S11.5: Interaction of egg viability, brood reduction and nest failure on the onset of incubation
Steven R. Beissinger
Department of Environmental Science, Policy & Management, 151 Hilgard Hall #3110, University of California, Berkeley, CA 94720-3110, e-mail beis@nature.berkeley.edu
Beissinger, S.R. 1999. Interaction of egg viability, brood reduction and nest failure on the onset of incubation. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
638-646. Johannesburg: BirdLife South Africa.Among the chief effects of shifts in the onset of incubation are changes in the amount of time an egg is exposed before incubation is initiated, the hatching spread among eggs, and the length of the time between fledging of the first and last young. I examine the interactions of three processes that I believe are likely to be among the most universal fitness benefits and costs associated with hatching asynchrony – egg viability, nest failure and brood reduction. I develop a simple model that examines the consequences of changing the onset of incubation on the preincubation exposure time, hatching spread and fledging spread on each egg within a clutch. The duration of preincubation exposure depends on the onset of incubation and the laying interval. Birds are constrained to lay no more than 1 egg a day and increasing the average interval between eggs greatly affects preincubation exposure. Preincubation exposure to ambient conditions may affect the viability of the developing embryo in the egg and its susceptibility to nest failure before the onset of incubation. Preincubation delays also affect hatching and fledging spreads, which in turn can affect the susceptibility of chicks to brood reduction shortly after hatching and mortality due to nest failure during the fledging period. A universal relationship emerges in the form of the trade-off between the duration of exposure of first laid eggs and the hatching or fledging spread of a clutch. Egg-laying intervals have a particularly important effect on this tradeoff. I develop an equation that examines the interaction of these factors on the onset of incubation to evaluate how fitness changes with changes in the onset of incubation, and discuss its importance in structuring research programs.
INTRODUCTION
Most vertebrates have little control over birthing intervals, and their offspring emerge or are born relatively synchronously. Birds, on the other hand, can influence birthing (hatching) intervals by varying the onset of incubation. Hatching synchrony is the primitive condition in birds, but most families hatch their eggs asynchronously (Stoleson & Beissinger 1995). The chief effects of shifts in the onset of incubation are to change the amount of time an egg is exposed before incubation is initiated, the hatching spread among eggs, and the length of the time between fledging of the first and last young (Clark & Wilson 1981; Hussell 1985; Stoleson & Beissinger 1995).
Initiating incubation before the clutch is complete causes asynchronous hatching and can often result in death of the smallest chicks through brood reduction. Many hypotheses have been suggested to account for the evolution of hatching asynchrony, perhaps because clear fitness benefits have often been difficult to demonstrate for offspring or parents of asynchronously hatching broods (Stoleson & Beissinger 1995, 1997). Delaying the onset of incubation and not providing parental care can have associated costs. Although hatching asynchrony leading to brood reduction has sometimes been considered a benefit of initiating incubation early (Mock et al. 1990; Nilsson 1993a,b), it could also be considered a cost that is associated with the benefits derived from early incubation, such as sustaining eggs, protecting nests and avoiding parasitism and minimising predation (Clark & Wilson 1981; Hussell 1985; Stoleson & Beissinger 1995; Beissinger et al. 1998).
This paper examines the interactions of three processes that I believe are likely to be among the most universal fitness benefits and costs associated with hatching asynchrony – egg viability, nest failure and brood reduction. I develop a simple model that examines the consequences of changing the onset of incubation on the preincubation exposure time, hatching spread and fledging spread for each egg within a clutch.
EFFECTS OF THE ONSET OF INCUBATION AND EGGLAYING INTERVALS ON FITNESS COMPONENTS
Preincubation Exposure
Consider egg number n in a clutch of N eggs that are laid at an average interval of i days between eggs. The number of days an egg is exposed (E) prior to the onset of incubation can be expressed as a product of the laying interval (i) and the difference between the egg number and the egg on which incubation is begun (I). For an egg where n < I, then
E = [(I - 1) * i – (n - 1) * i] which simplifies to
E = i * (I - n) if n < I and
E = 0 if n > I.
Thus, the duration of preincubation exposure will depend both on the timing of the onset of incubation and the laying interval (Fig. 1). Birds are constrained to lay no more than 1 egg a day and increasing the average interval between eggs greatly affects preincubation exposure. In many medium and large birds, intervals between the laying of eggs exceed 2 days (Astheimer 1985; Aparicio 1994) and preincubation delays can exceed a week (Vinuela & Carrascal 1999).
