S10.5: Time constraints and moult-breeding tradeoffs in large birds
Sievert Rohwer
Department of Zoology and Burke Museum, Box 353010, University of Washington, Seattle, Washington 98195, United States of America, fax 206 685 3039, e-mail rohwer@u.washington.edu
Rohwer, S. 1999. Time constraints and moult-breeding tradeoffs in large birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
568-581. Johannesburg: BirdLife South Africa.The primaries of birds grow at approximately 6 mm per day. In large birds the summed length of the primaries is so great that some species cannot breed successfully and also fully replace their primaries in a single year, even if feathers are replaced at several loci simultaneously. Thus, successive years of successful breeding may force some large birds to skip breeding to undergo an extensive moult that replaces all worn flight feathers. This is likely true for Laysan Albatross Diomedea immutabilis and Black-crowned Night Herons Nycticorax nycticorax. In Laysan Albatross the number of independent moult series within the primaries and secondaries, and the rules of feather replacement in these series seems adapted to moult-breeding tradeoffs. In Black-crowned Night Herons incomplete moults generate multiple loci of feather replacement within the primaries, known as stepwise moults. Unlike albatrosses and night herons, Pelagic Cormorants Phalacrocorax pelagicus have six months for moulting during winter; thus, adults need not skip breeding to replace overworn flight feathers. Dividing the primaries into more than one moult series seems advantageous over stepwise moulting, wherein multiple waves of feather replacement proceed through all the primaries, as a single large moult series. With multiple moult series the frequency of feather replacement in a series can be matched to the rate of feather wear in that series. Additionally, the inner primaries can be joined as a moult series with some of the outer secondaries, allowing greater efficiency in the replacement of all the flight feathers.
INTRODUCTION
A challenge to ornithologists is to make their studies of birds interesting to a diverse audience of biologists. Sadly, moult studies have so rarely risen to that challenge that even many ornithologists are uninterested in moult. But moult is a major issue in avian life histories. For small species it poses important energetic demands (Dietz et al. 1992; Lindstrom et al. 1993); for any species the replacement of wing and tail feathers must be costly to flight (Tucker 1991; Swaddle & Witter 1997; Mausman et al. 1988); and, for large birds the time demands of moult may constrain the frequency of breeding (Langston & Rohwer 1996, Shugart & Rohwer 1996).
In this paper I explore the basis for time constraints on moulting in large birds and consider adaptations and life history tradeoffs that may have resulted from the substantial time it takes large birds to replace their remiges. I also explore implications of the two major patterns of primary replacement to these life history tradeoff problems. Despite the Stresemanns' massive summary of flight feather replacement in birds (Stresemann & Stresemann 1966), progress in understanding the adaptive significance of variation in these patterns is hampered by inadequate descriptive data. Descriptions of wing quill moults rarely document the organisation of the wing into moult series, the direction of feather replacement within these series, and the frequency with which individual remiges are replaced. Improving such descriptions must become a priority for publications treating avian moult.
TIME CONSTRAINTS
Many workers have noticed the excessive time requirements for moult in large birds (e.g. Prevost 1983, Edelstam 1984). Fig. 1 summarises rates of primary feather generation reported by Prevost (1983: table 7) and others for 43 species. The data represent birds varying in size from small passerines to condors and, while small passerines grow their feathers at just 3.2 mm/day, the mean for large birds was only 6.3 mm/day. I know of no analysis of the reason for this constraint on feather growth rate. Examination of the cellular organization of follicles, where feathers are generated, suggests that the constraint lies in the rate at which the ring of collar cells of the feather follicle can divide, differentiate into cells that produce the particular structural elements of feathers, secrete the keratin microfibrils that constitute these elements, and die (see Lucas and Stettenheim 1972). Because every feather is proliferated from a single ring or short cylinder of actively dividing cells within a feather follicle, it is not surprising that the rate at which feathers grow is little affected either by feather size or by bird size.
