S11.2: The importance of the early onset of incubation for the maintenance of egg viability
Scott H. Stoleson
Rocky Mountain Research Station, USDA Forest Service, 2205 Columbia SE, Albuquerque, New Mexico, USA, fax 505 766 1046, e-mail sstoleso/rmrs_albq@fs.fed.us
Stoleson, S.H. 1999. The importance of the early onset of incubation for the maintenance of egg viability. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
600-613. Johannesburg: BirdLife South Africa.In the absence of incubation, the hatchability of avian eggs declines slowly. The rate of decline may be greater at high or very low than moderate temperatures because embryo development is temperature-dependent. Normally, development is delayed until eggs are warmed to 24-27 ░C by incubation. Once embryological development begins, prolonged deviation from normal incubation temperatures (34-38 ░C) results in abnormal development or death. If ambient temperatures are sufficiently high to initiate embryo development, or sufficiently low to kill embryos, parents may be obliged to initiate continuous incubation early to ensure the viability of eggs. Asynchronous hatching would result. This Egg Viability Hypothesis has been tested experimentally only four times in the field: twice with waterfowl in a high latitude temperate site, once with House Sparrows Passer domesticus in a Mediterranean climate, and once with Green-rumped Parrotlets Forpus passerinus in Venezuela where ambient temperatures regularly exceeded 30 ░C. For all but ducks at high latitudes, eggs exposed to ambient temperatures showed a decline in hatchability after three or more days of exposure. Hatchability was negatively correlated with duration of exposure. Rates of decline were greater for parrotlets than for ducks at high latitudes and cool temperatures, but similar to temperate coots and sparrows at high temperatures. These results suggest that the decline in egg viability varies with temperature, and is likely to be an important factor influencing the onset of incubation and hatching asynchrony in warm and very cold climates.
INTRODUCTION
Hatching asynchrony is common among birds, and occurs in variety of species in very different habits and environments (Stoleson & Beissinger 1995). Most empirical and theoretical work on the subject has focused on identifying an adaptive function for hatching patterns (Amundsen & Slagsvold 1991, and references therein). The adaptive significance of the early onset of incubation has received little attention, despite the fact that early incubation is the proximate cause of asynchronous hatching.
One hypothesis that may be broadly applicable among birds of disparate habits and habitats is the Egg Viability Hypothesis. It suggests that the early onset of incubation may reduce or eliminate hatching losses due to the loss of viability in early-laid eggs, and that hatching asynchrony is merely a consequence of maintaining egg viability (Arnold et al. 1987). It is based on the well-documented fact that the viability of eggs declines over time when left unincubated (Meijerhof 1992). By initiating incubation before a clutch is complete, parent birds reduce the time that first-laid eggs are left unincubated. In turn, the probability of hatching failure of those eggs due to declines in viability will be reduced or eliminated. Of course, if the initiation of incubation is delayed, hatching will be at least partially asynchronous, except for those species that have evolved mechanisms to synchronise hatching through embryonic vocalisations (e.g., Vince 1964, Davies & Cooke 1983, Schwagmeyer et al. 1991). The resulting size hierarchy among nestlings may have no adaptive significance, and may result in a handicap for smallest young because of their poor competitive abilities. Thus, there may exist a trade off: benefits accrued by increasing the viability of first laid eggs through early incubation may be offset by reduced survival of last-hatched offspring (Stoleson and Beissinger 1995, Vi˝uela 1997, Beissinger 1999).
Because the development and survival of avian embryos are strongly influenced by temperature (Romanoff & Romanoff 1972), it has been proposed that the rate of viability loss may be greater when ambient temperatures are high or very low (Arnold 1993, Veiga & Vi˝uela 1993, Stoleson & Beissinger 1995). In this paper I discuss the idea that hatching asynchrony is a consequence of early incubation to maintain egg viability, present experimental and observational data relevant to the concept, and speculate about how important a selective force it may be among birds.
Potential physiological mechanisms
Embryonic development in birds is temperature-dependent. Normally, little or no development takes place when temperatures are below 24░ to 27 ░C (Fig. 1). This threshold is known as physiological zero (Webb 1987). Under most conditions, when eggs are left unincubated at temperatures below this threshold, their development is suspended (Ewert 1992). In this way, birds may delay incubation until a complete clutch has been laid and hatch their eggs synchronously. Normally, the viability of eggs exposed to temperatures below physiological zero declines slowly (Decuypere & Michels 1992). At these temperatures, reduced viability of eggs is unlikely to constitute a major selective force, except perhaps for species with very large clutch sizes (Arnold et al. 1987).
