S11.4: Predicting the occurrence of synchronous and asynchronous hatching in birds
Bertram G. Murray, Jr.
Graduate Program in Ecology and Evolution, Rutgers University, 80 Nichol Avenue, New Brunswick, New Jersey 08901-2882, USA, fax 732 932 3222, e-mail bmurray@rci.rutgers.edu
Murray, B.G., Jr. 1999. Predicting the occurrence of synchronous and asynchronous hatching in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
624-637. Johannesburg: BirdLife South Africa.I propose three laws of evolution from which
I deduce the conditions under which we should expect to find synchronous and asynchronous
hatching. Synchronous hatching occurs in nidifugous species whose parents and chicks leave
the nest site shortly after hatching. In nidicolous species, asynchronous hatching is an
adaptation that increases the probability of rearing any young from a clutch, and brood
reduction is an adaptation that maximises the number of young that can be reared from a
successful brood. The variation in hatching patterns results from natural selection
favouring genotypes that maximise ![]() that is, the product of the mean number of
breeding seasons of individuals from successful clutches and the mean number of broods
reared per year, which represents lifetime reproductive success (in terms of number of
broods reared).
that is, the product of the mean number of
breeding seasons of individuals from successful clutches and the mean number of broods
reared per year, which represents lifetime reproductive success (in terms of number of
broods reared).
INTRODUCTION
Biologists tend to approach the study of life history traits piecemeal — that is, each trait is usually treated independently of the others. As a result biologists have a plethora of ad hoc hypotheses regarding the evolution of clutch size, mating systems, sexual size dimorphism, age of first breeding (often described as delayed maturity), and asynchronous hatching (to name a few). Stoleson and Beissinger (1995), for example, have identified 17 different hypotheses proposed to explain asynchronous hatching in birds. Slagsvold et al. (1995) added three more. Hypotheses seem to be immortal. Ornithologists rarely, if ever, reject a hypothesis once it has been proposed. This circumstance comes about because the hypotheses are ad hoc (i.e., not universally applicable) and therefore unfalsifiable (Popper 1968, 1979). In this paper I will be looking at asynchronous hatching in birds as an adaptive trait within the context of the evolution of a species’ life history. I provide a falsifiable deductive-nomological theory (Hempel & Oppenheim 1948) as an explanation of life-history evolution. Such a theory is either right or wrong because it is universally applicable and its predictions ‘may be independently tested everywhere, and at all times’ (Popper 1979, original italics).
In this paper I will be concerned with predicting patterns of the occurrence of synchronous and asynchronous hatching in birds. By prediction I mean to follow Popper (1968, 1979) in deducing predictions from guesses that are presumed to be laws, that is, from statements that I assume (for the sake of exploring the problem of hatching patterns in birds) to be universally true.
THREE LAWS OF EVOLUTION
At the beginning, then, let us consider some statements that I hypothesise to be universally true for living organisms.
First Law
Genotypes and phenotypes with the greatest Malthusian parameter increase more rapidly than those with smaller Malthusian parameters.
This is Darwin’s Theory of Natural Selection written in a form that allows quantification. The Malthusian parameter (m for genotypes, r for phenotypes) is the exponential rate of change in the number of individuals with a particular genotype or phenotype and is determined from the equation,
![]() (1)
(1)
where r represents either m for genotypes or r for phenotypes (depending on the kinds of input data that are available), lx is the probability of individuals (either males or females) of a specific genotype or phenotype surviving from birth (in live-bearing animals) or from being laid as an egg (in egg-laying animals) to age x, mx is the mean apparent fecundity of individuals (either males or females) of that genotype or phenotype and age x, and e is the base of the natural logarithms (see Murray [1990, 1995, 1997] for fuller treatment).
Elsewhere, I have shown that the Malthusian parameter of a genotype (or phenotype) is the only universal measure of fitness, that is, it is the only universal indicator of which of several alternative genotypes (or phenotypes) should prevail over evolutionary time (Murray 1990, 1995, 1997). This means that other demographic parameters (e.g., fecundity [including clutch and litter size], juvenile survival, adult survival, net reproductive rate [R0], and lifetime reproductive success [LRS], as calculated in Clutton-Brock [1988] and Newton [1989]), are not universal indicators of fitness. Therefore, these ‘components’ of fitness should not be used as indicators of fitness in evolutionary studies.
With regard to the problem of incubation behaviour and its effect on hatching sequence of the eggs, we should imagine that the different behaviours are associated with different genotypes, one genotype for beginning incubation with the first egg, another with the penultimate egg, another with the last egg, and so on. If we had the survival and fecundity data for females and males of the different genotypes, we could assess which of these genotypes should prevail in a particular situation by calculating the Malthusian parameter for each genotype by the methods I proposed earlier (Murray 1990, 1995, 1997).