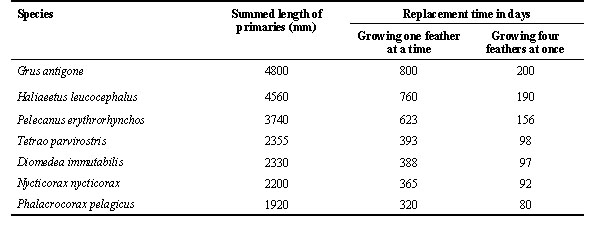
This constraint on the rate at which flight feathers grow dramatically affects the time required for large birds to replace all flight feathers. Table 1 presents the summed length of the primary feathers for seven large birds. If these species grew their primaries at 6 mm per day (Fig. 1) and if they grew them one feather at a time, from 320 to 800 days would be required for them to replace all of their primaries. If they grew four primaries simultaneously (an unusually high number for primaries), 80 to 200 days would still be required to replace all primaries. Most large birds do not overlap moult and breeding; most arrest moult during migration; and, many do not moult throughout the entire non breeding season. These constraints on overlapping moult with other demanding events in the annual cycle mean that most large birds cannot renew all of their flight feathers annually.
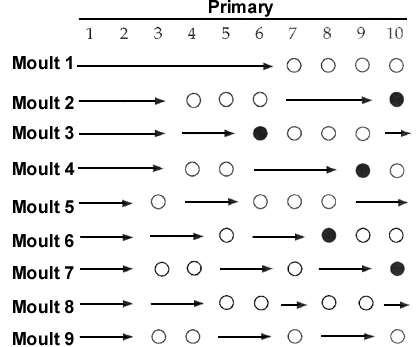
Three solutions to this rate-constraint problem are apparent in comparative patterns of flight feather replacement. First, many water birds that can forage and escape predators without flying, have long been known to lose their wing quills simultaneously. In such species the time required to regain flight becomes somewhat less than the time required to replace the longest primary of the wing. Second, other species that must maintain the ability to fly while moulting, divide the primaries into two or more moult series (Fig. 2). A moult series is defined as a set of remiges or rectrices that is replaced according to a single set of rules (Langston and Rohwer 1995; Yuri and Rohwer 1997). Having multiple moult series facilitates the replacement of different groups of primaries at different frequencies. Third, in still other species that must fly while moulting, the primaries are organised as a single moult series, but two or more waves of feather replacement proceed simultaneously through the primaries (Fig. 3). If every reinitation of moult includes P1, this results in the inner primaries being replaced more frequently than the outer primaries.
Large birds that maintain the ability of fly while moulting often replace primaries and secondaries at multiple loci simultaneously and often grow more than one feather simultaneously at these loci. Nonetheless, many such species cannot fully replace their flight feathers in the annual moult. When this is the case a life history tradeoff decision is faced by individuals at the start of the next breeding season. Should they breed and suffer yet another incomplete moult with its attendant survival cost, or should they skip breeding and rid their wings of worn feathers, thus increasing their survival (Langston & Rohwer 1996)? In the following section I examine the evidence that incomplete primary moults may drive large birds periodically to skip breeding in favour of moulting .
MOULT BREEDING TRADEOFFS
Laysan Albatross Diomedea immutabilis
This species breeds on the leeward Hawaiian Islands between 23o and 48o degrees N. Like all albatrosses, Laysans lay a single egg clutch, have an extremely long breeding season, and do not overlap moult and breeding (Fisher 1976; Brooke 1981; Furness 1988). Pairs that successfully fledged a chick in the preceding season are away from the breeding colony for less than four months each year; this gives them about 110 days to moult.
The pattern of primary replacement was documented in Laysan Albatrosses using a series of 191 specimens salvaged from the now banned North Pacific drift net fishery for squid (Langston and Rohwer 1995). Their primaries are organized into an inner and outer moult series (Fig. 2). Primaries 8-10 of the outer series are replaced annually; primaries 6-7 of the outer series are replaced much less frequently. Primaries 1-5 of the inner series (this series also includes several outer secondaries; Edwards and Rohwer, unpubl.) are replaced only about every third year (Langston and Rohwer 1995).
