S12.2: Are industrial fisheries a threat to seabird populations?
Robert W. Furness
Ornithology Group, Institute of Biomedical and Life Science, Graham Kerr Building, University of Glasgow, Glasgow G12 8QQ, UK, e-mail r.furness@bio.gla.ac.uk
Furness, R.W. 1999. Are industrial fisheries a threat to seabird populations? In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 676-687. Johannesburg: BirdLife South Africa.Many seabirds feed predominantly on abundant small shoaling pelagic fish that are targets of industrial fisheries, so seabirds might be in direct competition with industrial fisheries. Industrial fishing might reduce stock biomass, reduce the mean level or increase variability of recruitment, or alter food-web structure. Seabirds are generally long-lived, mostly producing few fledglings that will not recruit until several years old. Small pelagic fish exhibit short life spans, with early and highly variable recruitment, so seabird population sizes cannot track short-term changes in prey populations. Seabirds have a variety of buffering mechanisms to cope with such natural variations. These vary among species in strength and form. Theory predicts that seabird species would maintain high adult survival rates even in the face of moderately reduced fish abundances, but that breeding success and time budgets would show responses to food supply. Some data support this but in several cases even adult survival may be affected by reductions in pelagic fish stocks. Case studies, such as the North Sea Sandeel Ammodytes marinus fishery and Barents Sea Capelin Mallotus villosus fishery, provide detailed examples where effects of fishing might be expected. The lack of obvious effects of the North Sea Sandeel fishery on seabirds is highlighted and discussed.
INTRODUCTION
Although many oceanic or pelagic seabirds feed extensively on cephalopods or crustacea, most continental shelf and shallow seas seabirds feed predominantly on abundant, small, shoaling pelagic fish, at least during the breeding season (Furness and Monaghan 1987, Montevecchi 1993, Springer and Speckman 1997).
Small shoaling fish species are often targets of industrial fisheries for production of fish meal and oils. The removal of large quantities of these fish by industrial fisheries might reduce food supply to seabirds. One frequently quoted example of this has been in Peru, where the collapse of the heavily fished Peruvian Anchovy Engraulis ringens stock resulted in a dramatic decrease in numbers of guano seabirds (Duffy 1983).
Considered in abstract terms, industrial fishing could hypothetically affect seabird populations by a number of distinct processes. Fishing might reduce stock biomass, so that the prey density available to foraging seabirds, during the seasonal period of the fishery or subsequently, might fall below levels that would support high breeding success or survival. Such an effect would depend on whether seabirds require a certain minimum prey density to be available before foraging would be economic. Over a longer term, fishing might reduce the mean level, or increase variability, of recruitment into the fished stock. Such an effect would come about if fishing reduced spawning stock biomass and this reduction affected the level of recruitment of young fish. Thus the form of any relationship between spawning stock biomass and recruitment is of fundamental interest with regard to possible effects of such fisheries on seabird food supply. Finally, industrial fishing might alter food-web structure by affecting the competitive balance between fished and unfished, or between heavily fished and lightly fished stocks. For example, the relative abundance of two ecologically similar small planktivorous fish species might change such that species A decreased and species B increased over a period of heavy fishing on species A. If species A was the staple prey of seabirds while species B was unavailable to them or uneconomic, this change in community composition could have an adverse effect on seabirds, whereas if species B was the staple prey of seabirds, then a high fishing effort on species A might increase food availability to the seabirds and could result in an increase of seabird numbers as a result.
Seabirds are generally long-lived, mostly producing few fledglings that will only recruit if they survive to several years old (Furness and Monaghan 1987). Thus their populations can only increase slowly, and except in rare instances where mass mortality of adults occurs, they also usually decrease in numbers slowly. In stark contrast, small pelagic fish exhibit short life spans, with early and highly variable recruitment. Their populations therefore tend to fluctuate rapidly, and rather unpredictably, in abundance. Seabird population sizes cannot track short-term changes in prey population abundance. Thus, seabirds have a variety of buffering mechanisms to cope with such natural variations in food supply. These vary among species in strength and form (Furness 1996, Phillips et al. 1996a). Theory predicts that the most vulnerable seabird species to reductions in pelagic food supplies would be small surface-feeding seabird species with specialised and energetically expensive foraging methods and little spare time to allow any increase in foraging effort (Furness and Ainley 1984). An obvious example of such a seabird is the Arctic Tern Sterna paradisaea. This prediction is supported by case studies; Arctic Terns and Kittiwakes Rissa tridactyla in Shetland were particularly severely affected by the reduction in Sandeel stocks there in the late 1980s, whereas some of the larger seabirds continued to breed successfully despite the decline in Sandeel abundance (Heubeck 1989, Furness and Barrett 1991). Theory also predicts that seabird species would maintain high adult survival rates even in the face of moderately reduced fish abundances, but that breeding success and time budgets would show responses to food supply (Cairns 1987).