The impacts of preincubation exposure are evident throughout the nesting cycle and depend upon the immediate effect of a variety of factors. During egg laying, preincubation exposure to ambient conditions may affect viability of the developing embryo in the egg as well as its susceptibility to nest failure before the onset of incubation. Because eggs usually don’t develop very rapidly until they are warmed by a parent, preincubation delay may directly control hatching patterns, which often affects the likelihood of brood reduction and fledging success. Finally, preincubation delays affect fledging spreads directly, if we assume that hatching and fledging spreads are highly correlated, which in turn may affect the susceptibility of chicks to mortality during the fledging period. Below I examine each of these factors and develop functions that relate them to the onset of incubation.
Egg Viability
Unincubated eggs exposed to ambient conditions show a decrease in hatching success. Long known from incubator studies of domestic fowl, field studies have demonstrated a decline in viability that is related to the length of preincubation exposure in ducks, sparrows, and parrots (Arnold et al. 1987; Veiga 1992; Stoleson 1996, 1999). The threshold for the onset of a viability decline was typically 3 days in altricial species, but did not occur until 5 to 10 days in precocial waterfowl.
We can write an expression for the decline in viability [f(V)] as a function of preincubation delays:
f(V) = H if n < I and
1 + a * [i * (I - n)]b
f(V) = 0 if n > I
where H is the hatching success of unexposed eggs, and a and b are constants derived from logistic regression based on viability declines from empirical studies (Arnold et al. 1987; Stoleson 1996, 1999).
Fig. 2 shows a variety of functions [f(V)] that indicate how viability of eggs would be expected to decline with preincubation delays (E) for different thermal environments based on data from the few species that have been well studied. Embryos are sensitive to death from overheating if their temperature reaches 41°C (Webb 1987), which can occur rapidly if eggs are exposed directly to the sun. Eggs are tolerant of cooling to the point of freezing for short periods, but lose viability if exposed for more than one day (Webb 1987). Eggs also lose their viability after several days of exposure to temperatures below optimal incubation levels (34°-37°C) but above physiological zero (24°-26°C), which can be considered developmental zero (Webb 1987; Stoleson 1999). The greatest declines are expected in warm, tropical environments and when temperate species initiate second broods in mid-summer (Fig. 2). Eggs are expected to lose their viability very slowly under cool conditions that are typical of temperate springtime environments.
Brood reduction
In many bird species, the likelihood of a chick dying from brood reduction may partly be a function of its hatching spread, or the age interval between it and the oldest sibling (Magrath 1992; Bollinger 1994). This is also evident from results of experiments that compare synchronous and asynchronous broods (Amundsen & Slagsvold 1991; Stoleson & Beissinger 1995). For example, hatching last or 2nd to last creates a handicap that is difficult for Green-rumped Parrotlet Forpus passerinus chicks to survive in asynchronously hatching nests with a large hatching spread but not in synchronously hatching nests with a small hatching spread (Stoleson & Beissinger 1997).
Viewed from this perspective, birds exhibit a great variety of brood reduction patterns or functions [f(B)] in relation to hatching spread. Fig. 3 shows typical relationships between the probability of brood reduction for a particular chick in a brood and its hatching spread, although other patterns are certainly possible. For illustrative purposes, the exact values for the decline in survivorship are less important than the general shapes of the functions. The Green-rumped Parrotlet would be an example of incremental brood reduction, where beyond an age difference threshold the likelihood of mortality increases with increasing hatching spread. Such a relationship may also occur in a threshold or stepwise fashion. Adaptive brood reduction may be indicative of a few species for which asynchronous hatching leads to increased survivorship of young, as in some waterbirds and raptors with obligate brood reduction (Mock et al. 1990) or perhaps if asynchrony functions to create a hierarchy among the young (Hahn 1981). In these species, brood reduction occurs when young hatch too close together because chicks fight and injure each other or cause whole brood starvation more often than when hatching more asynchronously. Nevertheless, brood reduction would also be likely to occur if these chicks hatched too far apart. Finally, some species show no effect on fledging success from different hatching patterns, whereas others may experience a cost to whole brood survival from certain hatching patterns (Harper et al. 1992; Stoleson & Beissinger 1995).