This unusual mode of feather replacement seems to be an adaptation to differential rates of feather wear. The outermost primaries become excessively worn in just one year's time because they are frequently dragged in the water or on the sand when these birds are soaring at sea or approaching their nest sites. In contrast, the short inner primaries are functionally similar to the 31-34 secondaries and are not excessively worn after a year's use. Laysan Albatrosses grow their primaries at a mean rate of 5.4 mm/day, and birds that are replacing feathers in both of the primary molt series grow an average of 3.3 primaries simultaneously. From these numbers and from the summed length of the primaries, we can calculate that Laysan Albatrosses need 131 days to replace all ten primaries in a single bout of moulting (Langston and Rohwer 1996). This is more time than they are away from the breeding colony.
Although Laysan Albatrosses are nominally annual breeders, a surprise from two long-term studies of breeding birds is that about 25% of breeders do not return to breed the following year (Rice and Kenyan 1962; Fisher 1976). Since the annual mortality rate is only about 3%, most of these birds are alive and simply not breeding. What are they doing? Near the end of the moulting season on the high seas, Langston and Rohwer (1996) found that about 20% of Laysan Albatrosses had replaced all ten primaries. Either these birds had failed early or they had skipped a year of breeding so that they could spend enough time moulting to have replaced an accumulation of 2- and 3-year-old primaries and secondaries in their wings. Skipping a year of breeding to undergo a complete moult should prepare adults to return to an annual breeding cycle for the next few years.
Black-crowned Night Heron Nycticorax nycticorax
This species breeds on four continents, but its moult has been studied carefully only in eastern Washington, near the northern edge of its breeding range in North America (Shugart and Rohwer 1996). Few night herons winter in Washington and the adults are largely gone from the state by mid September. In two days of field work on 6-7 September 1991, at a locality where night herons are abundant, Shugart and I (unpubl.) saw just 14 adults (We saw over 150 juveniles.); 60% of which had no gaps in their primaries, suggesting that primary moult has already been arrested in most adults by early September. Eggs are laid in late April and early May and broods fledge in late July or early August, giving adults that successfully breed less than two months to moult. In night herons the primaries constitute a single moult series within which several waves of moult may proceed simultaneously in a proximal to distal direction (Fig. 3). The number of waves of feather replacement and number of feathers separating waves is variable (Shugart and Rohwer 1996).
Washington night herons grow their primaries at a mean of 6.0 mm a day. At this rate 92 days would be required to replace all primaries if four primaries were grown simultaneously. Few individuals replace so many feathers at once. Shugart and Rohwer (1996) assessed the number of primaries that had been replaced by 19 adults taken in Eastern Washington during early spring, prior to the commencement of moult. These specimens showed a bimodal distribution in the number of primaries they had replaced in the previous moult, with modes at 3.5 and 9 primaries. We suspect that these two modes correspond to birds that failed to breed successfully in the previous season and to birds that fledged young. Some birds were moulting as early as late May or June and apparently either had not attempted to breed or had failed very early in the previous season. Birds that successfully fledge young will have less than two months in which to moult, during which time they would not be able to replace even half of their primaries. Unfortunately there is no study of the frequency of breeding that would further elucidate this apparent tradeoff of moult and breeding in night herons.
Pelagic Cormorant Phalacrocorax pelagicus
This cormorant breeds along the shores of the North Pacific Ocean. On the west side of the Pacific it breeds from northern Chucotka, south through Kamchatka and the Kuril Islands, to northern Japan. On the east side it breeds from northern Alaska, south to central Baja California. Birds breeding in northern regions of the western Pacific migrate long distances to avoid ice in the winter, but birds of the mild eastern Pacific are largely resident. Like other cormorants (Potts 1971; Rasmussen 1988), the primaries of Pelagic Cormorants constitute a single moult series, through which several waves of feather replacement proceed in a proximal to distal direction in adults (Fig. 3; Filardi & Rohwer, unpubl.). Their primaries grow at a mean rate of 4.6 mm per day.
