in recoveries from this region followed the collapse of the Namibian sardine, and may indicate either much lower first-year survival or a changed dispersal of young birds.
Diet
In the 1950s, sardine formed 51-60% by mass of the diet of Cape Gannets off South Africa (Davies 1955; 1956; Rand 1959) and 93% by mass of their diet off Namibia (Matthews 1961). From 1978-1982, anchovy contributed 53% by mass of the diet off Namibia and sardine less than 1% (Crawford et al. 1985). From 1979-1990, sardine contributed 31% by mass of the food of Cape Gannets at Bird Island (Algoa Bay), saury Scomberesox saurus 27% and anchovy 24% (Klages et al. 1992).
The diet of the Cape Gannet has been monitored at Bird Island (Lambert’s Bay) and Malgas Island on a monthly basis since 1978 (Berruti et al. 1993). The biomass of anchovy was high at the start of this period, but decreased with fluctuations after 1988. Sardine began to increase in the 1980s. The contribution of anchovy to the diet of Cape Gannets is significantly correlated with survey estimates of anchovy abundance since these became available in 1984 (n = 14, r = 0.862, P < 0.001; Crawford & Dyer 1995; Fig. 9). The contribution of sardine to the diet of Cape Gannets reached its lowest level in 1982 and then started to increase again, following formation of the strong 1982/83 year class (Armstrong et al. 1987; Berruti et al. 1993; Fig. 10). It is significantly related to survey estimates of sardine biomass (n = 14, r = 0.719, P < 0.005).
In South Africa, the Cape Gannet has been able to cope with the recent change from anchovy to sardine, by switching its diet from one species to the other. From 1978-1982, anchovy contributed more than 50% of the diet off western South Africa, whereas sardine contributed more than 45% of the diet from 1993-1997.
Cape Cormorants
The Cape Cormorant breeds at 69 localities in southern Africa (Crawford 1997). In 1977-1981, 16 localities supported 99% of the population (Cooper et al. 1982). These include artificially-constructed platforms in central Namibia that provided alternative breeding localities after topographical changes in lagoons destroyed the isolation of natural breeding sites in the same vicinity (Cooper et al. 1982).
Population trend and movements
In Namibia, some 15000 pairs of Cape Cormorants bred in 1956 (Rand 1963, Cooper et al. 1982). This increased to 143000 in 1978 (Cooper et al. 1982). Numbers breeding decreased to fewer than 100000 pairs in 1988, 1993 and 1996 (Crawford, Cordes & Klages unpublished manuscript).
In South Africa, about 77000 pairs of Cape Cormorants bred in 1956. This increased to 104000 in 1978 (Cooper et al. 1982). More than 90000 pairs also bred in 1988 (Crawford 1991) and 1991 (Crawford & Dyer 1995). In 1989 and 1990 and from 1993-1996, fewer than 35000 pairs bred (Crawford & Dyer 1995; Crawford unpublished information).
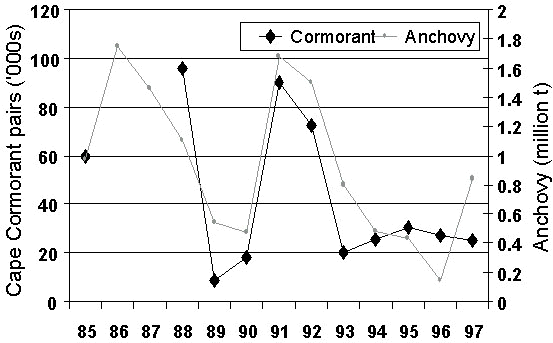
The trend for the entire population shows similarity to the trend in abundance of anchovy off South Africa (Fig. 11). Although, there is a variable proportion of Cape Cormorants breeding from year to year (Crawford & Dyer 1995), it seems that the population of Cape Cormorants increases when anchovy is abundant off southern Africa.
When anchovy is scarce, a decreased proportion of birds attempt breeding and many birds that start breeding desert their nests at an early stage resulting in low reproductive success (Crawford et al. 1992b). Off South Africa, the number of pairs that attempt breeding is significantly correlated with the biomass of anchovy off South Africa (Fig. 12, n = 10, r = 0.831, P < 0.005). Scarcity of food may precipitate high mortality of adults from disease (Crawford et al. 1992a).