Even at temperatures between physiological zero and freezing there are subtle differences in egg viability. Research on domestic poultry has shown that maximum hatchability is achieved when eggs are stored at 16 - 17░ C for periods of up to 7 days, and at 10 – 12 ░C for longer periods (Proudfoot & Hulan 1983, Meijerhof 1992). Exposure to temperatures below freezing is normally fatal to embryos, although there are reports of eggs of Mallard Anas platyrhynchos and domestic turkey Melleagris gallopavo hatching after being frozen (Webb 1987). Maintaining the viability of eggs where temperatures drop below freezing may be important for arctic and subarctic species, and for temperate latitude species that breed early in spring (e.g., Bubo owls).
Incubation temperatures tend to be very conservative in birds: almost all species incubate their eggs within a narrow range from 34░ to 40 ░C (Fig. 1; Drent 1975, Webb 1987, Rahn 1991). Much of the variation in this range can be attributed to methodological differences in measuring temperature. Attentiveness of parents varies with temperature (Weathers & Sullivan 1989). If ambient temperatures are within normal incubation temperatures, birds may not need to actively incubate eggs. Embryos are especially sensitive to temperatures above normal incubation range, however (Fig. 1). Even relatively short exposure to temperatures above 42░ - 45 ░C is fatal to embryos regardless of their developmental stage (Drent 1973, Bennett et al. 1981, Grant 1982, Webb 1987). Parental attentiveness increases greatly when temperatures exceed this level (e.g. Yom-Tov et al. 1978, Walsberg & Voss-Roberts 1983, Jehl & Mahoney 1987). Presumably, eggs exposed to ambient temperatures in the range of normal incubation for any length of time will begin to develop normally.
Between physiological zero and normal incubation temperatures, different structures within the avian embryo develop at different rates (Wilson 1991). Prolonged exposure of eggs to temperatures within this range results in abnormal development and, eventually, embryo mortality (Fig. 1; Romanoff & Romanoff 1972, Deeming & Ferguson 1992). Unfortunately, the effects of maintaining unincubated eggs in such ambient temperatures are poorly known (Grant 1982). Short term exposure to such temperatures may have no effect on embryos, or may initiate development. However, exposure for extended periods is likely to reduce the viability of eggs more rapidly than would occur below physiological zero because development will progress abnormally.
Once development begins, avian embryos become increasingly sensitive to changes in temperatures (Romanoff & Romanoff 1972, Deeming & Ferguson 1992). Parents may then be obliged to maintain steady incubation to ensure the survival of embryos (Haftorn 1988). Where ambient temperatures are sufficiently high to initiate development even in the absence of incubation, there may be strong selective pressure to initiate incubation early. The situation is likely to be more complex than suggested here, however, as nest attentiveness has been shown to increase gradually in many species (e.g. Haftorn 1981, Zerba & Morton 1983, Morton & Pereyra 1985, Meijer 1990).
EVIDENCE FOR VIABILITY EFFECTS
Various sources of evidence support the concept that potential declines in viability may be a factor influencing the onset of incubation in birds. These sources include studies of poultry egg storage, anecdotal accounts, and direct experimental tests of the hypothesis.
Egg viability in commercial poultry
Much applied research in poultry science has examined the effects of storage environment on the hatchability of eggs in artificial incubators. Generally the goal has been to identify those environmental factors that maximise egg viability over time. The length of time left unincubated is the major determinant of whether an egg hatches or not (Fasenko et al. 1991, Meijerhof 1992), but ambient conditions during storage are also important. Temperature is the most critical condition. Except for temperatures near or below freezing, the cooler the temperature, the greater the viability for a given storage time (Proudfoot & Hulan 1983, Wilson 1991, Decuypere & Michels 1992, Ancel et al. 1995). Hatching is also affected by humidity, gaseous environment, egg orientation and egg turning (Wilson 1991, Deeming 1992, Fasenko et al. 1992, Meijerhof 1992). Even when stored at cool temperatures, poultry eggs can show a reduction in viability after two or three days (Meijerhof 1992). There is no evidence that eggs stored for less than two or three days suffer any reduction in their viability, suggesting that a temporal threshold exists for any detectable viability loss to be manifested (Meijerhof 1992).
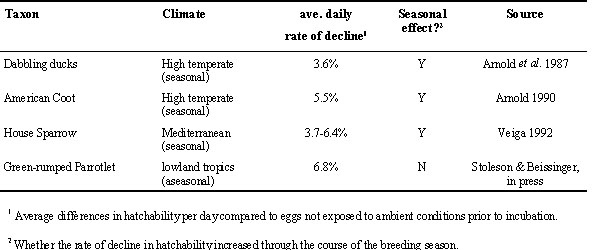
A number of studies have quantified the rate of viability loss under various storage conditions (summarized in Arnold et al. 1987: Table 2). The average daily decline in viability ranged from a 9.4% loss per day (Scott 1933) to an increase of 1.7% per day (Blatt and Cornwell 1972). The mean rate of viability decline was 2.8% per day. Poultry eggs stored at optimal temperatures lose their viability at a rate of 0.5% per day (Meijerhof 1992)
The poor success of efforts to increase egg viability in domestic species through artificial selection suggests that egg viability is a conservative trait (Crittenden & Bohren 1961, Nordskag & Hassan 1971). As such, it may be unlikely to vary greatly among avian taxa.