Virtually all experiments are conducted on birds of unknown genotype (except that we may sometimes assume that a species with a single phenotype has a single genotype) and poorly known demography. Indeed, experimental birds of one genotype are often forced to perform as members of another genotype (as when birds that normally begin incubating with the penultimate or earlier egg are made to begin incubating with the last egg). Thus, we can only imagine that experimental results obtained from manipulating birds of a single genotype reflect differences that may exist among genotypes. This may be the best that we can do at this time, but the point should be kept in mind. Manipulation experiments, furthermore, usually evaluate one parameter at a time, such as the number of young leaving the nest, which is, as I have pointed out, a misleading indicator of the fitness of a genotype (Murray 1990, 1992a, 1995, 1997).
Hussell (1972) has already pointed out the difficulty in interpreting results from experimentally enlarged broods because birds may sometimes be placed in situations that are outside their evolved abilities. Mock and Forbes (1994) and Stoleson (1997) concluded that long-term effects of hatching patterns on survival and reproduction have been ignored in evaluating hypotheses about asynchronous hatching and brood reduction, that is, that the results of short-term experiments on one or two variables may provide faulty indicators of fitness.
Second Law
In the absence of changes in selection forces, a population will reach and remain in an evolutionary steady state.
Over a period of years a population’s age-specific survival and fecundity vary in response to changes in environmental features such as the weather, the incidence of predators and parasites, and the abundance of competitors, food, and nest sites. Nevertheless, over that period of time, the population’s exponential growth rate (r) tends to be zero. Only for short periods of time will a population (defined as a group of interacting individuals of the same species within a circumscribed area) increase or decrease in numbers. The upper bound to increase is set by factors such as the availability of resources and incidence of predation and disease (Murray 1979), whereas the lower bound to decrease is set by extinction.
Nevertheless, any mutant genotype that increases the probability of its possessors’ surviving or breeding successfully should increase relative to alternative genotypes, while the population itself is not increasing. The favoured genotype will eventually become fixed and have a Malthusian parameter of zero (Murray 1990, 1997).
Third Law
Selection favours those females that lay as few eggs or bear as few young as are consistent with replacement because they have the highest probability of surviving to breed again, their young have the highest probability of surviving to breed, or both.
This law is contrary to current hypotheses regarding the evolution of clutch size (e.g., Winkler & Walters 1983, Murphy & Haukioja 1986, Godfray et al. 1991). I suggest that this hypothesis be evaluated by deducing its consequences and comparing them with empirical fact. This can be done most easily with the Murray-Nolan clutch-size equation.
The Murray-Nolan Clutch-Size Equation
An equation relating the various demographic parameters of a species’ life history has been proposed by Murray and Nolan (1989):
![]() (2)
(2)
where C is a genotype’s mean
clutch size, a is the primary sex ratio (male/female, assumed to be 1 in birds), lx is the probability of surviving from birth (in
birds, from the laying of the egg) to age x of those individuals from successful
clutches or litters, a is the average age of first breeding, w is the age of last breeding, and ![]() is the
mean number of broods successfully reared during a breeding season. (Eq. 2 can be used
with data from either sex; by convention, I will discuss it with respect to female
reproduction.)
is the
mean number of broods successfully reared during a breeding season. (Eq. 2 can be used
with data from either sex; by convention, I will discuss it with respect to female
reproduction.)
![]() is the annual reproductive success (ARS(b)),
measured as the number of broods reared,
is the annual reproductive success (ARS(b)),
measured as the number of broods reared,
![]()
where Pi is the probability of rearing brood i, ci is the number of clutches laid per female in producing brood i, and si is the probability that a brood i clutch produces any young at all (Murray 1991a, 1991b).
The annual reproductive success (ARS(k)), measured in number of young reared, is
![]()
where ki is the mean number of young produced by a successful brood i clutch.
![]() is the mean number of breeding seasons of the
females hatched in successful clutches. The mean lifetime reproductive success, then, in
terms of the number of broods reared, is the product of
is the mean number of breeding seasons of the
females hatched in successful clutches. The mean lifetime reproductive success, then, in
terms of the number of broods reared, is the product of ![]() and
and ![]() . Lifetime reproductive success, in
terms of number of young reared, is the product of
. Lifetime reproductive success, in
terms of number of young reared, is the product of ![]() ,
, ![]() and ki .
and ki .
These measures of lifetime reproductive success are quite different from that reported in the books edited by Clutton-Brock (1988) and by Newton (1989), as pointed out earlier (Murray 1992a).
According to Wootton et al. (1991), with whom I agree (Murray 1992b), Eq. 2 must be true, regardless of whatever hypothesis on the evolution of clutch size one supports. The evolutionary problem is to explain the cause-and-effect relationships implied by the equation.
From Law 2 and Eq. 2 we can infer that the evolution of a
larger clutch size (C) must result in a reduced lifetime reproductive success
(![]() ). Conversely, the evolution of traits extending life
expectancy (therefore increasing
). Conversely, the evolution of traits extending life
expectancy (therefore increasing ![]() ) or increasing the number
of broods reared during a breeding season (
) or increasing the number
of broods reared during a breeding season (![]() ) should result in the evolution of a
smaller clutch size.
) should result in the evolution of a
smaller clutch size.