Proportions of Cape Cormorants nesting at different islands off South Africa show substantial change between breeding seasons, suggesting considerable movement between different breeding colonies (Crawford et al. 1994).
Diet
In the 1950s, sardine formed 13-71% by mass of the food of Cape Cormorants off South Africa (Davies 1955, 1956, Rand 1960b) and 89% of its diet off Namibia (Matthews 1961). In 1973-1974, sardine contributed 84% by mass of the diet in Namibia and anchovy 15% (Berry 1976). In 1978-1980, the main diet at Namibian islands was pelagic goby (80% by mass; Crawford et al. 1985). From 1981 to 1985, pelagic goby was again the most important prey at Namibian islands, but anchovy was dominant at South African islands (Duffy et al. 1987). In all years between 1985 and 1992, except 1989, anchovy contributed at least half the diet of Cape Cormorants at South African islands and up to 99% by mass of the food eaten (Crawford & Dyer 1995).
DISCUSSION
The population parameters of long-lived seabirds buffer them against year-to-year variability in prey resources (Hunt et al. 1996). However, long-term (decadal-scale or greater) changes in food availability can result in drastic and rapid decreases of seabird colonies. This is shown, for example, in the decreases in African Penguins and Cape Gannets at Possession Island following the collapse of the Namibian sardine population (Fig. 3 & Fig. 6). Decadal-scale regimes of some populations of small pelagic fish are natural events, necessitating that seabird predators cope with such changes.
When there is a shift in the dominance of prey species, for example alternation in the relative abundance of sardine and anchovy, it is necessary for the seabird to be able to exploit the newly abundant prey organism. In Namibia, African Penguins at a number of colonies and Cape Gannets were unable to do this after the collapse of the sardine. Alternative prey species were either too deep in the water column, or beyond the foraging range of breeders (Crawford et al. 1985, 1987). Off South Africa, there was a different scenario. Cape Gannets effectively transferred their provisioning requirements from sardine to anchovy, and more recently back to sardine (Fig. 9 & Fig. 10). The South African gannet colonies have expanded. In the north-west Atlantic, Northern Gannets switched from warm-water to cold-water prey, at much the same time that Cape Gannets off western South Africa were switching from anchovy to sardine (Montevecchi & Myers 1997).
In addition to a change in the food species, the decadal changes in the food base often involve a change in the distribution of fish prey (Lluch-Belda et al. 1989; Alheit & Hagen 1997). A mechanism used by African Penguins and Cape Gannets to cope with this is for first-time breeders to emigrate from natal colonies to colonies where food is plentiful at the time. This was especially evident in the establishment of new colonies of African Penguins at Stony Point in 1982 (Broni 1982), Robben Island in 1983 (Crawford et al. 1995a) and Boulders in 1985 (Cooper 1985). Formation of these new colonies coincided to a remarkable extent with the start of the resurgence of sardine off South Africa that was initiated through the strong 1982/83 year class (Armstrong et al. 1987). The penguins reacted to this change well ahead of its detection by man. The immigration of young breeders to a colony may result in rapid growth of that colony (e.g. Fig. 5).
Once breeding, African Penguins have a high fidelity to mates (Randall 1983; Crawford et al. 1995a). On account of this they are generally sedentary in the vicinity of islands where they breed and are unlikely to move between islands (Randall et al. 1987). Similarly, Cape Gannets seldom breed at more than one colony. This means that the older and more experienced breeders have less flexibility than young birds to adapt to regime shifts in prey species. In long-lived seabirds, breeding success may increase with age and confidence in one’s mate. This has been shown for Short-tailed Shearwaters Puffinus tenuirostris, where improved breeding performance resulted from a bird’s cumulative breeding experience as well as its experience in breeding with a particular partner (Wooller et al. 1989). Established pairs need to return to the same locality to find each other.
Cape Cormorants move more freely between breeding localities than do African Penguins and Cape Gannets (Crawford et al. 1994). Adult survival (0.84 per annum; Crawford et al. 1992b) is lower than that of African Penguins (0.88-0.96; Randall 1983; Crawford et al. in press) or Cape Gannets (0.93; Crawford and Shannon unpublished information). This will reduce the lifespan of parents and decrease opportunity for accumulating experience with a particular mate. Conversely, the ability to move to different breeding localities provides flexibility to take immediate advantage of good feeding conditions that may arise. A probable younger age at breeding (about two years; Crawford unpublished information) and larger clutch (three eggs; Berry 1976) than African Penguins (4 years; 1.8 eggs; Crawford et al. in press) and Cape Gannets (four years; Crawford and Shannon unpublished information; one egg; Nelson 1978) gives Cape Cormorants the potential to increase more rapidly than the other two seabirds. They also decrease rapidly in periods of reduced availability of prey (e.g. Jackson 1930; 1931; 1932; 1933; 1934; 1935). Colonies of Cape Cormorants can be expected to vary to a greater degree than those of African Penguins and Cape Gannets.