In summary, poultry research has demonstrated repeatedly that the viability of unincubated eggs declines over time, and the rate of that decline is most dependent on temperature. There appears to be a threshold duration of about two to three days, before which no detectable loss of viability occurs. And finally, the loss of egg viability appears to be a conservative trait.
Anecdotal evidence
Declines in egg viability are likely to affect all avian species regardless of whether they hatch their eggs asynchronously or not. Eggs of precocial, nidifugous species are generally thought to hatch synchronously because chicks must leave the nest and follow parents immediately after hatching (Lack 1968). However, for numerous precocial species, incubation is initiated before the clutch is complete. Early incubation has been documented in various waterfowl (e.g., Arnold et al. 1987, Kennamer et al. 1990), shorebirds (e.g., Hussell & Page 1976), rheas (Cannon et al. 1986), and junglefowl (Meijer & Siemers 1993). For some of these species, synchronisation of hatching is achieved through a process of differential development of eggs induced by acoustic signals among embryos (Vince 1964, 1968). Other species hatch their eggs somewhat asynchronously (e.g., Cargill & Cooke 1981).
Regardless of hatching patterns, many precocial species initiate incubation before their clutch is complete. Many alternate hypotheses for hatching asynchrony (reviewed in Magrath 1990, Stoleson & Beissinger 1995) seem inadequate to explain early incubation in these species. The existence of a mechanism to reduce or eliminate hatching asynchrony is incompatible with hypotheses that posit an adaptive function to size disparities among nestlings. Hypotheses based on food limitations are untenable because young of these species are self-feeding at hatching. Hypotheses based on minimising the risk of total brood loss by adjusting the duration of laying and nestling periods (e.g., Clark & Wilson 1981, Murray 1994) are also untenable because precocial, nidifugous young leave the nest on hatching, so there is no nestling period per se. Only those hypotheses for which the critical period is laying (see Stoleson and Beissinger 1995: Table 1.1) may apply to these species. Of these, the Egg Viability Hypothesis, along with the Predation Hypothesis, are likely to be the most universal (although hypotheses are not mutually exclusive, and more than one may apply to any single species).
Except for species that hatch their eggs completely asynchronously, the Egg Viability Hypothesis suggests that the hatching success of early-laid eggs should be lower than that of later eggs. Few data are available to support or refute this prediction, but reduced viability in first eggs has been reported in several taxa (e.g., Veiga & Vi˝uela 1993, Potti & Merino 1996, Vi˝uela 1997).
Experimental evidence
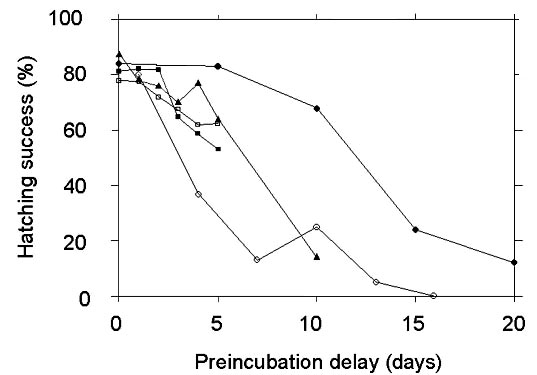
Very few experimental studies have been conducted to test the Egg Viability Hypothesis under field conditions (Fig. 2). These have differed somewhat in methodology, climate, and taxa of focus. However, all provide support for the hypothesis. In these experiments, eggs were experimentally subjected to varying lengths of preincubation delay at ambient temperatures to simulate a delay in the onset of incubation. Eggs were subsequently incubated either artificially or naturally.
Dabbling ducksArnold et al. (1987) first proposed the Egg Viability Hypothesis to explain why waterfowl begin incubation during laying yet hatch their clutches synchronously. They suggested that ducks and other species with self-feeding young may begin incubation to maintain high hatching success, and further suggested that declines in egg viability are an important constraint on clutch size in these species. They tested these ideas with breeding ducks (Anas spp.) in southern Manitoba, Canada (50 10' N, 99 47' W). Ambient temperatures increased seasonally, regularly exceeding physiological zero late in the breeding season (T. Arnold, personal communication). Eggs were hatched in an incubator in this study.