My argument is that a female that lays a clutch of N eggs has a lower probability of producing any young to nest leaving than if she had laid N - 1 eggs in her clutch (Fig. 1). Because birds lay no more than one egg per day and because the probability of a nest’s contents’ surviving from one day to the next is <1, the probability of a brood i clutch producing any young to nest-leaving (si) decreases as the clutch size becomes larger (Murray 1994, 1996), as indicated by,
![]() (5)
(5)
where f is the first day on which young leave the nest, ds is the daily survival of the nests’ contents, and i = 1, 2, . . ., n for first, second, and nth brood, respectively.
Accordingly, a larger clutch reduces the probability that brood i clutches will be successful (i.e., reduces s
1, s2, . . . , sn). A larger clutch also reduces the number of replacement clutches that could be laid after clutch failures (i.e., reduces c1) in single-brooded species and reduces the number of first, second, and later brood clutches in multibrooded species (reduces c1, c2, . . . , cn) during any finite period of time because of the longer time required to lay the eggs and rear the brood (at least one day longer for each additional egg). Furthermore, each additional egg could increase the egg and nestling periods by more than one day, if incubation time per egg of a clutch increases with the addition of eggs, as shown experimentally to be the case in the Pied Flycatcher Ficedula hypoleuca (Moreno & Carlson 1989) and Blue Tit Parus caeruleus (Smith 1989), again reducing si and ci.Evidence indicates that females are more likely to lay a replacement clutch following a small clutch than a large clutch (e.g., Slagsvold 1984; Smith et al. 1987; Tinbergen 1987; Lindén 1988) and that the time interval between consecutive clutches is shorter for smaller than for larger clutches (McGillivray 1983; Slagsvold 1984; Smith et al. 1987). Additional advantages of a small clutch could be better protection of young by parents after their leaving the nest (Safriel 1975) and the production of heavier young, which may increase the survival of young through their first year (Perrins 1965, 1980; Lack 1966; von Haartman 1971; Loman 1977; Murphy 1978; Garnett 1981).
The apparent benefit of a larger brood — that more fledglings are produced per successful clutch, ignoring the losses of failed clutches — is at least questionable, inasmuch as I have shown that a greater fecundity does not necessarily result in a greater Malthusian parameter (Murray 1997). Biologists, nevertheless, tend to think that natural selection favours the successful rearing of more young. The proposed paradox of hatching asynchrony, for example, is based on this assumption (Stoleson & Beissinger 1995), but the notion that the evolution of asynchronous hatching results in reducing the number of young produced from a nest seems paradoxical only when we think that rearing more young in a brood is an accurate indicator of fitness.
I propose these as laws, conjectures, or guesses, as Popper (1968, 1979) and Feynman (1965) call them, as being universally applicable statements from which other, testable statements may be deduced. Whether these statements form part of a satisfactory causal explanation depends on what kinds of predictions we can deduce from them and how well these conform to empirical fact. I have in fact discussed several predictions of this theory and provided supporting evidence elsewhere (Murray 1979, 1985, 1991b).
DISCUSSION
Although Law 3 states that natural selection
favours a small (i.e., replacement) clutch size, we can see that selection for a
smaller clutch size is a consequence of selection for greater lifetime reproductive
success (i.e., ![]() ). Traits that extend life or increase annual
reproductive success evolve because their Malthusian parameters are greater than those of
the prevailing traits and necessarily (because of Law 2) lead to the evolution of a
smaller clutch size. Put in yet another way, I am suggesting that natural selection
favours genotypes that maximise their possessors’ probability of successfully rearing
just enough offspring to sustain a population with a long-term growth rate of zero
because, in evolutionary time, a population can do no better. A genotype for a clutch size
smaller than replacement, of course, cannot sustain a population and soon becomes extinct.
). Traits that extend life or increase annual
reproductive success evolve because their Malthusian parameters are greater than those of
the prevailing traits and necessarily (because of Law 2) lead to the evolution of a
smaller clutch size. Put in yet another way, I am suggesting that natural selection
favours genotypes that maximise their possessors’ probability of successfully rearing
just enough offspring to sustain a population with a long-term growth rate of zero
because, in evolutionary time, a population can do no better. A genotype for a clutch size
smaller than replacement, of course, cannot sustain a population and soon becomes extinct.
With this background, what can we say about the evolution of incubation behaviour and hatching patterns? Once we get over the idea that natural selection favours production of more young, and instead we begin to understand that what is favoured are genotypes with the greatest Malthusian parameter, we can begin to look at what traits could lead to greater reproductive success and greater Malthusian parameters.
Let us consider now how natural selection for
hatching patterns could maximise ![]() . We have already noted that a smaller clutch increases
si (Fig. 1) and, therefore, Pi
, but the evolved clutch size cannot be smaller than replacement. Evolution toward greater
si is constrained by the necessity of producing enough young to sustain
the population in the face of predation, disease, and other sources of mortality.
. We have already noted that a smaller clutch increases
si (Fig. 1) and, therefore, Pi
, but the evolved clutch size cannot be smaller than replacement. Evolution toward greater
si is constrained by the necessity of producing enough young to sustain
the population in the face of predation, disease, and other sources of mortality.