Some data support this (Hamer et al. 1991, 1993, Phillips et al. 1996b, Furness and Camphuysen 1997, Harris and Wanless 1997) but in several cases even adult survival may be affected by reductions in pelagic fish stocks (Vader et al. 1990, Hamer et al. 1991, Harris and Bailey 1992, Krasnov and Barrett 1995, Barrett and Krasnov 1996).
Major industrial fisheries in Europe include the fisheries for Capelin Mallotus villosus in the Barents Sea (Gjøsæter 1997) and for Sandeels Ammodytes marinus in the North Sea (Gislason and Kirkegaard 1996). These two fish species are extremely abundant lipid-rich fish that are a major part of the diet of many seabirds, so seabirds might be in direct competition with industrial fisheries for these resources (Furness and Barrett 1991, Monaghan 1992). The fact that these fisheries are well documented and that there are very detailed data on the numbers and breeding success of seabirds in these regions, means that these cases provide the best opportunity to detect any effects of industrial fishing on seabird populations.
The Barents Sea Capelin stock has a historical biomass of 6-10 million tonnes, and serves as a food supply for Cod Gadus morhua, whales, seals and seabirds (Gjøsæter 1997). It supported an industrial fishery taking 1-3 million tonnes of Capelin between 1973 and 1984, but the Capelin stock collapsed to 20,000 t in 1987, recovered rather rapidly until 1992 but then collapsed again in 1993-95 (Gjøsæter 1997). Although the industrial fishery contributed to the first collapse by removing fish from a rapidly declining stock, the main cause of the collapse was high predation levels from increased stocks of Cod (Bogstad and Mehl 1997). Quantities of Capelin taken by seabirds (Mehlum and Gabrielsen 1995) were very small by comparison to quantities taken by Cod, marine mammals or the industrial fishery (Gjøsæter 1995, 1997), but the reduction in Capelin abundance resulted in an 80% decrease in numbers of common Guillemots Uria aalge in 1985-87, apparently as a result of starvation leading to mortality of young and adult birds in winter (Vader et al. 1990, Krasnov and Barrett 1995, Anker-Nilssen et al. 1997). However, some seabirds showed surprisingly little response to this huge decrease in Capelin stock. For example, seabirds on Hørnøya, north Norway, continued to achieve high breeding success and fed predominantly on Capelin during the period of minimum stock (Barrett and Furness 1990), possibly exploiting a local fjordic stock of Capelin rather than the Barents Sea stock.
In the North Sea, many fish stocks are very heavily exploited. An industrial fishery developed during the 1950s, first harvesting young Herring Clupea harengus, then taking Mackerel Scomber scombrus, and after these stocks had collapsed, fishing mainly for Norway Pout Trisopterus esmarkii and Sandeels. The Sandeel has become the main target of industrial fishing in the North Sea. The Kittiwake is one of the most abundant and widespread breeding seabirds in Europe, and it feeds very extensively on Sandeels during the breeding season (Furness 1990, Harris and Wanless 1997). Many features of its biology suggest that it should be particularly vulnerable to reductions in food supply, such as its surface feeding habits, the fact that one adult is always present to protect the nest site, its relatively small size, high foraging costs (prolonged flapping flight) and specialised diet (Furness and Ainley 1984), and empirical data confirm that Kittiwake breeding success is strongly affected by food abundance (Harris and Wanless 1990, 1997, Hamer et al. 1993). Thus the Kittiwake is the most obvious seabird to study in relation to effects of industrial fishing for Sandeels on seabirds in the North Sea. Relationships between Kittiwakes, Sandeel stocks and the Sandeel fishery are explored below.