The African Penguin has been decreasing throughout the 20th century. Early decreases were caused by exploitation of eggs and guano (Shelton et al. 1984). More recently production has been insufficient to balance mortality, the probable reason being insufficient food because of competition with fisheries and seals (Crawford in press). The Cape Gannet also decreased in the latter part of the 20th century. The main cause was the collapse of sardine off Namibia, and its non-replacement by an accessible alternative. This was probably brought about by excessive fishing of anchovy during the collapse of the sardine. Records of guano collected at Namibian islands (Crawford & Shelton 1978) indicate that earlier natural variability did not reduce colonies of Cape Gannets to the same extent as the collapse of the sardine in the 1970s. The population of Cape Cormorants increased during the period of anchovy dominance, but has decreased recently. The present population is thought to be roughly equivalent to that of the 1950s, when sardine was dominant and commercial purse-seine fisheries were developing.
ACKNOWLEDGEMENTS
Many contributed to gathering the information reported in this paper. I am grateful to all. I thank Sea Fisheries of Namibia and South Africa, Cape Nature Conservation, Eastern Cape Nature Conservation and National Parks Board of South Africa for permission to undertake the research, and for transport to and accommodation at the islands. The study was partly funded by the Sea Fishery Fund of South Africa. This paper is a product of SCOR WG-98.
REFERENCES
Alheit, J. & Hagen, E. 1997. Long-term climate forcing of European herring and sardine populations. Fish. Oceanogr. 6: 130-139.
Armstrong, M.J., Berruti, A. & Colclough, J. 1987. Pilchard distribution in South African waters, 1983-1985. In: Payne, A. I. L., Gulland, J. A. & Brink, K. H. (eds) The Benguela and comparable ecosystems. S. Afr. J. mar. Sci. 5: 871-886.
Baumgartner, T.R., Soutar, A. & Ferreira-Bartrina, V. 1992. Reconstruction of the history of Pacific sardine and northern anchovy populations over the past two millenia from sediments of the Santa Barbara Basin, California. Calif. Coop. Oceanic Ocean. Invest. Reps. 33: 24-40.
Benefit 1997. Benefit Science Plan. Windhoek; Benefit Secretariat: 90 pp.
Berruti, A., Underhill, L.G., Shelton, P.A., Moloney, C. & Crawford, R.J.M. 1993. Seasonal and interannual variation in the diet of two colonies of the Cape Gannet (Morus capensis) between 1977-78 and 1989. Colonial Waterbirds 16(2): 158-175.
Berry, H.H. 1976. Physiological and behavioural ecology of the Cape Cormorant Phalacrocorax capensis. Madoqua 9(4): 5-55.
Best, P.B., Crawford, R.J.M. & Van der Elst, R.P. 1997. Top predators in southern Africa’s marine ecosystems. Trans. Roy. Soc. S. Afr. 52(1): 177-225.
Broni, S.C. 1982. First recorded mainland breeding by the Jackass Penguin Spheniscus demersus. Cormorant 10(2): 120.
Butterworth, D.S. 1983. Assessment and management of pelagic stocks in the southern Benguela region. In: Sharp, G. D. & Csirke, J. (eds) Proceedings of the expert consultation to examine changes in abundance and species composition of neritic fish resources, San Jose, Costa Rica, April 1983. F. A. O. Fish. Rep. 291(2): 329-405.
Butterworth, D.S., Punt, A.E., Oosthuizen, W.H. & Wickens, P.A. 1995. The effects of future consumption by the Cape Fur Seal on catches and catch rates of the Cape hakes. 3. Modelling the dynamics of the Cape Fur Seal Arctocephalus pusillus pusillus. S. Afr. J. mar Sci. 16: 161-183.
Cairns, D.K. 1987. Seabirds as indicators of marine food supplies. Biological Oceanography 5: 261-271.