Experiments revealed that duck eggs showed no significant loss of hatchability after five days of preincubation exposure, but a significant loss by ten days (Arnold et al. 1987). The average daily decline in hatchability was 3.6%. In a second set of experiments, Arnold (1993) tested the effects of factors other than length of preincubation delay by holding all eggs for 15 days. The hatchability of eggs was significantly affected by laying date and temperature. Eggs showed greater hatchability earlier in the breeding season, and when exposed to lower temperatures. It is likely that temperature and laying date were highly correlated in these experiments.
American Coots
Arnold (1990) tested the Egg Viability Hypothesis as part of a study of breeding in the American Coot (Fulica americana) in Manitoba, Canada. Diurnal temperatures at the study site during the breeding season of the Coot regularly exceeded 30 ░C. Coot eggs showed a rapid decline in viability if left unincubated (average daily loss = 5.5%). No eggs hatched after 14 days of preincubation delay. As with the ducks, viability was affected by laying date and temperature. No obvious temporal threshold was detected, but that may be because eggs were assigned to preincubation delay intervals of 3 days, so the study lacked a high degree of resolution to detect a threshold of under 4 days.
House SparrowsVeiga and his associates studied egg viability in House Sparrows (Passer domesticus) in central Spain (40 N, 4 W) in a Mediterranean climate (Veiga 1992, Veiga & Vi˝uela 1993). Average ambient temperatures increased through the breeding season at this site, and regularly exceeded physiological zero later in the season. For this reason, Veiga analysed the effects of egg viability separately for early and late season nests. Unlike the ducks studied by Arnold, House Sparrows are altricial, and regularly hatch their eggs partially asynchronously. After exposure, eggs were incubated naturally.
The viability of experimental eggs declined with exposure time. The rate of decline differed between early and late season nests: the average daily rate of viability decline early in the season was 3.7%, while for late season nests it increased to 6.4%. Declines in viability were evident after as little as two days of preincubation exposure, although differences were not statistically significant until three or more days of exposure. Hatching success of control eggs in these experiments varied with laying order, especially later in the breeding season. Early-laid eggs (i.e., those exposed to ambient conditions prior to the onset of full incubation) were less likely to hatch than eggs laid later.
Green-rumped ParrotletsStoleson and Beissinger (in press) tested the Egg Viability Hypothesis in the altricial Green-rumped Parrotlet Forpus passerinus. This Neotropical parrot inhabits lowlands in Venezuela (8 34' N, 67 35' W) where temperature did not vary seasonally. The parrotlet differs from the species mentioned above in that it initiates incubation on the first egg (Beissinger & Waltman 1991). Clutch sizes in this species were unusually large for a lowland tropical species, ranging from 4 to 10 eggs (Beissinger & Waltman 1991). In these experiments, eggs were returned to active nests after exposure and incubated naturally.
The viability of parrotlet eggs declined fairly rapidly when left unincubated, averaging 6.8% loss per day. However, the decline was not linear, and no significant declines occurred until three or more days of exposure. No seasonal change in egg viability was detected. Monitoring of ambient temperatures revealed that physiological zero was exceeded on a daily basis, but temperatures reached normal incubation levels for only a small fraction of each day.
For almost all birds, normally-incubated eggs that fail to hatch fail either very early or very late in embryological development, and rarely show an intermediate level of development (Romanoff 1949, Ancel et al. 1995). Embryo mortality after intermediate levels of development are characteristic of gross anomalies induced by abnormal temperatures (Romanoff & Romanoff 1972). A significant portion of failed parrotlet eggs exposed to ambient temperatures showed intermediate levels of embryo development. This result is expected if unincubated eggs begin to develop in temperatures below normal incubation temperatures, and so develop abnormally.
Comparisons of results from experimental studiesDespite differences in study species and environments, the four experimental field studies of the Egg Viability Hypothesis show similar results (summarised in Table 1). The differences in results that did occur are consistent with what the hypothesis would predict based on environmental differences among the study sites.
All four studies demonstrated that the hatchability of eggs declined at a rate correlated to the length of preincubation exposure to ambient temperatures. The rates of decline varied from 3.6% to 6.8% per day. The lowest rates were demonstrated at a subarctic site, and the highest at a tropical lowland site. This is consistent with the suggestion that the rates of decline, and hence the probable importance of egg viability effects, are related to warm temperatures. All of the temperate sites, which had seasonal increases in temperature, showed seasonal increases in the rate of decline. The tropical site, with no seasonal temperature trend, showed no change in the rate of decline. For three studies there appeared to be an exposure threshold before which no decline in egg viability was detected. The threshold was three days for both House Sparrows and parrotlets, similar to that reported for domestic poultry (Meijerhof 1992). In ducks, loss of viability was detected between five and ten days of preincubation exposure. This occurred because Arnold et al. (1987) used 5-day intervals of exposure time, so the degree of resolution offered by their results is low. Similarly, Arnold (1990) used a 3-day interval of exposure in his study of American Coots. Although egg viability declined significantly between 1 and 4 days of exposure, it is unclear whether this was a constant decline or represents an exposure threshold. It is unknown if the difference in apparent threshold times is due to sample sizes, phylogeny, environmental effects, or some other factor.