METHODS
Data on the stock biomass and abundances of age classes of Sandeels, and of landings of Sandeels from the North Sea were obtained from the literature, especially from the International Council for the Exploration of the Sea (ICES 1997a). Data on Kittiwake numbers and breeding success at sites around the British Isles were obtained from the annual reports of the Joint Nature Conservation Committee (JNCC) Seabird Monitoring Programme. Estimates of annual consumption of Sandeels by predators in the North Sea were obtained from Tasker and Furness (1996) and Furness and Tasker (1997) for seabirds and from the ICES Multispecies Assessment Working Group (ICES 1997b) for predatory fish.
Relationships between Kittiwake breeding success and Sandeel stock sizes were examined by correlation analysis. One-tailed tests of significance were used since only positive correlations between success and food abundance were anticipated. Since the Shetland Sandeel stock has been considered to be distinct from the Sandeel stock in the rest of the North Sea (Bailey et al. 1991) the data for Shetland have been examined separately from those for the rest of the North Sea.
RESULTS
Sandeel catches in the North Sea
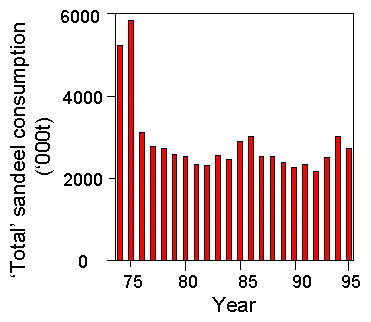
Sandeel catch by the North Sea industrial fisheries increased from a low level in the late 1950s up to a peak of 1039000 t in 1989 (Fig. 1). From the mid-1970s it has been consistently above 500000 t but has increased very little, whereas before the mid-1970s the Sandeel catch was below that level. The mean annual Sandeel catch during 1986-95 was significantly higher than during 1975-85 (t19=3.51, P<0.05). Thus any effects on Kittiwake breeding numbers or breeding success might be expected to become most evident after 1986.
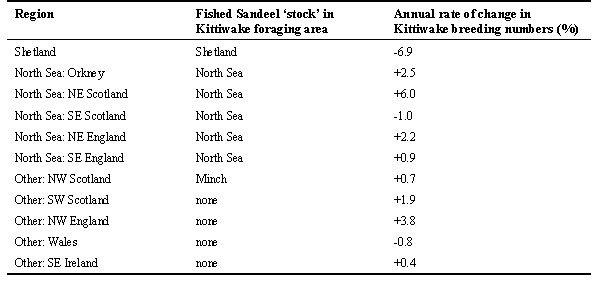
Changes in numbers of KittiwakesKittiwake breeding numbers have increased since the turn of the century in all areas of the British Isles. Between national censuses in 1969 and 1987, the increase in Kittiwake breeding numbers was high on the east coasts of England (+167%) and Scotland (+44%), but low in Shetland, Orkney and much of western Britain and Ireland (Lloyd et al. 1991). Between 1986 and 1995, rate of annual change in breeding Kittiwake numbers at selected monitoring colonies (Thompson et al. 1997) showed no difference (t8=0.66, n.s., Table 1) between regions on the North Sea coast, which are potentially affected by the North Sea Sandeel fishery (mean rate of increase 2.12% p.a., s.d. 2.57) and regions to the west of the British Isles, where Sandeel fishing is negligible or nonexistent (mean rate of increase 1.2% p.a., s.d. 1.74), but Kittiwake numbers have been decreasing at Shetland (-6.9% p.a.) which is a faster decline than seen in any other region, consistent with the collapse of the Shetland Sandeel stock during the late 1980s.
Breeding success of KittiwakesKittiwake productivity monitoring data for the years 1986-96 show that the mean breeding productivity in Shetland (mean 0.53 chicks per nest, s.d. 0.28) was significantly lower than in other regions (mean 0.84 chicks per nest, s.d. 0.29) of the British Isles (t64=3.27, P<0.05). However, excluding Shetland, breeding success was significantly higher at colonies adjacent to the North Sea Sandeel fishery (mean 0.97 chicks per nest, s.d. 0.28) than in colonies in western parts of the British Isles (mean 0.65 chicks per nest, s.d. 0.20) (t53=4.57, P<0.05).