We can write an expression for hatching spread (HS) of an individual egg that depends on its number in the clutch (n), the laying interval (i), and the egg on which incubation was initiated (I). If we assume that the hatching spread is perfectly inversely correlated with laying intervals, it reflects the number of days beyond the onset of incubation that an egg is laid. Then for egg n where n > I :
HS = [(n – 1) * i – E1] which simplifies to
HS = i * (n – I) if n > I and
HS = 0 if n < I
Such an expression can be coupled to an appropriate brood reduction function f(B) from Fig. 3 to evaluate how the onset of incubation affects brood reduction.
Nest failure
Predation and nest failure are affected by changes in the onset of incubation in only two periods of the nesting cycle – during egg-laying and after fledging of the first young (Hussell 1985; Murray 1994; Stoleson & Beissinger 1995). Hatching synchrony should be favoured when the rate of failure during fledging exceeds the rate of failure during laying, whereas the opposite relationship selects for hatching asynchrony (Clark & Wilson 1981; Hussell 1985).
We can model the magnitude of these effects by compounding the daily survival rate: (1) during the laying period (De) by the preincubation exposure; or (2) during the fledging period (Df) by the fledging spread, or the number of days between the fledging of the first and last young. I have simplified the model by assuming that the fledging spread is determined by the preincubation exposure, although this is not always the case (e.g., Stoleson & Beissinger 1997). Thus, the probability of survival for an egg during laying (Pe) or for a chick during the fledging period (Pf) can then be written as:
Pe = De[i * (I - n)] for n < I if n < I and
Pe = 1 if n > I
Pf = Df[i * (n - I)] if n > I and
Pf = 1 if n < I
Failure rates prior to incubation are often assumed to be lower than failure rates during the fledging period. However, good measures of nest failure before the onset of incubation are often hard to obtain, because visits by researchers to the nests of some species result in nest abandonment. As a result, rates of failure during the incubation period are often used in computing the nest failure model (Clark & Wilson 1981; Murray 1994). Experiments with the Green-rumped Parrotlet, which begins incubation on the first egg and lays a large clutch (Beissinger & Waltman 1991), added nest sites with eggs to mimic the effect of eggs left unguarded during laying and found high rates of nest destruction (Beissinger et al. 1998). Eggs left unguarded for 3 days were destroyed at 40.6% of experimental parrotlet nests compared to egg loss at only 4.5% of the simultaneously observed egg-laying control pairs that had already started to incubate. Eggs were destroyed primarily by other parrotlets – male-female and male-male pairs - presumably as part of an effort to obtain a nest site or mate.
INTERACTION OF FACTORS WITH CHANGES IN THE ONSET OF INCUBATION
A universal relationship emerges in the form of the trade-off between the duration of exposure of first laid eggs and the hatching or fledging spread of a clutch (Fig. 4). The longer the preincubation delay, the shorter the hatching or fledging spread, whereas shorter preincubation delays are coupled with longer hatching spreads. Egg-laying intervals have a particularly important effect on this tradeoff.
The fitness (W) of different preincubation delays and hatching patterns depends on the biology of a particular species. In particular, the onset of incubation will depend on the rate of decline in the viability of eggs with exposure to ambient temperatures [f(V)], how hatching spreads affect the likelihood of brood reduction [f(B)], and the rate of nest failure prior to the onset of incubation (De) and during the fledging period (Df). The interaction of these factors on the onset of incubation can be examined by combining them into a single equation to evaluate how fitness (W) changes with changes in the onset of incubation (I):
![]()
This expression incorporates egg viability, brood reduction and nest failure rates as they affect each egg within a clutch, and is based on the effects of preincubation delays.
Although such an equation can be evaluated analytically, it would not take into account changes in the fitness of a chick caused by the death of an older sibling. This is the only kind of insurance function that occurs as a result of changes in the onset of incubation or hatching asynchrony (Stoleson & Beissinger 1995); other functions reported by Forbes et al. (1997) indicate the effects of insurance for clutch size evolution. For example, analytical approaches would not consider increases in the likelihood of survival for a chick when the oldest sibling dies (Werschkul 1979; Mock & Parker 1986; Beissinger & Waltman 1991). An alternative approach is to use simulation models that can incorporate changes in hatching order, and presumably the likelihood of brood reduction, that can result from the death of an older sibling.