Cooper, J.1985. New breeding locality data for southern African seabirds. Jackass Penguin Spheniscus demersus. Cormorant 13(1): 81.
Cooper, J., Brooke, R.K., Shelton, P.A. & Crawford, R.J.M. 1982. Distribution, population size and conservation of the Cape Cormorant Phalacrocorax capensis. Fish. Bull. S. Afr. 16: 121-143.
Cooper, J., Williams, A.J. & Britton, P.L. 1984. Distribution, population sizes and conservation of breeding seabirds in the Afrotropical region. In: Croxall, J. P., Evans, P. G. H. & Schreiber, R. W. (eds) Status and conservation of the world’s seabirds. International Council for Bird Preservation Technical Publication 2: 403-419.
Cordes, I., Crawford, R.J.M., Williams, A.J. & Dyer, B.M. in press. Decrease of African Penguins at the Possession Island group, 1956-1995 - contrasting trends for colonial and solitary breeders. Marine Ornithology.
Crawford, R.J.M. 1991. Factors influencing population trends of some abundant vertebrates in sardine-rich coastal ecosystems. S. Afr. J. mar. Sci. 10: 365-381.
Crawford, R.J.M. 1997. Cape Cormorant. In: Harrison, J. A., Allan, D. G., Underhill, L. G., Herremans, M., Tree, A. J., Parker, V. & Brown, C. J. (eds) The atlas of southern African birds. Johannesburg; BirdLife South Africa: 32-33.
Crawford, R.J.M. in press. Responses of African Penguins to regime changes of sardine and anchovy in the Benguela system. S. Afr. J. mar. Sci.
Crawford, R.J.M., Allwright, D.M. & Heÿl, C.W. 1992a - High mortality of Cape Cormorants (Phalacrocorax capensis) off western South Africa in 1991 caused by Pasteurella multocida. Colon. Waterbirds 15(2): 236-238.
Crawford, R. J. M., Boonstra, H. G. v. D., Dyer, B. M. & Upfold, L. 1995a. Recolonization of Robben Island by African Penguins, 1983-1992. In: Dann, P., Norman, I. & Reilly, P. (eds) The penguins: ecology and management. Chipping Norton; Surrey Beatty & Sons: 333-363.
Crawford, R.J.M. & Cochrane, K. L. 1990. Onset of breeding by Cape Gannets Morus capensis influenced by availability of nesting material. Ostrich 61: 147-149.
Crawford, R.J.M. Cruickshank, R.A., Shelton, P.A. & Kruger, I. 1985. Partitioning of a goby resource amongst four avian predators and evidence for altered trophic flow in the pelagic community of an intense, perennial upwelling system. S. Afr. J. mar. Sci. 3: 215-228.
Crawford, R. J. M. & Dyer, B. M. 1995. Responses by four seabird species to a fluctuating availability of Cape anchovy Engraulis capensis off South Africa. Ibis 137: 329-339.
Crawford, R.J.M., Dyer, B. M. & Brooke, R. K. 1994. Breeding nomadism in southern African seabirds - constraints, causes and conservation. Ostrich 65(2): 231-246.
Crawford, R.J.M.,
Ryan, P.G. & Williams, A.J. 1991. Seabird consumption and production in the Benguela and Western Agulhas ecosystems. S. Afr. J. mar. Sci. 11: 357-375.Crawford, R.J.M., Shannon, L.J. & Whittington, P.A. in press. Population dynamics of the African Penguin Spheniscus demersus at Robben Island. Marine Ornithology.
Crawford, R.J.M., Shannon, L.V. & Pollock, D.E. 1987. The Benguela ecosystem. 4. The major fish and invertebrate resources. In: Barnes, M. (ed.) Oceanography and marine biology. An annual review 25. Aberdeen; University Press: 353-505.
Crawford, R.J.M., & Shelton, P.A. 1978. Pelagic fish and seabird interrelationships off the coasts of South West and South Africa. Biol. Conserv. 14: 85-109.
Crawford, R.J.M. & Shelton, P.A. 1981. Population trends for some southern African seabirds related to fish availability. In: Cooper, J. (ed.) Proceedings of the symposium on birds of the sea and shore, 1979. Cape Town; African Seabird Group: 15-41.