Breeding productivity of Kittiwakes monitored in Shetland (1986-96) showed a significant correlation with the (log transformed) number of 1-group Sandeels caught at Shetland per 30 minute survey tow (r9=0.75, P<0.05), and with the (log transformed) total of 1- plus 2- plus 3-group Sandeels caught per 30 minute tow (r9=0.77, P<0.05) (Fig. 2).
Over the period 1986-95, breeding productivity of Kittiwakes in Orkney showed a significant correlation with VPA estimated numbers of 1-group Sandeels in the North Sea (r8=0.46, P<0.05), as did breeding productivity of Kittiwakes in east Scotland (r8=0.42, P<0.05), and in east England (r8=0.49, P<0.05). However, there were no significant correlations between VPA estimated numbers of 1-group Sandeels in the North Sea and breeding productivity of Kittiwakes in southwest Britain and southeast Ireland (r8=0.09, n.s.) or in west Scotland and northern Ireland (r8=0.01, n.s.).
Consumption of Sandeels by major predators and the industrial fishery
The ICES Multispecies Assessment Working Group (ICES 1997b) estimated that over the last three decades, Mackerel, Whiting Merlangius merlangus, Haddock Melanogrammus aeglefinus, gurnards and the industrial fishery were the largest consumers of Sandeels in the North Sea, but the amounts taken by these consumers varied considerably over years, primarily as a result of changes in predator population sizes. In particular, the North Sea stock of Mackerel collapsed in the early 1970s and has failed to recover since, so that the mass of Sandeels consumed by North Sea Mackerel has fallen dramatically, from almost two million tonnes in 1974 to less than 100,000 t each year from 1986-93 (Fig. 3). In addition, the Atlantic stock ‘western Mackerel’ migrates into the northern North Sea to a variable extent from year to year and consumes North Sea Sandeels. Consumption by this stock is variable across years as a result (Fig. 4).
Consumption of Sandeels by other predators is estimated to be much less than by Mackerel. Gurnards are one of the major predators of Sandeels but their consumption shows no trend over the period of interest. However, there has been a downward trend in consumption by Whiting and by Haddock as these stocks have decreased from the 1970s to the 1990s. During this period, however, the industrial catch of Sandeels has grown. Adding together the industrial catch with the consumption by Mackerel (North Sea and western stocks when in the North Sea), Whiting, Haddock and seabirds, the summed consumption of Sandeels (Fig. 5) shows virtually no overall change from 1976 to 1995, but with exceptionally high levels in 1974 and 1975 as a result of particularly high consumption by western Mackerel in those years.
DISCUSSION
The decline in breeding numbers of Kittiwakes at Shetland since 1986 contrasts with the general increasing trend in most other areas of the British Isles (Table 1). This decline has been attributed to predation of both Kittiwake chicks and adults by Great Skuas Catharacta skua (Heubeck et al. 1997) as a result of reduced Sandeel abundance in the late 1980s. Thus low stocks of Sandeels can impact Kittiwakes both directly by reducing breeding success through food shortage (Fig. 2, see also Hamer et al. 1993, Harris and Wanless 1997), but also through the indirect effect of increased predator impact (see also Regehr and Montevecchi 1997). The decrease in Sandeel abundance at Shetland had little or no effect on the breeding success of Common Guillemots, but did affect their population during winter (Heubeck et al. 1991), so the timings and mechanisms of effects of low food abundance can vary from species to species.
In contrast to the clear effect of reduced Sandeel abundance on Kittiwakes in Shetland, Kittiwakes breeding on the east of Britain, which might be expected to display responses to changes in Sandeel stocks through the rest of the North Sea, showed no difference in mean population growth rate from Kittiwakes monitored at colonies on the west of Britain and in Ireland. Furthermore, sustained high catches of Sandeels since 1986 occurred as Kittiwake numbers at the monitoring colonies continued to increase on the North Sea coast. Breeding success of Kittiwakes on the North Sea coast was significantly higher than on the west of Britain and Ireland, suggesting that food supply to Kittiwakes in the North Sea was at least as good as elsewhere despite the industrial fishery. One possible reason for this result may be the fact that the quantity of Sandeels removed by the industrial fishery is less than the quantity that used to be removed by major fish predators. Since the stocks of these major fish predators have fallen, the total consumption of Sandeels has remained almost constant since 1976 (Fig. 5). Given that the Mackerel stocks were much higher during the late 1960s and early 1970s, it seems that Sandeel consumption by fish predators would have been much higher before 1976, so that the industrial fishery appears to have filled a niche vacated by North Sea Mackerel when their stock collapsed. These data suggest that the Mackerel is a keystone predator in the North Sea pelagic food web, and that removal of the Mackerel has improved the food supply to seabirds. Whether recovery of Mackerel stocks in the North Sea would be compatible with a sustained industrial fishery and continued historically high populations of seabirds is unknown, but seems unlikely.