An illustration of this approach is presented in Stoleson & Beissinger (1995) for the House Sparrow (Passer domesticus). They examined the trade-offs that a parent makes when initiating incubation by using a stochastic simulation model that incorporated the effects of egg viability, brood reduction, and nest failure. Results for early season nests showed that the greatest fitness was achieved by initiating incubation in the middle of the 5-egg clutch, but that little difference occurred by starting on eggs 2, 3 or 4. As the nesting season progressed and the effects of declining viability increased with increasing air temperatures, the incubation pattern that yielded the highest fitness shifted earlier toward the second and third eggs. The effects of viability and brood reduction were of similar magnitude in this example, but certainly would not be expected to be equal in other cases.
In conclusion, the preincubation delay that defines the onset of incubation can be directly incorporated into models of hatching asynchrony that include multiple factors. The value of examining models of interacting factors on the onset of incubation is that they can provide a clear and consistent method to explore how different factors potentially affect the onset of incubation and subsequent hatching patterns. Not only do they help to identify the trade-offs that parents face when initiating incubation, but such models can be most useful in structuring research programs. Experimental studies of hatching asynchrony typically produce completely synchronous and completely asynchronous broods (Amundsen & Slagsvold 1991; Stoleson & Beissinger 1995), and have rarely examined intermediate patterns of hatching asynchrony that characterise most species of birds. Evaluation of intermediate patterns of hatching becomes critical when trying to parameterise models. Finally, using models to help structure research programs allows studies of hatching asynchrony to progress beyond single factor thinking that has characterised the field to an understanding of hatching patterns based on the way that multiple causes interact during the nesting cycle.
REFERENCES
Amundsen, T. & Slagsvold, T. 1991. Asynchronous hatching in the Pied Flycatcher: an experiment. Ecology 72: 797-804.
Aparicio, J.M. 1994. The effect of variation in the laying interval on proximate determination of clutch size in the European Kestrel. Journal of Avian Biology 25: 275-280.
Arnold, T.W., Rohwer, F.C. & Armstrong, T. 1987. Egg viability, nest predation, and the adaptive significance of clutch size in Prairie Ducks. American Naturalist 130: 643-653.
Astheimer, L.B. 1985. Long laying intervals: a possible mechanism and its implications. Auk 102: 401-409.
Beissinger, S.R. & Waltman, J.R. 1991. Extraordinary clutch size and hatching asynchrony of a neotropical parrot. Auk 108: 863-871.
Beissinger, S.R., Tygielski, S. & Elderd, B. 1998. Social constraints on the onset of incubation in a neotropical parrot: a nestbox addition experiment. Animal Behaviour 55: 21-32.
Bollinger, P.B. 1994. Relative effects of hatching order, egg-size variation, and parental quality on chick survival in Common Terns. Auk 111: 263-273.
Clark, A.B. & Wilson, D.S. 1981. Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Quarterly Review of Biology 56: 253-277.
Forbes, S., Thorton, S., Glassey, B., Forbes, M. & Buckley, N.J. 1997. Why parents birds play favourites. Nature 390: 351-352.
Hahn, D.C. 1981. Asynchronous hatching in the Laughing Gull: cutting losses and reducing rivalry. Animal Behaviour 29: 421-427.
Harper, R.G., Juliano, S.A. & Thompson, C.F. 1992. Hatching asynchrony in the House Wren, Troglodytes aedon: a test of the brood-reduction hypothesis. Behavioral Ecology 3: 76-83.
Hussell, D.J.T. 1985. On the adaptive basis for hatching asynchrony: brood reduction, nest failure and asynchronous hatching in Snow Buntings. Ornis Scandinavica 16: 205-212.
Magrath, R.D. 1992. Roles of egg mass and incubation pattern in the establishment of hatching hierarchies in the blackbird (Turdus merula). Auk 109: 474-487.
Mock, D.W. & Parker, G.A. 1986. Advantages and disadvantages of egret and heron brood reduction. Evolution 40: 459-470.
Mock, D.W., Drummond, H. & Stinson, C.H. 1990. Avian siblicide. American Scientist 78: 438-449.
Murray, B.G. 1994. Effect of selection for successful reproduction on hatching synchrony and asynchrony. Auk 111: 806-813.