Crawford, R.J.M., Shelton, P.A., Cooper, J. & Brooke, R.K. 1983. Distribution, population size and conservation of the Cape Gannet Morus capensis. S. Afr. J. mar. Sci. 1: 153-174.
Crawford, R.J.M., Underhill, L.G., Raubenheimer, C.M., Dyer, B.M. & Märtin, J. 1992b. Top predators in the Benguela ecosystem - implications of their trophic position. In: Payne, A. I. L., Brink, K. H., Mann, K. H. & Hilborn, R. (eds) Benguela trophic functioning. S. Afr. J. mar. Sci. 12: 675-687.
Crawford, R.J.M., Williams, A.J., Hofmeyr, J.H., Klages, N.T.W., Randall, R.M., Cooper, J., Dyer, B.M. & Chesselet, Y. 1995b. Trends of African Penguin Spheniscus demersus populations in the 20th century. S. Afr. J. mar. Sci. 16: 101-118.
Crawford, R.J.M., Williams, A.J., Randall, R.M., Randall, B.M., Berruti, A. & Ross, G.J.B. 1990. Recent population trends of Jackass Penguins Spheniscus demersus off southern Africa. Biol. Conserv. 52(3): 229-243.
David, J.H.M. 1987. Diet of the South African Fur Seal (1974-1985) and an assessment of competition with fisheries in southern Africa. S. Afr. J. mar. Sci. 5: 693-713.
Davies, D.H. 1955. The South African pilchard (Sardinops ocellata). Bird predators, 1953-4. Investl Rep. Div. Fish. S. Afr. 18: 32 pp.
Davies, D.H. 1956. The South African pilchard (Sardinops ocellata) and maasbanker (Trachurus trachurus). Bird predators, 1954-55. Investl Rep. Div. Fish. S. Afr. 23: 40 pp.
Duffy, D.C., Wilson, R.P. & Wilson, M.P. 1987. Spatial and temporal patterns of diet in the Cape Cormorant off southern Africa. Condor 89: 830-834.
Hewitson, J.D. 1988. Catch trends in the multispecies pelagic fishery off Namibia in 1987. Colln scient. Pap. Int. Commn SE. Atl. Fish. 15(2): 7-17.
Hunt, G.L., Barrett, R.T., Joiris, C. & Montevecchi, W.A. 1996. Seabird/fish interactions: an introduction. In Hunt, G. L. & Furness, R. W. (eds) Seabird/fish interactions, with particular reference to seabirds in the North Sea. ICES Cooperative Research Report 216: 2-5.
Jackson, H. 1930. A valuable asset to the farmer: guano and other island products. Fmg S. Afr. 5: p. 428.
Jackson, H. 1931. The Government Guano Islands. Fmg S. Afr. 6: p. 359.
Jackson, H. 1932. The Government Guano Islands. Fmg S. Afr. 7: p. 380.
Jackson, H. 1933. Report on the Government Guano Islands. Fmg S. Afr. 8: p. 519.
Jackson, H. 1934. Report on the Government Guano Islands. Fmg S. Afr. 9: p. 581.
Jackson, H. 1935. The Government Guano Islands. Fmg S. Afr. 10: p. 596.
Klages, N. T. W. 1994. Dispersal and site fidelity of Cape Gannets Morus capensis. Ostrich 65(2): 218-224.
Klages, N.T.W., Willis, A.B. & Ross, G.J.B. 1992. Variability in the diet of the Cape Gannet at Bird Island, Algoa Bay, South Africa. In: Payne, A. I. L., Brink, K. H., Mann, K. H. & Hilborn, R. (eds) Benguela trophic functioning. S. Afr. J. mar. Sci. 12: 761-771.
Lluch-Belda, D., Crawford, R.J.M., Kawasaki, T., MacCall, A.D., Parrish, R.H., Schwartzlose, R.A. & Smith, P.E. 1989. World-wide fluctuations of sardine and anchovy stocks: the regime problem. S. Afr. J. mar. Sci. 8: 195-205.
Lluch-Belda, D., Schwartzlose, R.A., Serra, R., Parrish, R., Kawasaki, T., Hedgecock, D. & Crawford, R.J.M. 1992. Sardine and anchovy regime fluctuations of abundance in four regions of the world oceans: a workshop report. Fish. Oceanogr. 1: 339-347.