I include this species to illustrate the advantage of being able to moult throughout a long non breeding season. At 48o N in the eastern Pacific, Pelagic Cormorants are able to moult throughout the winter, and adults require a little more than 200 days to complete their moult. Most begin moulting in August or September and have completed their moult by March or April (Filardi and Rohwer, unpubl.). Despite the very long time required to fully replace the primaries almost all adults from this region manage to do so. Thus, there is no reason to expect that incomplete moults in Pelagic Cormorants from the eastern Pacific would force a Spring tradeoff decision between moult and breeding.
We have found very few adult Pelagic Cormorants in collections from northern regions of the western Pacific, but some did have worn, unreplaced primaries. Furthermore, young birds from the western Pacific begin their first replacement of primaries, not in the winter after they were fledged, as they often do on the east side of the Pacific, but in the following Spring (Filardi & Rohwer, unpubl.). Thus for birds breeding in northern areas of the western Pacific, adults may sometimes be forced to skip breeding to moult, and immatures may require an extra season to replace their juvenile primaries.
CONCLUSIONS
It is critical to emphasise that no study has yet demonstrated that the tradeoff between moult and breeding proposed for albatrosses and night herons operates at the level of individuals. Such data is essential to prove that tradeoff decisions are being made between moult and breeding. Among annually breeding albatrosses, herons, and large raptors the critical questions is: "Are individuals with many worn flight feathers skipping breeding to moult?" Should this be confirmed, the importance of studying moult in the context of life history tradeoffs in big birds will be solidly established. Such a discovery will also raise interesting problems of co-ordination between pair mates (Langston & Rohwer 1996). Most large birds mate for life, and some, such as Laysan Albatrosses, require a full year to reform pairs (Rice and Kenyon 1962). In such species, it would seem essential that both members of a pair skip breeding to moult in the same year; otherwise the number of years of skipped breeding would nearly double. How and if this is accomplished is unexplored.
Why two major patterns of primary replacement?
We know remarkably little about the functional significance of splitting the primaries into multiple moult series (Fig. 2) compared to treating the primaries as a single series with multiple waves of feather replacement (Fig. 3 & Fig 4). To a substantial degree, comparative studies in this field are impeded by the very poor quality of moult descriptions.
Quality of data
One important problem is that the primaries are typically assumed to constitute a single moult series. This need not be true. In Laysan Albatrosses and in Black-footed Albatrosses Diomedea nigripes, the outer secondaries and the inner primaries form a moult series that is independent of the series that includes the outer five primaries (Table 2; Langston and Rohwer 1996; Edwards and Rohwer unpubl.). The same is also true of some falcons, owls and parrots (Table 4).
Another critical problem is that the definition of moult series and the description of the sequence of feather replacement within these series has been largely devoid of good standards of quantification. Table 2 illustrates for primaries a quantitative scheme for reporting such data from museum specimens. This scheme allows assessment of the reliability of conclusions about the identity of separate moult series and about the direction of feather replacement within these series. Each growing feather is treated as focal and tallied just once in the four rows of choices. Direction of replacement is inferred by the status of adjacent feathers. If an adjacent growing feather is the same length, then direction of replacement is recorded as ambiguous. If the growing feather is surrounded by old feathers it is recorded as a point of initiation in the current replacement of primaries. Yuri and Rohwer (1997) provide a fuller discussion of the complexity of identifying moult series.
For Laysan Albatrosses, the directionality data in Table 2 (from Langston and Rohwer, 1995) make it clear that primaries 6-10 constitute an outer moult series, whose feathers are replaced in a proximal to distal direction. Primaries 1-5, as well as some outer secondaries, constitute a separate moult series, whose feathers are replaced sequentially in a distal to proximal direction (Fig. 2). The data for adult Black-crowned Night Herons summarised in the bottom half of Table 2 (from Shugart and Rohwer 1996, and unpubl.) illustrate the messier patterns that arise when the primaries constitute a single moult series, with multiple, proximal to distal waves of feather replacement (Fig. 3). In this species new waves are initiated with each moult at P1 (thus it has the highest value for point of initiation), but moult picks up again wherever it was last arrested in waves had have not merged and that had not reached P10 (Shugart and Rohwer 1996). Fig. 4 illustrates a symbolic way to analyse stepwise moults; this figure summarises the same sequence of moults that are presented more realistically in Fig. 3.