Early incubation and asynchronous hatching increase si
, the probability of rearing any young from a clutch (Fig. 2), and,
thus, should be the norm among birds. Variations on this idea are the
‘predation’ or ‘nest failure’ hypothesis (Tyrväinen 1969; Hussell
1972, 1985; Clark & Wilson 1981; Stoleson & Beissinger 1995) or, more
cumbersomely, the ‘reduction-of-total-nest-failure’ hypothesis (Murray 1994).
The si for a clutch of a given size can be increased by beginning
incubation prior to the laying of the last egg (Fig. 2), but this
has a cost of reduced probability of survival of later laid eggs (Beissinger & Waltman
1991; Stoleson & Beissinger 1997), reducing ![]() . The number of young
surviving from a successful clutch (ki) could be increased by starting
incubation with the last egg, but this has the cost of reducing the probability of rearing
any young at all (smaller si). Thus, both
. The number of young
surviving from a successful clutch (ki) could be increased by starting
incubation with the last egg, but this has the cost of reducing the probability of rearing
any young at all (smaller si). Thus, both ![]() and
and ![]() cannot both be
simultaneously maximised. Incubation should start early enough to increase si
but late enough for the parent(s) to rear, on average, the number of young that maximises
the Malthusian parameter. The variation in the start of incubation—some species
beginning incubation with the first egg, others with the last, and others in
between—is, I hypothesise, a balance between
cannot both be
simultaneously maximised. Incubation should start early enough to increase si
but late enough for the parent(s) to rear, on average, the number of young that maximises
the Malthusian parameter. The variation in the start of incubation—some species
beginning incubation with the first egg, others with the last, and others in
between—is, I hypothesise, a balance between ![]() and
and ![]() in
maximising
in
maximising ![]() .
.
In addition, because the survival of young from a successful
clutch affects all subsequent values of lx,
we should expect to find adaptations that increase the number of young that leave a
successful nest, increasing ![]() . One such adaptation would be the
reduction of brood size when the parents are confronted with a limited food supply (i.e.,
the ‘brood reduction hypothesis’ [Lack 1947, 1954]). According to my theory,
asynchronous hatching and brood reduction are independent adaptations, the first
increasing
. One such adaptation would be the
reduction of brood size when the parents are confronted with a limited food supply (i.e.,
the ‘brood reduction hypothesis’ [Lack 1947, 1954]). According to my theory,
asynchronous hatching and brood reduction are independent adaptations, the first
increasing ![]() and the second increasing
and the second increasing ![]() .
.
Unfortunately, the kinds of data necessary
for testing this theory are lacking, but we can consider the extreme cases
quasi-quantitatively. First, some species have precocial young, which leave the nest
shortly after hatching. Selection should favour synchronous or nearly synchronous
hatching, especially if chicks and parents move some distance from the nest shortly after
hatching (Clark & Wilson 1981; Magrath 1990; Slagsvold 1990; Murray 1994). Synchronous
hatching may seem contradictory to the theory, but synchronous hatching is favoured in
precocial, nidicolous species because ![]() would be greatly reduced if
chicks hatched more than slightly asynchronously.
would be greatly reduced if
chicks hatched more than slightly asynchronously.
Incubation, however, does not always begin simultaneously for all eggs in nidifugous species. Variation in the start of incubation may be an incidental consequence of such things as warm temperatures stimulating development (Ward 1965; Jayakar & Spurway 1965) or parents being present on the nest while protecting its contents from predators (Bruning 1973) or other nest intruders (Beissinger et al. 1998). Nevertheless, in species with precocial young, synchrony of hatching seems enhanced by clicking sounds associated with breathing or vibrations emanating from the eggs in the few days before hatching (Vince 1969; Freeman & Vince 1974). These sounds seem to speed up or slow down development and synchronise hatching.
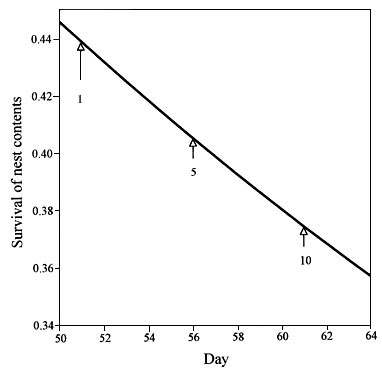
At the other extreme are species, such as the cavity-nesting Green-rumped Parrotlet Forpus passerinus, which begins incubating its large clutch from the day the first egg is laid (Beissinger & Stoleson 1991; Beissinger & Waltman 1991). This species has a long nest period, an average of 20 days incubation and 31 days brooding, before young leave the nest (Beissinger & Waltman 1991). In natural cavities, the mean si is about 0.44 (Beissinger & Bucher 1992). This corresponds to a mean ds of about 0.984, assuming that one egg is laid each day and incubation and brooding periods in larger clutches are not longer than in smaller clutches. These assumptions are not exactly so (Stoleson & Beissinger 1997; Beissinger et al. 1998), but if we assume they are for the purpose of illustration, si is 0.4393 when incubation begins on day 1 and 0.3739 when it begins on day 10 (Fig. 3), a drop of 15%. Surely, it is better for parrotlets to begin incubation before the last day.