Matthews, J.P. 1961. The pilchard of South West Africa Sardinops ocellata and the marsbanker Trachurus trachurus. Bird predators, 1957-1958. Investl Rep. Mar. Res. Lab. S.W. Afr. 3: 35 pp.
Montevecchi, W.A. & Myers, R.A. 1997. Centurial and decadal oceanographic influences on changes in Northern Gannet populations and diets in the north-west Altantic: implications for climate change. ICES Journal of Marine Science 54: 608-614.
Nelson, J.B. 1978. The Sulidae. Gannets and boobies. Oxford; Oxford University Press.
Newman, G.G. & Crawford, R.J.M. 1980. Population biology and management of mixed-species pelagic stocks off South Africa. Rapp. P.-v. Reun. Cons. perm. int. Explor. Mer 177: 279-291.
Newman, G.G., Crawford, R.J.M. & Centurier-Harris, O.M. 1979. Fishing effort and factors affecting vessel performance in the South African purse-seine fishery, 1964-1972. Investl Rep. Sea Fish. Brch S. Afr. 120: 34 pp.
Oatley, T.B. 1988. Change in winter movements of Cape Gannets. In: MacDonald, I. & Crawford, R. J. M. (eds) Long-term data series relating to southern Africa’s renewable natural resources. S. Afr. Natl. Sci. Progr. Rep. 157: 40-42.
Rand, R.W. 1959. The biology of guano-producing sea-birds. The distribution, abundance, and feeding habits of the Cape Gannet, Morus capensis, off the south-western coast of the Cape Province. Investl Rep. Div. Fish. S. Afr. 39: 36 pp.
Rand, R.W. 1960a. The biology of guano-producing sea-birds. The distribution, abundance, and feeding habits of the Cape Penguin, Spheniscus demersus, off the south-western coast of the Cape Province. Investl Rep. Div. Fish. S. Afr. 41: 28 pp.
Rand, R.W. 1960b. The biology of guano-producing sea-birds. 3. The distribution, abundance, and feeding habits of the cormorants Phalacrocoracidae off the southwest coast of the Cape Province. Investl Rep. Div. Fish. S. Afr. 42: 32 pp.
Rand, R.W. 1963. The biology of guano-producing seabirds. 5. Composition of colonies on the South West African islands. Investl Rep. Div. Sea Fish. S. Afr. 46: 26 pp.
Randall, R.M. 1983. Biology of the Jackass Penguin Spheniscus demersus (L.) at St Croix Island, South Africa. PhD Thesis, University of Port Elizabeth, South Africa.
Randall, R.M. & Randall, B.M. 1986. The diet of Jackass Penguins Spheniscus demersus in Algoa Bay, South Africa, and its bearing on population declines elsewhere. Biol. Conserv. 37: 119-134.
Randall, R.M., & Ross, G.J.B. 1979. Increasing population of Cape Gannets on Bird Island, Algoa Bay, and observations on breeding success. Ostrich 50(3): 168-175.
Randall, R.M., Randall, B.M., Cooper, J., La Cock, G.D. & Ross, G.J.B. 1987. Jackass Penguin Spheniscus demersus movements, inter-island visits, and settlement. J. Fld Orn. 58(4): 445-455.
Shaughnessy, P.D. 1984. Historical population levels of seals and seabirds on islands off southern Africa, with special reference to Seal Island, False Bay. Investl Rep. Sea Fish. Res. Inst. 127: 61 pp.
Shelton, P.A., Crawford, R.J.M., Cooper, J. & Brooke, R.K. 1984. Distribution, population size and conservation of the Jackass Penguin Spheniscus demersus. S. Afr. J. mar. Sci. 2: 217-257.
Soutar, A. & Isaacs, J.D. 1974. Abundance of pelagic fish during the 19th and 20th centuries as recorded in anaerobic sediment off the Californias. Fishery Bull., Wash. 72(2): 257-273.
Wilson, R.P. 1985. Seasonality in diet and breeding success of the Jackass Penguin Spheniscus demersus. J. Orn., Lpz. 126(1): 53-62.
Wooller, R.D., Bradley, J.S., Skira, I.J. & Serventy, D.L. 1989. Short-tailed Shearwater. In: Newton, I (ed.) Lifetime reproduction in birds. London; Academic Press: 405-417.
Fig. 1. Catches of sardine and anchovy off South Africa, 1950-1997.

Fig. 2. Catches of sardine and anchovy off Namibia, 1950-1997.