Environmental changes can affect seabird populations through ‘bottom-up’ effects on foodwebs (Ainley et al. 1995). Aebischer et al. (1990) showed that Kittiwakes, as well as marine organisms at other trophic levels, responded to long term variations in environmental conditions in the North Sea. Changes in Sandeel abundance in Shetland during the late 1980s are thought to have been driven by changes in oceanographic patterns affecting recruitment of Sandeels at Shetland, and not by the industrial fishery for Shetland Sandeels (Wright 1996). There is evidence that seabird distribution at sea can be a response to Sandeel distribution (Wright and Begg 1997), but over recent decades, the industrial fishery for Sandeels in the North Sea has tended to harvest predominantly from areas of the North Sea where seabird foraging densities are low (Wright et al. 1997). This comes about because most feeding on Sandeels by seabirds occurs during the breeding season, when seabirds are constrained to forage in the vicinity of their colonies, and the majority of seabirds breeding in the North Sea are found in the northwestern sector, where industrial fishing for Sandeels has traditionally been very slight. The possibility that the industrial fishery may move into areas characterised by high breeding aggregations of seabirds could result in local depletion of Sandeel stocks to the detriment of local seabird populations.
The fact that breeding performance of Kittiwakes in Orkney, in east Scotland and in east England each show significant correlation with VPA estimated numbers of 1-group Sandeels in the North Sea is noteworthy. Firstly, the Kittiwakes in Orkney will not be foraging in the southern North Sea while breeding; they will only be sampling Sandeels from relatively close to Orkney (data from Shetland and the Isle of May suggest that Kittiwakes generally forage within 50 km at most from colonies while rearing chicks). Although traditionally the North Sea Sandeel stock is treated as a single stock, it is likely that there are separate stocks with somewhat independent dynamics in different parts of the North Sea. These broad correlations between Kittiwake performance and aggregated Sandeel data for all the North Sea suggest that Sandeel stock dynamics are fairly coherent from Orkney to east England at least. If spatially resolved Sandeel stock data were available on a scale more similar to the foraging ranges of Kittiwakes one might expect correlations to be even clearer. The possibility that Kittiwake breeding performance may provide clues as to the number of Sandeel stocks around the British Isles deserves further consideration, alongside molecular investigations to discriminate Sandeel stocks.
Although similar analyses have not yet been performed for other seabird species, one may predict that the breeding performance of many other species will show less response to sandeel abundance because they are more strongly buffered against changes in food supply, for example through having the ability to dive to considerable depth to search for fish (as in auks), through having more of their daily activity budget spent in resting so they can increase work rate (as in shags Phalacrocorax aristotelis), through being able to switch to alternative foods (as in gannets Morus bassanus) (Furness and Monaghan 1987). Possibly terns might show stronger effects of changes in food supply, since they appear to be more susceptible than kittiwakes (Furness and Ainley 1984), but their population biology is more difficult to monitor since they have a tendency to move colony site and to be highly sensitive to human disturbance (Lloyd et al. 1991).
ACKNOWLEDGEMENTS
This work was carried out under sponsorship from IFOMA. The Secretary General of ICES and Dr. J. Rice (Chair, ICES Multispecies Assessment Working Group) kindly gave permission to use ICES data on Sandeel stocks and on multispecies assessment.
REFERENCES
Aebischer, N.J., Coulson, J.C. & Colebrook, J.M. 1990. Parallel long-term trends across four marine trophic levels and weather. Nature 347: 753-755.
Ainley, D.G., Sydeman, W.J. & Norton, J. 1995. Upper trophic level predators indicate interannual negative and positive anomalies in the California Current food web. Marine Ecology Progress Series 118: 69-79.