DISCUSSION
Implications of the Egg Viability Hypothesis
Results of experimental field work and poultry research clearly show that differences in the rate of loss of viability are linked, at least in part, to temperature differences. Higher temperatures appeared to produce greater declines in egg viability. The exact mechanistic cause of this relationship remains to be determined. One likely possibility is that higher temperatures increase the rate of embryo development due to passive incubation; the more prolonged the exposure to high temperatures, the greater the degree of embryo development. Embryos become increasingly sensitive to changes in temperatures with increased development (Webb 1987). Thus, the greater susceptibility of embryos to mortality at higher temperatures may be due to greater development, and hence sensitivity (Veiga 1992).
This effect of temperature on viability has numerous implications for avian breeding biology. One should expect birds to facultatively adjust the onset of incubation, when possible, to temperature in order to maintain egg viability. As temperatures increase seasonally in nontropical climates, birds may be expected to advance the onset of incubation to counteract the expected higher decline in egg viability. One obvious consequence is that the degree of hatching asynchrony should also increase seasonally. Such seasonal increases in asynchrony have been documented in numerous taxa (e.g., Nisbet & Cohen 1975, Slagsvold & Lifjeld 1989, Arnold 1993, Murphy 1994, Yogev et al. 1996). However, it is not a universal phenomenon (see Bryant 1978).
If incubation patterns cannot be changed, then one would expect the average hatchability of eggs to decline with increasing ambient temperatures. Very few studies have reported seasonal variation in hatching success, and their results are mixed (Arnold 1993). Temperatures also increase with decreasing latitude, so that a latitudinal decline in egg hatchability would be predicted by the Egg Viability Hypothesis. In fact, Koenig (1982) demonstrated a significant positive correlation between latitude and average hatchability of eggs for a sample of 42 bird families.
Arnold et al. (1987) argued that the risk of egg failure due to viability losses constrained clutch size in waterfowl. At a certain point, the benefits of delaying the onset of incubation to lay another egg are offset by the reduced hatchability of first-laid eggs. This constraining effect on clutch size is likely to apply to other taxa in which a high degree of hatching asynchrony or an early onset of incubation are not possible. If so, then in some avian taxa, clutch size should show decreases inversely correlated with ambient temperatures. Such trends should occur seasonally or with decreasing latitude (Arnold et al. 1987). Seasonal and latitudinal trends in clutch size are common (Lack 1954, Rowe et al. 1994, Young 1994), and numerous explanations have been proposed, including variation in food availability, parental experience, prospects for offspring survival, and others. Of course, these hypotheses are not mutually exclusive, and evidence suggests that all may apply to at least some species. The Egg Viability Hypothesis should be included as another factor influencing clutch size variation in birds. It also provides an explanation for inconvenient exceptions to general rules based on the factors listed above. For example, the fact that the Barn Owl Tyto alba shows no latitudinal trend in clutch size has been viewed as anomalous (Wilson et al. 1986). However, because this species begins incubation on the first egg, it is not subject to viability constraints on clutch size. Similarly, first-egg incubation may partly explain how the Green-rumped Parrotlet is able to lay and hatch clutches of up to 10 eggs when 2-3 eggs are the norm for most Neotropical species (Beissinger & Waltman 1991).
Just as there is a tradeoff between clutch size and reduced viability of early eggs in waterfowl (Arnold et al. 1987), there is likely to be a tradeoff between reduced viability of early-laid eggs and reduced probability of survival of later-hatched young due to hatching asynchrony (Stoleson & Beissinger 1995). With an earlier onset of incubation, and therefore greater viability of early eggs, the greater the size disparities within a brood and the greater the likelihood of mortality for the smallest young. The optimal timing for the onset of incubation will depend on this tradeoff, as well as any additional costs or benefits derived from early incubation (e.g. limited breeding opportunities; Beissinger & Waltman 1991) or a nestling size hierarchy (e.g. brood reduction; Mock & Forbes 1994). Thus, egg viability is likely to be just one of several factors influencing the onset of incubation in birds, and its relative importance is likely to vary with environmental conditions.
Recommendations for future research
Although the Egg Viability Hypothesis has been tested in the field with four very different taxa, much remains to be learned about the importance of early incubation for the maintenance of egg viability. Research needs to be conducted on a broader range of species to determine how universal viability loss is among taxa. Also, a single species should be examined across a broad range of environments to help control for variation among taxa. Such a study may permit the discrimination of specific effects of temperature, latitude, and season on hatching asynchrony and clutch size. It may also be advantageous to examine the effects of egg viability in precocial species in a hot climate, as this would address the importance of the early onset of incubation without the complications of hatching asynchrony creating competitive disparities among offspring.