The specific day on which incubation should start is more
difficult to predict, simply because we do not have enough information. The general
prediction, however, is that incubation should start at the time that results in
maximising ![]() .
.
In manipulation experiments, Stoleson and Beissinger (1997) showed that parrotlet parents were capable of rearing as many as eight chicks, even with simulated synchronous hatching. Although these results are interesting, I doubt that they falsify the theory because we do not know ci or si for either natural or experimental clutches of different size.
In between the synchronously hatching precocial, nidifugous
species and the completely asynchronously hatching parrotlet are the many species in which
incubation begins after the first egg and before the last egg is laid. For example, in
many passerines, incubation begins with the penultimate egg. Thus, most chicks are ready
to leave the nest earlier than if incubation had started with the final egg, increasing
the probability that one or more young would survive, for example, a predator- or
weather-induced early departure from the nest (increasing si
and ![]() ), while decreasing the probability that all chicks
will survive to nest-leaving (decreasing ki and
), while decreasing the probability that all chicks
will survive to nest-leaving (decreasing ki and ![]() ). If no predators arrive and the weather is
benign, nestlings could remain in the nest until the younger chicks are ready to leave,
increasing ki and
). If no predators arrive and the weather is
benign, nestlings could remain in the nest until the younger chicks are ready to leave,
increasing ki and ![]() . According to this hypothesis,
. According to this hypothesis, ![]() is
maximised.
is
maximised.
From the theory we might predict that, among nidicolous species with the same nest period, asynchronous hatching should be greater in species with a lower probability of surviving another day (ds) and, therefore, a lower si. This expectation may not occur, however, because we have to consider the effect of early incubation on ki, that is on the success of late hatched chicks.
For example, the Prairie Warbler Dendroica discolor has an si of between 0.20 and 0.22, and the nest period is about 26 days for a four-egg clutch (Nolan 1978). From Eq. 5, mean ds is about 0.942 (i.e., si on day 26 is 0.212). Both ds and si are lower than in the parrotlet, yet hatching spans from less than 12 hours in some nests to more than 24 hours (Nolan 1978), so asynchrony of hatching is slight. Nevertheless, a head start of even a few hours may enable an earlier hatched nestling to survive a predator attack that occurs just before the later hatched chicks are ready to leave the nest. In the Prairie Warbler the advantages of an even earlier start to incubation may well be offset by increased probability of losses of later hatched chicks (i.e., a reduced ki).
The nesting cycle of the Least Flycatcher Empidonax minimus is similar to that of the Prairie Warbler: from the laying of the first egg to nest leaving of the first chick is only slightly longer, about 28 days for a four-egg clutch (Briskie & Sealy 1989). Nest survival (si), however, is higher (0.38), as is mean ds (about 0.965). Nevertheless, incubation begins earlier and hatching spread is longer (mean, 36.4 hours for four-egg clutch).
This between-species comparison of hatching success and
degree of hatching asynchrony would seem not to agree with an apparent expectation from
the theory—that a higher probability of nest failure should be associated with
greater asynchrony. Just the reverse occurs in these species. Evolution, however, is a
within-species process. We should be comparing the patterns between genotypes. Within each
species we need to know which hatching pattern maximises ![]() , and we do not.
, and we do not.
Within a species, in which mean ds would apply to all members of a population, we should expect an earlier start to incubation and more asynchronous hatching in larger clutches, for which there is some evidence (Hussell 1972, 1985; Slagsvold 1986; Skagen 1987; Briskie & Sealy 1989), again increasing si.
Finally, with regard to theoretical
expectations, this theory could explain the well-known within-species decrease in clutch
size toward the end of the breeding season (Lack 1954, 1966; Klomp 1970) and the possible
earlier start to incubation and greater asynchrony in late season broods compared with
early season broods (Gibb 1950; Slagsvold 1986; Skagen 1987). A smaller clutch and earlier
incubation would each increase si, which would increase the probability
of reproductive success of the individual bird and increase ![]() for the genotype. Thus, the
reduction in clutch size and the earlier incubation could be adaptations that increase the
likelihood of successful breeding as the breeding season draws to a close. It is better to
rear a few young rather than to rear no young at all in trying to rear a bigger brood.
for the genotype. Thus, the
reduction in clutch size and the earlier incubation could be adaptations that increase the
likelihood of successful breeding as the breeding season draws to a close. It is better to
rear a few young rather than to rear no young at all in trying to rear a bigger brood.
Research needs
In order to test these predictions we need high quality natural history data, such as good empirical values for lx (the probability of surviving from birth to age x of individuals from successful clutches), a (mean age of first breeding), ci (the number of clutches laid per female in producing a successful brood i clutch), si (the probability that a brood i clutch will be successful), Pi (the mean number of brood i clutches reared per female), and ki (the mean number of young reared in successful brood i), especially for clutches of different size. We need these data because they are the parameters that are an explicit part of the theory, and we could make much better guesses about the effect of differences in these parameters on the Malthusian parameter, but these data are essentially unreported in the literature. The theory cannot be tested with idiosyncratic measures of ‘reproductive success’ or ‘fitness.’ Many investigators with long-term life-history data could calculate values for these parameters.