Anker-Nilssen, T., Barrett, R.T. & Krasnov, J.V. 1997. Long- and short-term responses of seabirds in the Norwegian and Barents Seas to changes in stocks of prey fish. Proceedings Forage Fishes in Marine Ecosystems. Anchorage; Alaska Sea Grant College Program: 683-698.
Bailey, R.S., Furness, R.W., Gauld, J.A. & Kunzlik, P.A. 1991. Recent changes in the population of the Sandeel (Ammodytes marinus Raitt) at Shetland in relation to estimates of seabird predation. ICES marine Science Symposia 193: 209-216.
Barrett, R.T. & Furness, R.W. 1990. The prey and diving depths of seabirds on Hørnøy, north Norway after a decrease in Barents Sea Capelin stocks. Ornis Scandinavica 21: 179-186.
Barrett, R.T. & Krasnov, J.V. 1996. Recent responses to changes in fish stocks of prey species by seabirds breeding in the southern Barents Sea. ICES Journal of Marine Science 53: 713-722.
Bogstad, B. & Mehl, S. 1997. Interactions between Atlantic Cod (Gadus morhua) and its prey species in the Barents Sea. Proceedings Forage Fishes in Marine Ecosystems. Anchorage; Alaska Sea Grant College Program: 591-615.
Cairns, D.K. 1987. Seabirds as indicators of marine food supplies. Biological Oceanography 5: 261-271.
Duffy, D.C. 1983. Environmental uncertainty and commercial fishing: effects on Peruvian guano birds. Biological Conservation 26: 227-238.
Furness, R.W. 1990. A preliminary assessment of the quantities of Shetland Sandeels taken by seabirds, seals, predatory fish and the industrial fishery in 1981-83. Ibis 132: 205-217.
Furness, R.W. 1996. A review of seabird responses to natural or fishery-induced changes in food supply. In Greenstreet, S.P.R. and Tasker, M.L. (Eds.) Aquatic Predators and their Prey. Oxford, Fishing News Books, 166-173.
Furness, R.W. & Ainley, D.G. 1984. Threats to seabird populations presented by commercial fisheries. ICBP Technical Report 2: 701-708.
Furness, R.W. & Barrett, R.T. 1991. Seabirds and fish declines. National Geographic Research and Exploration 7: 82-95.
Furness, R.W. & Camphuysen, C.J. 1997. Seabirds as monitors of the marine environment. ICES Journal of Marine Science 54: 726-737.
Furness, R.W. & Monaghan, P. 1987. Seabird ecology. Glasgow; Blackie.
Furness, R.W. & Tasker, M.L. 1997. Seabird consumption in Sand Lance MSVPA models for the North Sea, and the impact of industrial fishing on seabird population dynamics. Proceedings Forage Fishes in Marine Ecosystems. Anchorage; Alaska Sea Grant College Program: 147-169.
Gislason, H. & Kirkegaard, E. 1996. The industrial fishery and the North Sea Sandeel stock. Seminar on the Precautionary Approach to North Sea Fisheries Management, Oslo, 9-10 September 1996.
Gjøsæter, H. 1995. Pelagic fish and the ecological impact of the modern fishing industry in the Barents Sea. Arctic 48: 267-278.
Gjøsæter, H. 1997. The Barents Sea Capelin stock (Mallotus villosus): A brief review. Proceedings Forage Fishes in Marine Ecosystems. Anchorage; Alaska Sea Grant College Program: 469-484.
Hamer, K.C., Furness, R.W. & Caldow, R.W.G. 1991. The effects of changes in food availability on the breeding ecology of Great Skuas in Shetland. Journal of Zoology, London 223: 175-188.
Hamer, K.C., Monaghan, P., Uttley, J.D., Walton, P. and Burns, M.D. 1993. The influence of food supply on the breeding ecology of Kittiwakes Rissa tridactyla in Shetland. Ibis 135: 255-263.
Harris, M.P. & Bailey, R.S. 1992. Mortality rates of Puffin Fratercula arctica and Guillemot Uria aalge and fish abundance in the North Sea. Biological Conservation. 60: 39-46.