In summary, the viability of eggs declines without incubation. The rate of this decline appears to be greater at higher temperatures. Experimental work has demonstrated declines in viability in several disparate avian taxa, but more research is needed to ascertain that the phenomenon is universal among birds. Loss of viability may be minimised by the early onset of incubation, but this also results in hatching asynchrony and the increased likelihood of partial brood loss. The timing of incubation and resulting hatching pattern of young is likely to represent a tradeoff between these two opposing selective factors, as well as others that influence parental fitness. The impact of egg viability decline may also be a factor influencing latitudinal and seasonal variation in clutch size, hatching asynchrony, and egg hatchability.
ACKNOWLEDGMENTS
Many of the ideas presented here were formulated with Steve Beissinger, and benefited from discussions with, and comments from, T. Amundsen, T. Arnold, C. Bosque, J. Gibbs, B. Hall, P. Stoleson and J. Vi˝uela. I thank M. Apostol, K. Brittin, D. Casagrande, J. Clemmons, B. Elderd, D. Gaylor, A. Pacheco, S. Spector and S. Tygielski for helping to collect field data on parrotlets. Comments from S. Beissinger improved an earlier draft. Writing of this manuscript was supported by the USDA Forest Service.
REFERENCES
Amundsen, T. & Slagsvold, T. 1991. Hatching asynchrony: facilitating adaptive or maladaptive brood reduction? Acta XX Congressus Internationalis Ornithologici 3: 1707-1719.
Ancel, A., Liess, S. & Girard, H. 1995. Embryonic development of the domestic guinea fowl (Numida meleagris). Journal of Zoology, London 235: 621-634.
Arnold, T.W. 1990. Food limitation and the adaptive significance of clutch size in American coots (Fulica americana). Ph.D. dissertation, Department of Zoology, University of Western Ontario, London.
Arnold, T.W. 1993. Factors affecting egg viability and incubation time in prairie dabbling ducks. Canadian Journal of Zoology 71: 1146-1152.
Arnold, T.W., Rohwer, F.C. & Armstrong, T. 1987. Egg viability, nest predation, and the adaptive significance of clutch size in prairie ducks. American Naturalist 130: 643-653.
Batt, B.D.J. & Cornwell, G.W. 1972. The effects of cold on mallard embryos. Journal of Wildlife Management 36:745-751.
Beissinger, S. R. 1999. Interaction of egg viability, brood reduction and nest failure on the onset of incubation. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 638-646. Johannesburg: BirdLife South Africa.
Beissinger, S. R. & Waltman, J.R. 1991. Extraordinary clutch size and hatching asynchrony of a neotropical parrot. Auk 108: 863-871.
Bennett, A. F. Dawson, W.R., & Putnam, R.W. 1981. Thermal environment and tolerance of embryonic Western Gulls. Physiological Zoology 54: 146-154.
Bryant, D.M. 1978. Establishment of weight hierarchies in the broods of House Martins Delichon urbica. Ibis 120: 16-26.
Cannon, M.E., Carpenter, R.E. & Ackerman, R.A. 1986. Synchronous hatching and oxygen consumption of Darwin's Rhea eggs (Pterocnemia pennata). Physiological Zoology 59: 95-108.
Cargill, S.M. & Cooke, F. 1981. Correlation of laying hatching sequences in clutches of the Lesser Snow Goose (Anser caerulescens caerulescens). Canadian Journal of Zoology 59: 1201-1204.
Clark, A B. & Wilson, D.S. 1981. Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Quarterly Review of Biology 56: 253-277.
Crittenden, L.B. & Bohren, B.B. 1961. The genetic and environmental effects of hatching time, egg weight, and holding time on hatchability. Poultry Science 40: 1736-1750.
Davies, J.C., & Cooke, F. 1983. Intraclutch hatch synchronization in the Lesser Snow Goose. Canadian Journal of Zoology 61: 1398-1401.
Decuypere, E. & Michels, H. 1992. Incubation temperature as a management tool: a review. World's Poultry Science Journal 48: 28-38.
Deeming, D.C. 1992. Reasons for the dichotomy in egg turning in birds and reptiles. Pp. 307-323 in Egg incubation: Its effects on embryonic development in birds and reptiles (D. C. Deeming & M. W. J. Ferguson, Eds). Cambridge, Cambridge University Press.
Deeming, D.C. & Ferguson, M.W.J. 1992. Physiological effects of incubation temperature on embryonic development in reptiles and birds. Pp. 147-173 in Egg incubation: Its effects on embryonic development in birds and reptiles (D. C. Deeming & M. W. J. Ferguson, Eds.). Cambridge, Cambridge University Press.
Drent, R.H. 1973. The natural history of incubation. Pp. 262-322 in Breeding biology of birds (D. S. Farner, Ed.). Washington DC, National Academy of Science.