Second, we need accurate data on the lengths of the
incubation and brooding periods. I think it is possible that young birds may remain in the
nest after they are physically capable of living outside the nest. I am sure that many
ornithologists have had the experience, as I have, of approaching a nest, only to have the
young explode out of it with the slightest touch. Were these young ready to leave the nest
the day before but remained in the nest until the youngest chick was ready, or did I
‘fledge’ them prematurely (i.e., before they were ready)? I think they
were ready but waiting. In this regard, Briskie and Sealy (1989) report that the oldest
Least Flycatcher nestlings usually do not leave the nest until a day or two after they are
capable of leaving successfully. Therefore, I wonder whether, when we are careful to
measure incubation and brooding periods only in ‘undisturbed’ nests, we may in
fact be overestimating the nest period. Fully ready chicks waiting in their nests for
siblings to complete development may be an adaptation that increases ki
(and ![]() ) without much additional risk to themselves.
) without much additional risk to themselves.
Without more accurate data, testing any theory could only be difficult. I point out, however, that a hypothesis, applicable to species A but not to species B, and another hypothesis, applicable to species B but not to species A, are not falsifiable because each is ad hoc (Popper 1968, 1979). All we could do is compile lists of species for which hypothesis A or hypothesis B is applicable. With regard to incubation behaviour and asynchrony of hatching we would have 20 such lists (Stoleson & Beissinger 1995; Slagsvold et al. 1995). My philosophy of science makes me favour my universal theory.
CONCLUSIONS
By approaching the problem of understanding
incubation behaviour and asynchronous hatching from the point of view of Popper (1968,
1979)—by constructing falsifiable hypotheses in the form of the deductive-nomological
model (Hempel & Oppenheim 1948) and utilizing laws assumed to be universally
applicable—I propose that early incubation and asynchronous hatching are adaptations
that increase the probability of rearing any young from a clutch (i.e., increases
si and ![]() ), which has been referred to as the
‘predation’ or ‘nest failure’ hypothesis (Clark & Wilson 1981;
Hussell 1972, 1985; Stoleson & Beissinger 1995) and the
‘reduction-of-total-nest-failure’ hypothesis (Murray 1994). Brood reduction is
an adaptation that maximises the number of young that can be reared from a successful
brood (i.e., increasing ki and
), which has been referred to as the
‘predation’ or ‘nest failure’ hypothesis (Clark & Wilson 1981;
Hussell 1972, 1985; Stoleson & Beissinger 1995) and the
‘reduction-of-total-nest-failure’ hypothesis (Murray 1994). Brood reduction is
an adaptation that maximises the number of young that can be reared from a successful
brood (i.e., increasing ki and ![]() ). The incubation and hatching patterns are
fine-tuned, and thus vary among species, by selection favouring the combination of
behaviours that has the greatest
). The incubation and hatching patterns are
fine-tuned, and thus vary among species, by selection favouring the combination of
behaviours that has the greatest ![]() . This variation results from specific differences in
length of the total nesting period, intensity of predation, inclement weather, and other
sources of nest loss, the length of the breeding season, and many other factors.
. This variation results from specific differences in
length of the total nesting period, intensity of predation, inclement weather, and other
sources of nest loss, the length of the breeding season, and many other factors.
ACKNOWLEDGMENT
I thank S. R. Beissinger for his helpful comments on an earlier manuscript.
REFERENCES
Beissinger, S.R. & Bucher, E.H. 1992. Sustainable harvesting of parrots for conservation. In: Beissinger, S. R., & Snyder, N. F. R. (eds) New World parrots in crisis: solutions from conservation biology. Washington, D. C.; Smithsonian Institution Press: 73-115.
Beissinger, S.R. & Stoleson, S.H. 1991. Nestling mortality patterns in relation to brood size and hatching asynchrony in Green-rumped Parrotlets. Acta XX Congressus Internationalis Ornithologici: 1727-1733.
Beissinger, S.R. & Waltman, J.R. 1991. Extraordinary clutch size and hatching asynchrony of a neotropical parrot. Auk 108: 863-871.
Beissinger, S.R., Tygielski, S. & Eldred, B. 1998. Social constraints on the onset of incubation in a neotropical parrot: a nestbox addition experiment. Animal Behaviour 55: 21-32.
Briskie, J.V. & Sealy, S.G. 1989. Nest-failure and the evolution of hatching asynchrony in the Least Flycatcher. Journal of Animal Ecology 58: 653-665.
Bruning, D. 1973. Breeding and rearing rheas in captivity. International Zoological Yearbook 13: 163-172.
Clark, A.B. & Wilson, D.S. 1981. Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Quarterly Review of Biology 56: 253-277.