Stepwise moult of the primaries
Having the primaries organised as a single series where multiple waves of moult may proceed from P1 to P10 (stepwise molts) seems suboptimal for several reasons. First, in every species in which adults feature such moults, the first moult of primaries by young birds begins as a single wave that starts at P1 (Table 3). In large birds this may mean that the outermost juvenile primaries are retained two or more years, by which time they become excessively worn (Fig. 3 & Fig. 4).
Second, when the first moult of juvenile primaries results in only about half of the primaries being replaced, the next episode of moult will be characterized by two waves, if moult is reinitiated at the next juvenile primary to be replaced and at P1. This results in the inner primaries, that receive less wear, being replaced more frequently than the outer primaries, that receive more wear (Fig. 3 & Fig. 4).
Third, when the first moult of juvenile primaries is complete, the next episode of primary moult will be characterized by only a single wave of feather replacement. This is potentially a serious handicap to species that may breed for the first time after replacing the juvenile primaries because incomplete moults will not have set up multiple waves of feather replacement in the primaries. I speculate that the omission of one or more of outer primaries 6-9 from the first replacement of the juvenile primaries (Table 3) is an adaptation to set up an outer wave of feather replacement in the primaries of young birds. In this way some outer primaries can be replaced following the first breeding attempt when first-time breeders would have little time for moulting.
Finally, even in adults, stepwise moulting results in the seemingly maladaptive replacement of the inner primaries almost annually and the outer primaries less frequently (Fig. 3 & Fig. 4). In night herons 100% of 19 adults had replaced P1 in the preceding moult, but only about 50% of these birds had replaced P8, P9, or P10 (Shugart and Rohwer 1996).
Multiple moult series in the primaries
The striking theoretical advantage of breaking the primaries into two or more moult series (Table 4) is that feathers can be replaced more frequently in some series than others (Fig. 2). Thus, Laysan and Black Footed Albatrosses moult their outermost primaries annually, but they moult their inner primaries and their secondaries much less frequently (Langston and Rohwer 1995). They accomplish this in two ways. First, moult is initiated in the inner series of primaries only every second or third year. Second, moult in the outer series is not initiated at P6, the first feather of this series, except in years when much time is available for moulting. When less time is available, moult in the outer series is initiated at P7 or, more frequently, P8, but it always proceeds to P10 (Fig. 2). Thus the three longest primaries that receive unusual amounts of wear are replaced annually (Langston and Rohwer 1995).
Another likely advantage of breaking the primaries into two moult series is that inner primaries and outer secondaries can be combined into a single moult series. This should facilitate breaking the shorter flight feathers of the wing into the number of moult series that would optimise the frequency with which these feathers are replaced. The result should be better spacing of gaps caused by moulting feathers, better control of the size of these gaps, and a matching of the rate of wear with the frequency of feather replacement in different series. The net effect should be reduced energetic, aerodynamic, and time costs of moulting. Ultimately this should mean more years of breeding, because the mortality costs of moults would be reduced and because fewer years of breeding would be lost for moulting.
So far as I know the combination of inner primaries and outer secondaries into a single moult series is established only for Laysan and Black footed Albatrosses (Langston and Rohwer 1995; Edwards and Rohwer, unpubl.). But, I shall be surprised if this is not a common pattern. Most birds moult their outer secondaries in a distal to proximal direction. Furthermore, most species that divide the primaries into two series, have reversed the direction of primary replacement in the inner moult series to match the distal to proximal replacement of the secondaries (Table 4). Unfortunately, few moult studies have simultaneously considered the sequence and timing of replacement of both primaries and secondaries; thus, there has been little opportunity for integrated replacement of the outer primaries and the inner secondaries to have been observed.