Drent, R.H. 1975. Incubation. Pp. 333-420 in Avian Biology, vol. 5 (D. S. Farner & J. R. King, Eds.). New York, Academic Press.
Ewert, M A. 1992. Cold torpor, diapause, delayed hatching and aestivation in reptiles and birds. Pp. 173-191 in Egg incubation: Its effects on embryonic development in birds and reptiles (D. C. Deeming & M. W. J. Ferguson, Eds.). Cambridge, Cambridge University Press.
Fasenko, G.M., Robinson, F.E., Armstrong, J.G., Church, J.S., Hardin, R.T. & Petitte, J.N. 1991. Variability in preincubation embryo development in domestic fowl 1. Effects of nest holding time and method of egg storage. Poultry Science 70: 1876-1881.
Fasenko, G.M., Hardin, R.T., Robinson, F.E. & Wilson, J.L. 1992. Relationship of hen age and egg sequence position with fertility, hatchability, viability and preincubation embryonic development in broiler breeders. Poultry Science 71: 1374-1383.
Grant, G.S. 1982. Avian incubation: egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Ornithological Monographs 30: 1-75.
Haftorn, S. 1981. Incubation during the egg-laying period in relation to clutch-size and other aspects of reproduction in the Great Tit Parus major. Ornis Scandinavica 12: 169-185.
Haftorn, S. 1988. Incubating female passerines do not let the egg temperature fall below the "physiological zero temperature" during their absences from the nest. Ornis Scandinavica 19:97-110.
Hussell, D.J.T. & Page, G.W. 1976. Observations on the breeding biology of Black-bellied Plovers on Devon Island, Northwest Territories, Canada. Wilson Bulletin 88: 632-653.
Jehl, J.R., Jr. & Mahoney, S.A. 1987. The roles of thermal environment and predation in habitat choice in the California Gull. Condor 89: 850-862.
Kennamer, R.A., Harvey, W.F. IV & Hepp, G.R. 1990. Embryonic development and nest attentiveness of Wood Ducks during egg laying. Condor 92: 587-592.
Koenig, W.D. 1982. Ecological and social factors affecting hatchability of eggs. Auk 99: 526-536.
Lack, D. 1954. The Natural Regulation of Animal Numbers. London, Oxford University Press.
Lack, D. 1968. Ecological adaptations for breeding in birds. London, Methuen & Co.
Magrath, R.D. 1990. Hatching asynchrony in altricial birds. Biological Review 65: 587-622.
Meijer, T. 1990. Incubation development and clutch size in the Starling. Ornis Scandinavica 21: 163-168.
Meijer, T. & Siemers, I. 1993. Incubation development and asynchronous hatching in Junglefowl. Behaviour 127: 309-321.
Meijerhof, R. 1992. Pre-incubation holding of hatching eggs. World's Poultry Science Journal 48: 57-81.
Mock, D.W. & Forbes, L.S. 1994. Life-history consequences of avian brood reduction. Auk 111: 115-123.
Morton, M.L. & Pereyra, M.E. 1985. The regulation of egg temperatures and attentiveness patterns in the Dusky Flycatcher (Empidonax oberholseri). Auk 102: 25-37.
Murphy, M.T. 1994. Breeding patterns of Eastern Phoebes in Kansas: adaptive strategies or physiological constraint? Auk 111: 617-633.
Murray, B.G. 1994. Effect of selection for successful reproduction on hatching synchrony and asynchrony. Auk 111: 806-813.
Nisbet, I.C.T. & Cohen, M.E. 1975. Asynchronous hatching in Common and Roseate Terns, Sterna hirundo and Sterna dougallii. Ibis 117: 374-379.
Nordskag, A.W. & Hassan, G.M. 1971. Direct and maternal effects of egg size genes on hatchability. Genetics 67: 267-278.
Potti, J. & Merino, S. 1996. Causes of hatching failure in the Pied Flycatcher. Condor 98: 328-336.
Proudfoot, F.G. & Hulan, H.W. 1983. Agriculture Canada Publication, 1573/E: Care of hatching eggs before incubation.
Rahn, H. 1991. Why birds lay eggs. In: Deeming, D.C. & Ferguson, M.W.J. (eds) Egg incubation: its effects on embryonic development in birds and reptiles: 345-360. Cambridge, Cambridge University Press.
Romanoff, A.L. 1949. Critical periods and causes of death in avian embryonic development. Auk 66: 264-270.
Romanoff, A.L. & Romanoff, A.J. 1972. Pathogenesis of the Avian Embryo. New York, J. Wiley & Sons.
Rowe, L., Ludwig, D. & Schluter, D. 1994. Time, condition, and the seasonal decline of avian clutch size. American Naturalist 143: 698-722.