Clutton-Brock, T.H. (ed.). 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL; University of Chicago Press: 538pp.
Feynman, R. 1965. The Character of Physical Law. Cambridge, MA; MIT Press: 173pp.
Freeman, B.M. & Vince, M.A. 1974. Development of the avian embryo: a behavioural and physiological study. London; Chapman and Hall: 362pp.
Garnett, M.C. 1981. Body size, its heritability and influence on juvenile survival among Great Tits, Parus major. Ibis 123: 31-41.
Gibb, J. 1950. The breeding biology of the Great and Blue titmice. Ibis 92: 507-539.
Godfray, H. C. J., Partridge, L. & Harvey, P. H. 1991. Clutch size. Annual Review of Ecology and Systematics 22: 409-429.
Haartman, L. von. 1971. Population dynamics. In: Farner, D. S., & King, J. R. (eds) Avian biology, vol. 1. New York; Academic Press: 391-459.
Hempel, C.G. & Oppenheim, P. 1948. Studies in the logic of explanation. Philosophy of Science 15: 135-175.
Hussell, D. J.T. 1972. Factors affecting clutch size in arctic passerines. Ecological Monographs 42: 317-364.
Hussell, D. J.T. 1985. On the adaptive basis for hatching asynchrony: brood reduction, nest failure and asynchronous hatching in Snow Buntings. Ornis Scandinavica 16: 205-212.
Jayakar, S.D. & Spurway, H. 1965. The Yellow-wattled Lapwing, Vanellus malabaricus (Boddaert), a tropical dry-season nester. II. Additional data on breeding biology. Journal of the Bombay Natural History Society 62: 1-14.
Klomp, H. 1970. The determination of clutch-size in birds. A review. Ardea 58: 1-124.
Lack, D. 1947. The significance of clutch-size. Parts I and II. Ibis 89: 302-352.
Lack, D. 1954. The natural regulation of animal numbers. London; Oxford University Press: 343pp.
Lack, D. 1966. Population studies of birds. London; Oxford University Press: 341pp.
Lindén, M. 1988. Reproductive trade-off between first and second clutches in the Great Tit Parus major: an experimental study. Oikos 51: 285-290.
Loman, J. 1977. Factors affecting clutch and brood size in the crow, Corvus cornix. Oikos 29: 294-301.
Magrath, R.D. 1990. Hatching asynchrony in altricial birds. Biological Reviews 65: 587-622.
McGillivray, W.B. 1983. Intraseasonal reproductive costs for the House Sparrow (Passer domesticus). Auk 100: 25-32.
Mock, D.W. & Forbes, S.L. 1994. Life-history consequences of avian brood reduction. Auk 111: 115-123.
Moreno, J. & Carlson, A. 1989. Clutch size and the costs of incubation in the Pied Flycatcher Ficedula hypoleuca. Ornis Scandinavica 20: 123-128.
Murphy, E.C. 1978. Seasonal variation in reproductive output of House Sparrows: the determination of clutch size. Ecology 59: 1189-1199.
Murphy, E.C. & Haukioja, E. 1986. Clutch size in nidicolous birds. Current Ornithology 4: 141-180.
Murray, B.G., Jr. 1979. Population dynamics: alternative models. New York; Academic Press: 212pp.
Murray, B.G., Jr. 1985. Evolution of clutch size in tropical species of birds. In: Buckley, P. A., Foster, M. S., Morton, E. S., Ridgely, R. S., & Buckley, F. G. (eds) Neotropical Ornithology. Ornithological Monographs, 36: 505-519.
Murray, B.G., Jr. 1990. Population dynamics, genetic change, and the measurement of fitness. Oikos 59: 189-199.
Murray, B.G., Jr. 1991a. Measuring annual reproductive success, with comments on the evolution of reproductive behavior. Auk, 108: 942-952.
Murray, B.G., Jr. 1991b. Sir Isaac Newton and the evolution of clutch size in birds: a defense of the hypothetico-deductive method in ecology and evolutionary biology. In: Casti, J. L., & Karlqvist, A. (eds) Beyond belief: randomness, prediction, and explanation in science. Boca Raton, FL; CRC Press: 143-180.
Murray, B.G., Jr. 1992a. The evolutionary significance of lifetime reproductive success. Auk 109: 167-172.
Murray, B.G., Jr. 1992b. The evolution of clutch size: a reply to Wootton, Young, and Winkler. Evolution 46: 1581-1584.
Murray, B.G., Jr. 1994. Effect of selection for successful reproduction on hatching synchrony and asynchrony. Auk 111: 806-813.
Murray, B.G., Jr. 1995. A method for projecting genotypic change in populations with complex genetic and demographic structure. Oikos 73: 415-418.
Murray, B.G., Jr. 1996. Erratum: Murray (1994). Auk 113: 724.
Murray, B.G., Jr. 1997. Population dynamics of evolutionary change: demographic parameters as indicators of fitness. Theoretical Population Biology 51: 180-184.
Murray, B.G., Jr. & Nolan, V., Jr. 1989. The evolution of clutch size. I. An equation for predicting clutch size. Evolution 43: 1699-1705.