Nilsson, J.-Å. 1993a. Bisexual incubation facilitates hatching asynchrony. American Naturalist 142: 712-717.
Nilsson, J.-Å. 1993b. Energetic constraints on hatching asynchrony. American Naturalist 141: 158-166.
Stoleson, S.H. 1996. Hatching asynchrony in the Green-rumped Parrotlet: a multiple hypothesis analysis. Ph.D. dissertation, Yale University, New Haven, CT.
Stoleson, S.H. 1999. The importance of the early onset of incubation for the maintenance of egg viability. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 600-613. Johannesburg: BirdLife South Africa.
Stoleson, S.H. & Beissinger, S.R. 1995. Hatching asynchrony and the onset of incubation in birds, revisited: when is the critical period? In: Power, D.M. (ed.) Current Ornithology. Vol. 12; Plenum Press: 191-270.
Stoleson, S.H. & Beissinger, S.R. 1997. Extreme hatching asynchrony, brood reduction, and food limitation in a neotropical parrot: an experimental study. Ecological Monographs 67: 131-154.
Veiga, J.P. 1992. Hatching asynchrony in the House Sparrow: a test of the egg-viability hypothesis. American Naturalist 139: 669-675.
Viñuela, J. & Carrascal, L.M. 1999. Hatching patterns in altricial birds: a preliminary comparative analysis. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 584-599. Johannesburg: BirdLife South Africa.
Webb, D.R. 1987. Thermal tolerance of avian embryos: a review. Condor 89: 874-898.
Werschkul, D.F. 1987. Nestling mortality and the adaptive significance of early locomotion in the Little Blue Heron. Auk 96: 116-130.
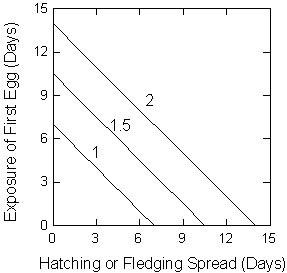
Fig. 1. The relationship between the exposure of the first egg and the egg number on which incubation is begun for various laying intervals (1, 1.5, and 2 days between successive eggs). Laying intervals are the numbers appearing below each line.
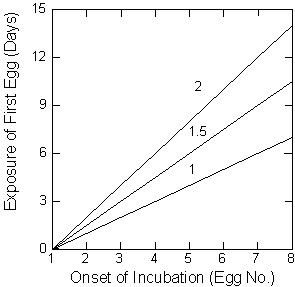
Fig. 2. Likely declines in egg viability [f(V)] in relation to preincubation exposure (days before the onset of incubation) for different thermal environments. Temperate spring decline based on data for waterfowl from Arnold et al. (1987), tropical and temperate summer based on data from sparrows and parrots from Veiga (1992) and Stoleson (1996, 1999), and very hot or cold based on Webb (1987).
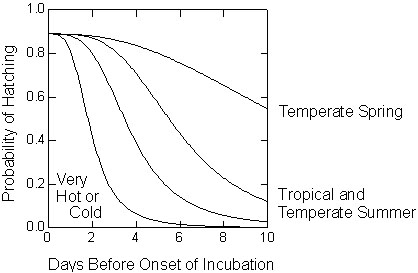
Fig. 3. Examples of likely patterns of brood reduction [f(Br)] as a function of the hatching spread (difference in age between the oldest nestling and the nestling of interest). As age differences between older and younger siblings increase, the effect of hatching spread on survivorship may result in incremental or threshold penalties. Adaptive brood reduction occurs when survivorship is highest over medium hatching spreads. Some species show no differences in nestling survival with hatching patterns, whereas others may experience a decrease in whole brood survival at a cost of c due to certain hatching patterns.
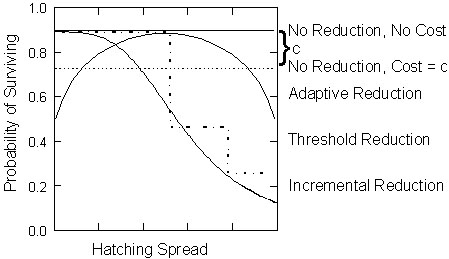
Fig. 4. The tradeoff between the exposure of the first egg versus the hatching or fledging spread, which reflects the age differences among the chicks, for different laying intervals (1, 1.5 and 2 days between successive eggs). Laying intervals are the numbers appearing below each line.