Schwagmeyer, P.L., Mock, D.W., Lamey, T.C., Lamey, C.S. & Beecher, M.D. 1991. Effects of sibling contact on hatch timing in an asynchronously hatching bird. Animal Behaviour 41: 887-894.
Scott, H.M. 1933. The effect of age and holding temperatures on hatchability of turkey and chicken eggs. Poultry Science 12: 49-54.
Slagsvold, T. & Lifjeld, J.T. 1989. Constraints on hatching asynchrony and egg size in Pied Flycatchers. Journal of Animal Ecology 58: 837-845.
Stoleson, S.H. & Beissinger, S.R. 1995. Hatching asynchrony and the onset of incubation in birds, revisited: when is the critical period? In: Power, D.M. (ed.) Current Ornithology, vol. 12, 191-270. New York, Plenum Press.
Stoleson, S.H. & Beissinger, S.R. In press. Egg viability as a constraint on hatching synchrony at high ambient temperatures. Journal of Animal Ecology.
Veiga, J.P. 1992. Hatching asynchrony in the House Sparrow: a test of the egg-viability hypothesis. American Naturalist 139: 669-675.
Veiga, J.P. & Vi˝uela, J. 1993. Hatching asynchrony and hatching success in the House Sparrow: evidence for the egg viability hypothesis. Ornis Scandinavica 24: 237-242.
Vince, M.A. 1964. Social facilitation of hatching in the Bobwhite Quail. Animal Behaviour 12: 531-534.
Vince, M.A. 1968. Retardation as a factor in the synchronization of hatching. Animal Behaviour 16: 332-335.
Vi˝uela, J. 1997. Laying order affects incubation duration in the Black Kite (Milvus migrans): counteractng hatching asynchrony? Auk 114: 192-199.
Walsberg, G.E. & Voss-Roberts, K.A. 1983. Incubation in desert-nesting doves: mechanisms for egg-cooling. Physiological Zoology 56: 88-93.
Weathers, W.W. & Sullivan, K.A. 1989. Nest attentiveness and egg temperature in the Yellow-eyed Junco. Condor 91: 628-633.
Webb, D.R. 1987. Thermal tolerance of avian embryos: a review. Condor 89: 874-898.
Wilson, H.R. 1991. Physiological requirements of the developing embryo: temperature and turning. Pp. In: Tullett, S.G. (ed.) Poultry science symposium, 22: Avian incubation.145-156. London, Butterworth-Heineman.
Wilson, R.T., Wilson, M.P. & Durkin, J.W. 1986. Breeding biology of the Barn Owl Tyto alba in central Mali. Ibis 128: 81-90.
Yogev, A., Ar, A. & Yom-Tov, Y. 1996. Determination of clutch size and the breeding biology of the Spur-winged Plover (Vanellus spinosus) in Israel. Auk 113: 68-73.
Yom-Tov, Y., Ar, A. & Mendelssohn, H. 1978. Incubation behavior of the Dead Sea Sparrow. Condor 80: 340-343.
Young, B.E. 1994. Geographic and seasonal patterns of clutch-size variation in House Wrens. Auk 111: 545-555.
Zerba, E. & Morton, M.L. 1983. The rhythm of incubation from egg laying to hatching in Mountain White-crowned Sparrows. Ornis Scandinavica 14: 188-197.
Table 1. Experimental field studies of egg viability.

Fig. 1. The effects of temperature ranges on avian embryonic development. Limits of ranges are approximate, and may vary among taxa.
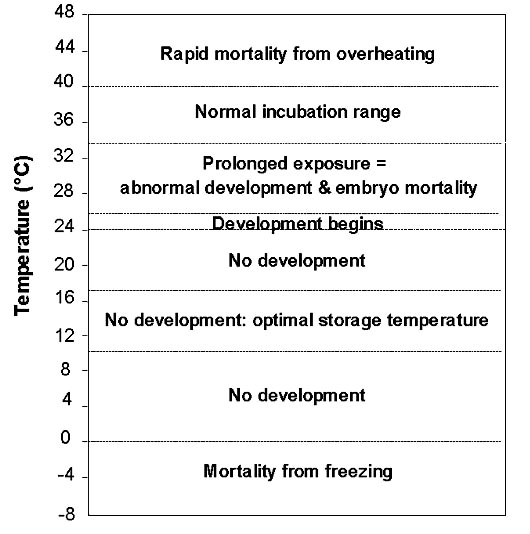
Fig. 2. Relationship between preincubation delay and hatching success from four experimental field studies. Results for American Coots are represented by open circles, for ducks by filled circles, for Green-rumped Parrotlets by filled triangles, and for House Sparrows by open squares (early season) and filled squares (late season). Experimental eggs were incubated in incubators for ducks and coots, and incubated by parent birds for sparrows and parrotlets.