CONCLUSION
Progress in interpreting the broad comparative patterns of flight feather replacement is presently seriously impeded by lack of data. Rarely do moult studies provide satisfactory quantitative summaries of data. Rarely do they establish whether or not the primaries are divided into multiple series or simply feature multiple waves of feather replacement in a single series. Rarely is the relative frequency of replacement established on a feather by feather basis. Finally, rarely are all the remiges analysed as a single unit.
The probable importance of moult-breeding tradeoffs remains undocumented at the level of individuals in any large bird. Furthermore, the adaptive significance of breaking the primaries into multiple series of feather replacement cannot be assessed without comparative data on the frequency of feather replacement within these series. Clearly much useful work remains to be done in this important and, increasingly, general-interest field of avian biology!
ACKNOWLEDGMENTS
Thanks to Jon Herron for help making figures, to Christopher Thompson and Lukas Jenni for help with references, and to Brigitte Rohwer for typing. Measurements were taken from the extended wing collection at the University of Washington Burke Museum.
REFERENCES
Brooke, R.K. 1981. Modes of moult of flight feathers in albatrosses. Cormorant 9: 13-18.
Cramp, S. (ed). 1985. Handbook of the birds of Europe the Middle East and North Africa. Vol. IV Terns to Woodpeckers. Oxford Univ. Press, Oxford.
Dietz, M.W., Daan, S. & Masman D. 1992. Energy Requirements for Molt in the Kestrel Falco tinnunculus. Physiolgical Zoology 65: 1217-1235.
Dorward, D.F. 1962. Comparative biology of the White Booby and the Brown Booby Sula spp. at Ascension. Ibis 103b: 174-220.
Fisher, H.I 1976. Some dynamics of a breeding colony of Laysan Albatrosses. Wilson Bulletin 88: 121-142.
Forsman, E.D. 1981. Molt of the Spotted Owl. Auk 98: 735-742.
Furness, R.W. 1988. Influences of status and recent breeding experience on the moult strategy of the Yellow-nosed Albatross Diomedea chlororhynchos. Journal of Zoology 215: 719-727.
Hanmer, D.B. 1980. Mensural and moult data of eight species of kingfisher from Moçambic and Malawi. Ostrich: 51: 129-150.
Houston, D.C. 1975. The moult of the White-backed and Rüppell's Griffon Vultures Gyps africanus and G. rueppellii. Ibis 117: 474-488.
Kemp, A.C. 1976. A study of the ecology, behaviour and systematics of Tockus hornbills (Aves: Bucerotidae). Transvaal Museum Memoir 20, Pretoria.
Langston, N. E. & Rohwer, S. 1995. Unusual patterns of incomplete primary molt in Laysan and Black-footed Albatrosses. Condor 97:1-19.
Langston, N.E. & Rohwer, S. 1996. Molt-breeding tradeoffs in albatrosses: life history implications for big birds. Oikos 76: 498-510.
Lenton, G.M. 1984. Moult of Malayan Barn Owls Tyto alba. Ibis 126: 188-197.
Lindström, A., Visser, G.H. & Daan, S. 1993. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiological Zoology 66:490-510.
Lucas, A.M. & Stettenheim P. R. 1972. Avian Anatomy, Integument Part II. Agricultural Handbook 362, U.S. Government Printing Office, Washington D.C.
Mausman, D., Daan, S. & Duskra, C. 1988. Time allocation in the Kestrel (Falco tinnunculus) and the principle of energy minimization. Journal of Animal Ecology 57: 411-432.
Mundy, P.J. 1982. The comparative biology of southern African vultures. Johannesburg: Vulture Study Group.
Potts, G.R. 1971. Moult in the Shag Phalacrocorax aristotelis, and the ontogeny of the "Staffelmauser". Ibis 113: 298-305.
Prevost, Y. 1983. The moult of the osprey Pandion haliaetus. Ardea 71: 199-209.
Rasmussen, P.C. 1988. Stepwise molt of remiges of Blue-eyed and King Shags. Condor 90: 220-227.