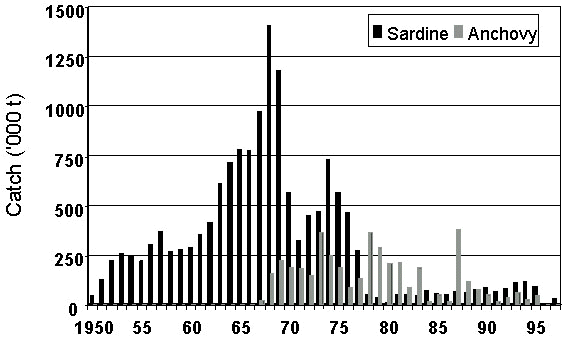
Fig. 3. Numbers of African Penguins at Possession Island compared with Namibia’s sardine catch, 1956-1996 (from information in Cordes et al. in press).
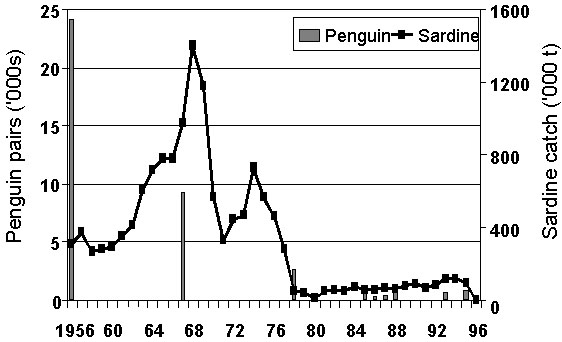
Fig. 4. Numbers of African Penguins at Dyer Island compared with estimates of the biomass of anchovy off South Africa, 1953-1997. The latter were obtained from a relationship between the contribution of anchovy to the diet of Cape Gannets off western South Africa and survey estimates of anchovy biomass for 1984-1997. Penguin abundance is updated from Crawford et al. (1995b).
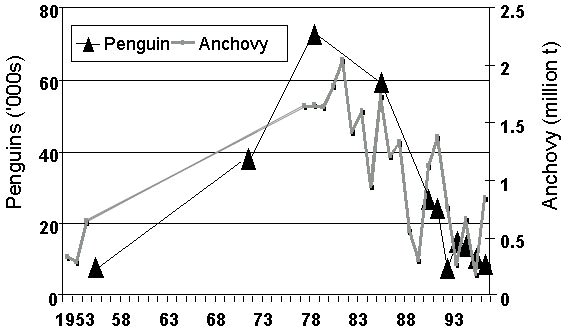
Fig. 5. Numbers of African Penguins at Robben Island compared with survey estimates of the biomass of sardine off South Africa, 1983-1997 (updated from Crawford et al. in press; M. Barange, Sea Fisheries Research Institute, South Africa unpublished information).
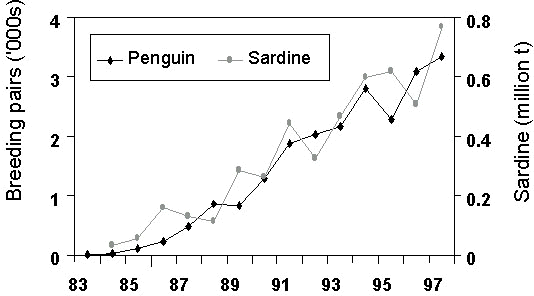
Fig. 6. Breeding area of Cape Gannets at Possession Island compared with Namibia’s sardine catch, 1956-1996 (from Crawford, Cordes and Klages unpublished manuscript).
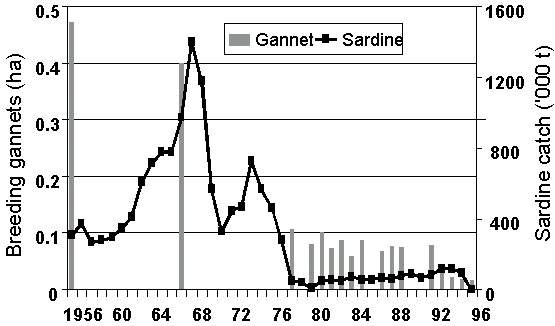
Fig. 7. Breeding area of Cape Gannets at Bird Island, Lambert’s Bay, 1956-1996 (updated from Crawford & Dyer 1995).