Harris, M.P. & Wanless, S. 1990. Breeding success of Kittiwakes Rissa tridactyla in 1986-88: Evidence for changing conditions in the northern North Sea. Journal of Applied Ecology 27: 172-187.
Harris, M.P. & Wanless, S. 1997. Breeding success, diet, and brood neglect in the Kittiwake (Rissa tridactyla) over an 11-year period. ICES Journal of Marine Science 54: 615-623.
Heubeck, M. 1989. Seabirds and Sandeels: proceedings of a seminar held in Lerwick, Shetland, 15-16 October 1988. Lerwick, Shetland Bird Club, 35pp.
Heubeck, M., Harvey, P.V. & Okill, J.D. 1991. Changes in the Shetland Guillemot Uria aalge population and the patTern of recoveries of ringed birds, 1959-1990. Seabird 13: 3-21.
Heubeck, M., Mellor, R.M. & Harvey, P.V. 1997. Changes in the breeding distribution and numbers of Kittiwakes Rissa tridactyla around Unst, Shetland and the presumed role of predation by Great Skuas Stercorarius skua. Seabird 19: 12-21.
ICES 1997a. Report of the Working Group on the assessment of Demersal Stocks in the North Sea and Skagerrak, ICES headquarters 7-15 October 1996. ICES CM1997/Assess:6, 633 pp.
ICES 1997b. Report of the multispecies assessment working group, ICES headquarters 11-19 August 1997. ICES CM1997/Assess:16, 235pp.
Krasnov, J.V. & Barrett, R.T. 1995. Large-scale interactions among seabirds, their prey and humans in the southern Barents Sea. In: Skjoldal, H.R., Hopkins, C., Erikstad, K.E. and Leinaas, H.P. (Eds.) Ecology of fjords and coastal waters. Elsevier Science B.V. 443-456.
Lloyd, C, Tasker, M.L. & Partridge, K. 1991. The status of seabirds in Britain and Ireland. London, Poyser, 355pp.
Mehlum, F. & Gabrielsen, G.W. 1995. Energy expenditure and food consumption by seabird populations in the Barents Sea region. In: Skjoldal, H.R., Hopkins, C., Erikstad, K.E. and Leinaas, H.P. (Eds.) Ecology of fjords and coastal waters. Elsevier Science B.V. 457-470.
Monaghan, P. 1992. Seabirds and Sandeels: the conflict between exploitation and conservation in the northern North Sea. Biodiversity and Conservation 1: 98-111.
Montevecchi, W.A. 1993. Birds as indicators of change in marine prey stocks. In: Furness, R.W. and Greenwood, J.J.D. (Eds.) Birds as monitors of environmental change. London; Chapman and Hall. 217-265.
Phillips, R.A., Furness, R.W. & Caldow, R.W.G. 1996a. Behavioural responses of Arctic Skuas Stercorarius parasiticus to changes in Sandeel availability. In Greenstreet, S.P.R. and Tasker, M.L. (Eds.) Aquatic Predators and their Prey. Oxford, Fishing News Books, 17-25.
Phillips, R.A., Caldow, R.W.G. and Furness, R.W. 1996b. The influence of food availability on the breeding effort and reproductive success of Arctic Skuas Stercorarius parasiticus. Ibis 138: 410-419.
Regehr, H.M. & Montevecchi, W.A. 1997. Interactive effects of food shortage and predation on breeding failure of Black-legged Kittiwakes: indirect effects of fisheries activities and implications for indicator species. Marine Ecology Progress Series 155: 249-260.
Springer, A.M. & Speckman, S.G. 1997. A forage fish is what? Summary of the symposium. Proceedings Forage Fishes in Marine Ecosystems. Anchorage; Alaska Sea Grant College Program: 773-794.
Tasker, M.L. & Furness, R.W. 1996. Estimation of food consumption by seabirds in the North Sea. In: Hunt, G.L. Jr. and Furness, R.W. (Eds.) Seabird/fish interactions, with particular reference to seabirds in the North Sea. ICES Cooperative Research Report No. 216. Copenhagen; InTernational Council for the Exploration of the Sea: 6-42.
Thompson, K.R., Brindley, E. & Heubeck, M. 1997. Seabird numbers and breeding success in Britain and Ireland, 1996. Peterborough; Joint Nature Conservation Committee: 64pp.