Rice, D.W. & Kenyon, K.W. 1962. Breeding cycles and behavior of Laysan and Black-footed Albatrosses. Auk 79: 517-567.
Schmutz, J.K. 1992. Molt of flight feathers in Ferruginous and Swainson's Hawks. Journal of Raptor Research 26: 124-135.
Shugart, G.W. & Rohwer, S. 1996. Serial descendant primary molt or Staffelmauser in Black-crowned Night-herons. Condor 98: 222-233.
Siegfried, W.R. 1971. Plumage and moult of the Cattle Egret. Ostrich, supplement 9: 153-164.
Snyder, N.F.R., Johnson, E.V. & Clendenen, D.A. 1987. Primary molt of California Condors. Condor 89: 468-485.
Stresemann, E. & Stresemann, V. 1966. Die Mauser der Vögel. Journal für Ornithologie 107: Sonderheft.
Sutter, E.R. 1984. Ontogeny of the wing moult pattern in the White Stork Ciconia ciconia. Proceedings of the Fifth Pan-African Ornithological Congress.
Swaddle, J.P. & Witter M.S. 1997. The effects of molt on the flight performance, body mass, and behavior of European Starlings (Sturnus vulgaris): an experimental approach. Canadian Journal of Zoology 75: 1135-1146.
Tucker, V.A. 1991. The effect of molting on the gliding performance of a Harris' Hawk (Parabuteo unicinctus). Auk 108: 108-113.
Willoughby, E.J. 1966. Wing and tail molt of the Sparrow Hawk. Auk 83: 201-206.
Yuri, T. & Rohwer, S. 1997. Molt and migration in the northern Rough-winged Swallow. Auk 114: 249-262.
Table 1. Examples illustrating the great combined length of the 10 primaries in large birds.

Table 2. Analysis of the direction of primary replacement using each growing feather as a focal feather.1
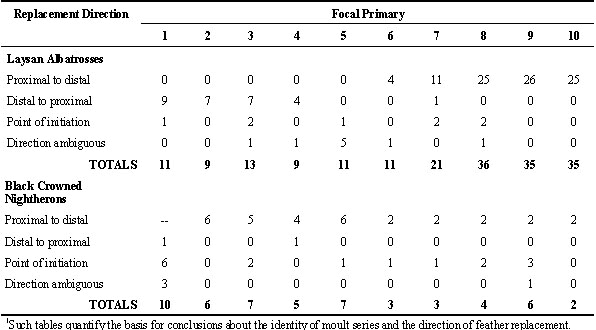
Table 3. Species in which the first moult of primaries proceeds as a single wave from P1 to P10. 1
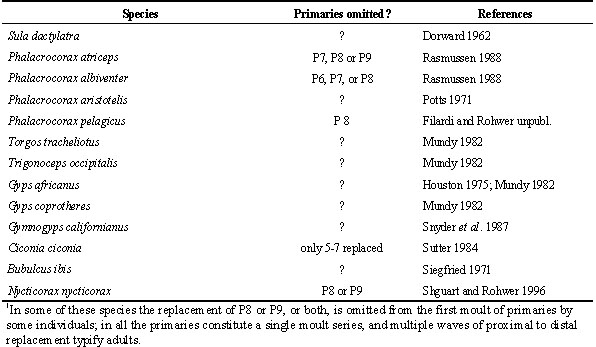
Table 4. Some species in which the primaries are divided into two or more moult series.
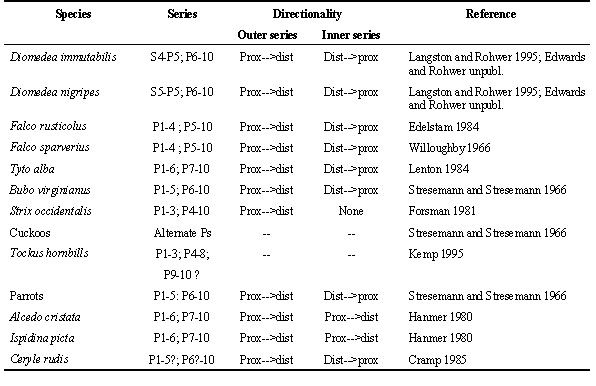
Fig. 1. Primary growth rate (mm/day) in 43 species of birds. Means were used when available; for the ranges in Prevost (1983), I used mid points. Other sources were: Filardi and Rohwer unpubl., Forsman 1981, Langston and Rohwer 1996, Shugart and Rohwer 1996, and Snyder et al. 1987.