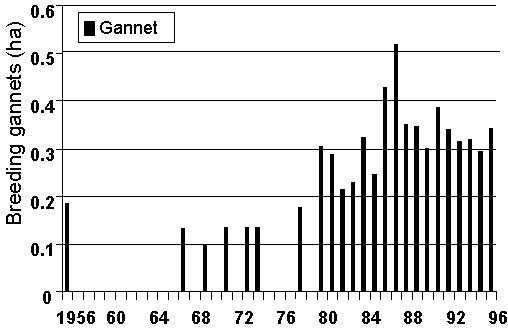
Fig. 8. Breeding area of Cape Gannets at Malgas Island, 1956-1996 (updated from Crawford & Dyer 1995).
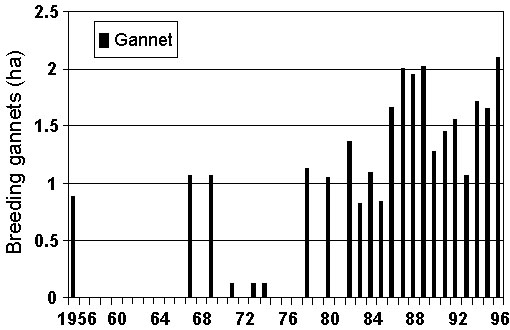
Fig. 9. The contribution of anchovy to the diet of Cape Gannets off western South Africa compared with survey estimates of the biomass of anchovy off South Africa, 1978-1997 (updated from Crawford & Dyer 1995; M. Barange, Sea Fisheries Research Institute, South Africa unpublished information).
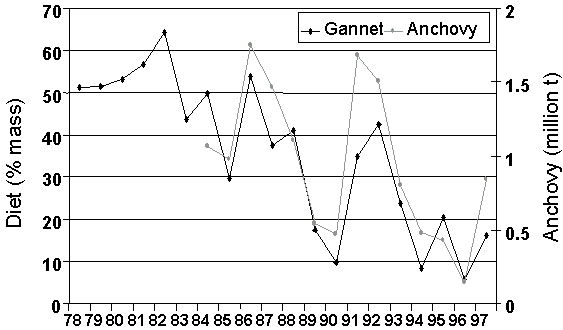
Fig. 10. The contribution of sardine to the diet of Cape Gannets off western South Africa compared with survey estimates of the biomass of sardine off South Africa, 1978-1997 (updated from Crawford & Dyer 1995; M. Barange, Sea Fisheries Research Institute, South Africa unpublished information).
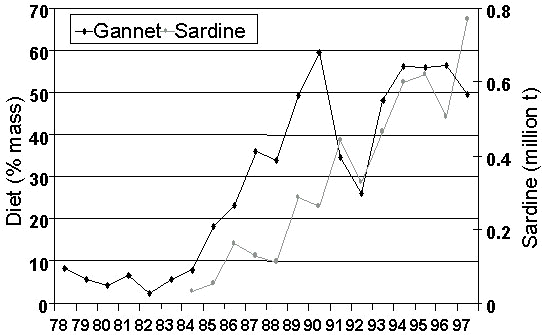
Fig. 11. Trends in the overall population of Cape Cormorants compared with estimates of the biomass of anchovy off South Africa, 1953-1996. The latter were obtained from a relationship between the contribution of anchovy to the diet of Cape Gannets off western South Africa and survey estimates of anchovy biomass for 1984-1997 (from Crawford, Cordes and Klages unpublished manuscript).
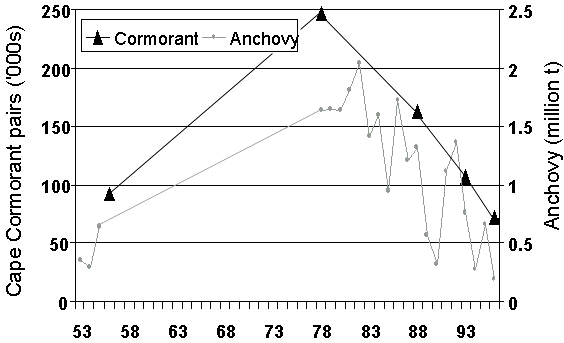
Fig. 12. Numbers of Cape Cormorants breeding off western South Africa compared with survey estimates of the biomass of anchovy off South Africa, 1985-1997 (updated from Crawford & Dyer in press).