Newton, I. (ed.). 1989. Lifetime reproduction in birds. San Diego, CA; Academic Press: 479pp.
Nolan, V., Jr. 1978. The ecology and behavior of the Prairie Warbler Dendroica discolor. Ornithological Monographs 26. Lawrence, KS: Allen Press.
Perrins, C.M. 1965. Population fluctuations and clutch-size in the Great Tit, Parus major L. Journal of Animal Ecology 34: 601-647.
Perrins, C.M. 1980. The survival of young Great Tits Parus major. Proceedings of the International Ornithological Congress 17: 159-174.
Popper, K.R. 1968. The logic of scientific discovery. Rev. ed. New York; Harper & Row: 480pp.
Popper, K.R. 1979. Objective knowledge: an evolutionary approach. Rev. edition. London; Oxford University Press: 395pp.
Safriel, U.N. 1975. On the significance of clutch size in nidifugous birds. Ecology 56: 703-708.
Skagen, S.K. 1987. Hatching asynchrony in American Goldfinches: an experimental study. Ecology 68: 1747-1759.
Slagsvold, T. 1984. Clutch size variation of birds in relation to nest predation: on the cost of reproduction. Journal of Animal Ecology 53: 945-953.
Slagsvold, T. 1986. Asynchronous versus synchronous hatching in birds: experiments with the Pied Flycatcher. Journal of Animal Ecology 55: 1115-1134.
Slagsvold, T. 1990. Fisher’s sex ratio theory may explain hatching patterns in birds. Evolution 44: 1009-1017.
Slagsvold, T., Amundsen, T. & Dale, S. 1995. Cost and benefits of hatching asynchrony in blue tits Parus caeruleus. Journal of Animal Ecology 64: 563-578.
Smith, H.G. 1989. Larger clutches take longer to incubate. Ornis Scandinavica 20: 156-158.
Smith, H.G., Källander, H. & Nilsson, J.-Å. 1987. Effect of experimentally altered brood size on frequency and timing of second clutches in the Great Tit. Auk 104: 700-706.
Stoleson, S.H. 1997. Double jeopardy and the parameterization of brood reduction models: a comment on Mock and Forbes (1994). Auk 111: 137-140.
Stoleson, S.H. & Beissinger, S.R. 1995. Hatching asynchrony and the onset of incubation in birds, revisited. Current Ornithology 12: 191-270.R. 1997. Hatching asynchrony, brood reduction, and food limitation in a neotropical parrot. Ecological Monographs 67: 131-145.
Tinbergen, J.M. 1987. Costs of reproduction in the Great Tit: intraseasonal costs associated with brood size. Ardea 75: 111-122.
Tyrväinen, H. 1969. The breeding biology of the Red-wing (Turdus iliacus L.). Annales Zoologici Fennici 6: 1-46.
Vince, M.A. 1969. Embryonic communication, respiration and the synchronization of hatching. In: Hinde, R. A. (ed) Bird vocalizations: their relations to current problems in biology and psychology. Cambridge; Cambridge University Press, Cambridge: 233-260.
Ward, P. 1965. The breeding biology of the Black-faced Dioch Quelea quelea in Nigeria. Ibis 107: 326-349.
Winkler, D.W. & Walters, J.R. 1983. The determination of clutch size in precocial birds. Current Ornithology 1: 33-68.
Wootton, J.T., Young, B.E. & Winkler, D.W. 1991. Ecological versus evolutionary hypotheses: demographic stasis and the Murray-Nolan clutch size equation. Evolution 45: 1947-1950.
Fig. 1. The probability that any young will survive to leave the nest as a function of clutch size. The survival of a nest’s contents is a function of its daily survival rates (ds < 1) (see Eq. 5). For a given schedule of daily survival rates, and assuming that incubation begins with the laying of the last egg, the probability of nest success (si) increases as the clutch size decreases—a four-egg clutch has a greater si than a five-egg clutch. By laying a smaller clutch a female increases her probability of rearing any young. The minimum allowable clutch size is the replacement clutch size.
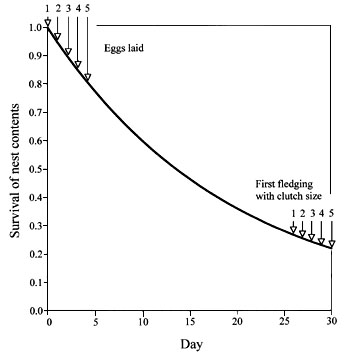
Fig. 2. The probability that any young from a clutch of a given size will survive to leave the nest as a function of the start of incubation. The survival of a nest’s contents is a function of the daily survival rates (ds < 1) (see Eq. 5). For a given clutch size, the probability of nest success (si) increases as incubation begins earlier—any clutch has a higher probability of producing any young to leave the nest when incubation starts earlier. The earliest that incubation can start, however, is constrained by the increasing probability of mortality of later hatched chicks.
Fig. 3. The probability of nest success (si) for a species with a ten-egg clutch starting incubation with the first, fifth, and tenth egg.