Vader, W., Barrett, R.T., Erikstad, K.E. & Strann, K.B. 1990. Differential responses of Common and Thick-billed Murres to a crash in the Capelin stock in the southern Barents Sea. Studies in Avian Biology 14: 175-180.
Wright, P. 1996. Is there a conflict between Sandeel fisheries and seabirds? A case study at Shetland. In Greenstreet, S.P.R. and Tasker, M.L. (Eds.) Aquatic Predators and their Prey. Oxford, Fishing News Books, 154-165.
Wright, P., Barrett, R.T., Greenstreet, S.P.R., Olsen, B. & Tasker, M. 1997. Effect of fisheries for small fish on seabirds in the easTern Atlantic. In: Hunt, G.L. Jr. and Furness, R.W. (Eds.) Seabird/fish interactions, with particular reference to seabirds in the North Sea. ICES Cooperative Research Report No. 216. Copenhagen; International Council for the Exploration of the Sea: 44-55.
Wright, P.J. & Begg, G.S. 1997. A spatial comparison of Common Guillemots and Sandeels in Scottish waters. ICES Journal of Marine Science 54: 578-592.
Table 1. Rates of growth of Kittiwake breeding numbers from 1986-95 in monitoring areas of colonies in different regions around the British Isles (data from Thompson et al. 1997).

Fig 1. Sandeel landings from the North Sea each year from 1961 to 1995 (data from ICES CM1992/Assess:9 and ICES Cooperative Research Report 221).
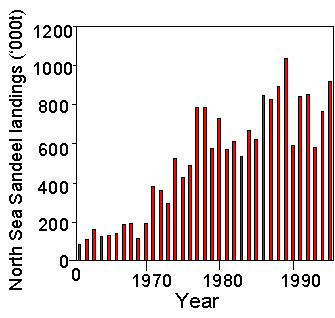
Fig 2. Breeding performance of Kittiwakes (chicks per nest) in monitoring plots at Shetland colonies in relation to the natural logarithm of the numbers of Sandeels (thousands) of 1-, 2- and 3-group fish caught per 30 minute tow (data on Kittiwake breeding from Thompson et al. 1997; data on Sandeel abundance from ICES 1997a).
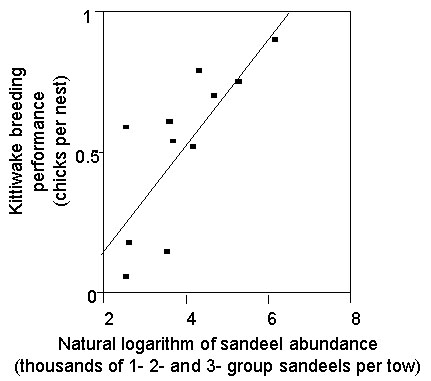
Fig 3 . Consumption of Sandeels in the North Sea by North Sea Mackerel, as estimated by ICES Multispecies Assessment Working Group (ICES 1997a).
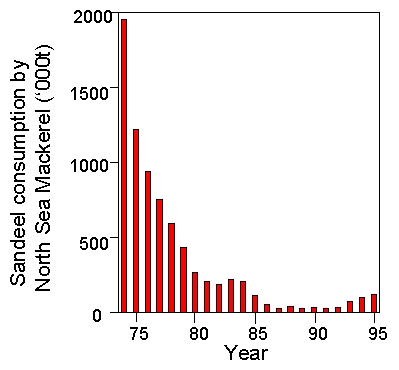
Fig 4. Consumption of Sandeels in the North Sea by the western Mackerel stock as estimated by the ICES Multispecies Assessment Working Group (ICES 1997a).
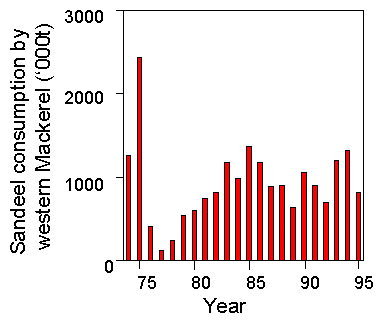
Fig 5. Consumption of Sandeels in the North Sea by major consumers showing trends over the study period (Mackerel, Whiting, Haddock, seabirds and the industrial fishery) from 1974 to 1995.