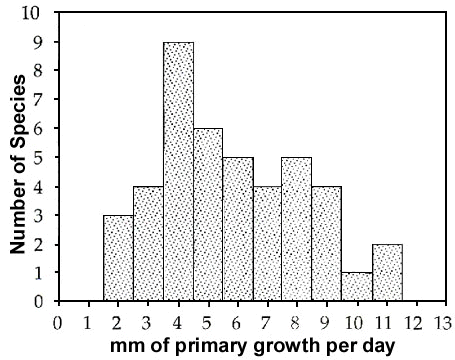
Fig. 2. An example of five successive primary moults in a species with the primaries divided into outer and inner moult series. Moult is activated every year in the outer series, but less frequently in the inner series. Because the direction of feather replacement in the inner primaries has been reversed to match that of the secondaries, the inner primaries and some of the outer secondaries may be combined into a single, longer moult series.
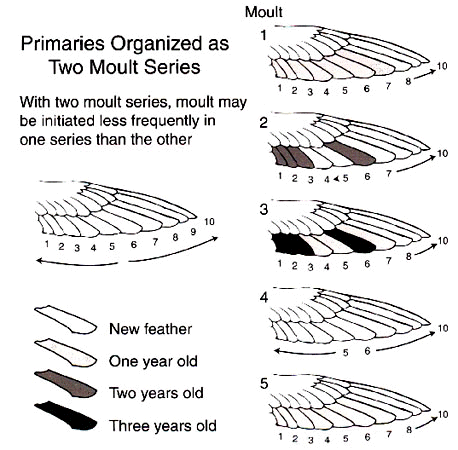
Fig. 3. A worked example of nine successive moults of 6 primaries illustrating the development of multiple waves of feather replacement through a single moult series. This example illustrates what is meant by stepwise moulting of the primaries. In moult 2 there would be two waves of primary replacement; at the completion of moult 2, P10 would be destined for two years of wear. In moult 3 there would be three waves of primary replacement but the two inner waves would fuse; at the completion of moult 3, P6 would be destined for two years of wear. In moult 4 there would be just two waves of feather replacement because of the fusion of the inner waves in moult 3. If waves of moult that meet do not fuse, an excessive number of waves of feather replacement will develop in the primaries. The concentration of two year old feathers in the outer primaries continues until moults 8 and 9, which then become a repeating cycle. Of course no bird would replace a fixed number of primaries in successive moults, but this example serves to illustrate problems that may arise with stepwise moults.
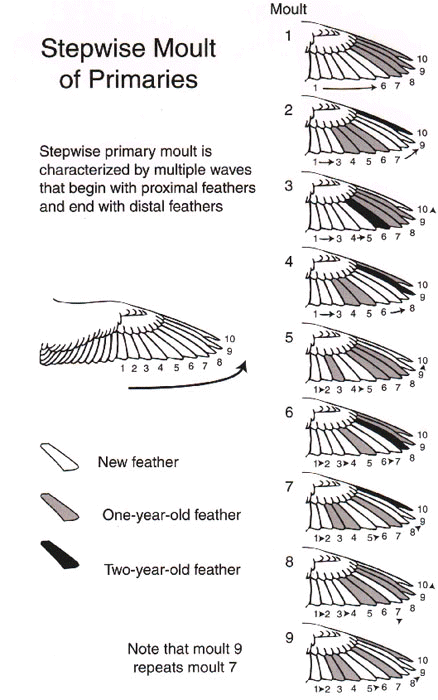
Fig. 4. A schematic version of Fig. 3 that helps analyse stepwise moulting when multiple waves are generated by incomplete moults.