S10.3: Intraspecific variation of moult: Adaptive significance and ways of realisation
Georgui A. Noskov, Tatiana A. Rymkevich & Natalia P. Iovchenko
Biological Research Institute of St. Petersburg University, Oranienbaumskoje sh. 2, Stary Peterhoff, St Petersburg, 198904, Russia, e-mail george@ area.usr.pu.ru; tatiana@ area.usr.pu.ru; natalia@ni1339.spb.edu
Noskov, G.A., Rymkevich, T.A. & Iovchenko, N.P. 1999. Intraspecific variation of moult: Adaptive significance and ways of realisation. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
544-563. Johannesburg: BirdLife South Africa.Intraspecific variation of moult finds its expression in differences of moult timing, rate, extent, and as a consequence, duration as well as position in the annual cycle and connection with specific parts of the distribution area. More northern and high altitude populations and birds moulting later reduce the duration and extent of feather replacement up to the total suppression of moult in the breeding ranges. Correspondingly, the duration and extent of prenuptial moult in the wintering ranges is increased. It has been demonstrated, experimentally, that these adaptive changes of moult parameters are achieved by photoperiodic control of (1) moult onset (for postjuvenile moult within genetically-fixed minimal and maximal values of the onset age); (2) rates of feather replacement (by changes of daylength within the photoperiodic interval of moult; (3) moult extent (within the part of feathering that is capable of responding to hormonal factors). The system of moult regulation, by changing of circannual rhythms of neurohumoral system, parameters of photoperiodic interval of moult and internal rhythms of feather follicle growth and rest, allows populations to adapt the annual cycle quickly to environmental conditions varying considerably within the distribution area. Thus, moult appears to be a changeable event playing the part of the buffer in the annual cycle.
INTRODUCTION: MOULT IN THE ANNUAL CYCLE OF SEASONAL EVENTS
The annual cycle of seasonal events in birds, as a genetically determined sequence of reproduction, moults and migrations, with an autonomous circannual rhythm, is a specific peculiarity of this group of animals. There is all reason to believe that the phenomenon of the annual cycle was formed in the early stages of the evolution of the class. The appearance of the phenomenon of the annual cycle allowed birds to solve two seemingly incompatible problems. On the one hand, it allowed them to create a biological clock on the basis of the sequence of seasonal events common for all birds during the year. On the other hand, it allowed them to form mechanisms for modification of the timing and duration of these seasonal events, even allowing for the exclusion of some of them from the annual cycle by means of photoperiodic reactions in conformity with existing environmental conditions. Among the seasonal events, moult is the most flexible process with regards to seasonal and geographical transformations. Precisely owing to this process, the adaptation of the annual cycle to the local environment conditions is realised.
METHODS
This paper is based on the results of a 30-year field and experimental investigation of moult. The majority of data (about 130000 records of moulting birds) was collected at the Ladoga Ornithological Station (60° 41¢ N, 32° 56¢ E), where from 1968 every year from April-May to October-November some thousands of birds are trapped and ringed. Moreover about 3000 records of moult in 70 species were collected in 14 other regions of Palaearctic (among them: Polar Ural, Yakutia, Far East, Sakchalin, Tien Shan, the Caucasus, Crimea, the Black Sea). In the experiment conditions the moult was investigated in 2500 individuals of 45 species. Moult was recorded according to methods of registration of moult developed in St. Petersburg University (Noskov & Gaginskaya 1972; Noskov & Rymkevich 1977). For data processing some new indices of moult such as ‘moult progress’ (S) and ‘moult completeness’ (C) were used (Rymkevich et al. 1987; Mogilner & Rymkevich 1990; Rymkevich & Bojarinova 1996).
SEASONAL VARIATION OF PARAMETERS OF POSTJUVENILE MOULT
Seasonal variation of postjuvenile moult parameters, as a rule, is connected with the variation of the dates of hatching during the breeding season: birds hatched late in the season start their postjuvenile moult at an earlier age, moult more rapidly and renew fewer feathers than early-hatched young of the same population.
Age of onset of postjuvenile moult
Many studies on free-living passerine and non-passerine birds show that age of onset of postjuvenile moult can vary considerably and depends on the dates of hatching during the breeding season (e.g. Evans 1966; Noskov & Gaginskaya 1969; Noskov 1975; King, 1972; Ligon & White 1974; Rymkevich 1976, 1977 ; Pearson 1975; Noskov & Rymkevich 1978, 1985, 1988a,b; Iovchenko 1988; 1993a; Rymkevich 1990; Rymkevich & Bojarinova, 1996). Experimental investigations have shown that age of onset of postjuvenile moult was under photoperiodic control (e.g. Miyazaki 1934; Noskov 1970; Gwinner et al. 1971; Rymkevich 1974, 1977; Pinkovski 1976; Noskov, 1977; Savinich 1984; Noskov, Smirnov, 1986; Iovchenko, Smirnov 1987). Photoperiodic reaction can change age of moult onset only within certain limits. Such genetically fixed limits we proposed to call ‘minimal and maximal age of onset of postjuvenile moult’. Maximal age is characterized by more expressed individual variation than minimal. For example, in the Great Tit Parus major, maximal age varies between 56-77 days and minimal age only between 35-39 days (Rymkevich, Bojarinova, 1996). Experiments on many species show that young birds start moulting at maximal age before the daylength decreases to a certain value (Fig. 1). At the same time, feather replacement can not start earlier than the minimal age no matter how much the daylength is reduced.
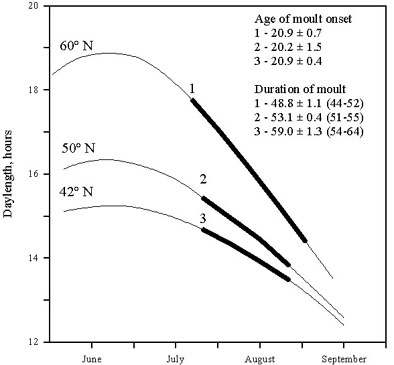
Values of minimal and maximal age of moult onset are specific for a species and its populations. Differences in age of moult onset can reach some tens of days in species with long breeding seasons (sedentary, wandering and short-distance migrants) or reduce to 2-3 days, or even be absent in some long-distance migrants (Fig. 2).
Thus, by regulating the age of onset of postjuvenile moult, the differences in the progress of the annual cycle of early and late-hatched birds, can be significantly decreased by the beginning of the autumn migration.
Duration and rates of postjuvenile moult
Seasonal variation in duration of postjuvenile moult was found in many studied species . It is especially typical for nomadic and short-distance migrants (those species in which early-hatched young moult sufficiently long). Duration of moult, first of all, depends on the rates of involving different feather follicles in moult (Lesher & Kendeigh, 1941). In the majority of species, the rates are under photoperiodic control. Many experimental studies (Noskov & Rymkevich 1978; 1985; 1988a,b) allowed us to define the main features of photoperiodic control of moult:
(1) The normal dynamics of postjuvenile moult are ensured by photoperiodic reaction to shortening daylength. Keeping birds under constant long daylength prolongs moult (Fig. 3). Under constant short daylength, moult is very intensive in the beginning but it slows down considerably in the end. Only under shortening daylengths are the rates of moult uniform during the whole process.
(2) Changing daylength within a range of its shorter values or more rapid shortening daylength ensures the higher rates, and as a consequence, a shorter duration of moult (Fig. 4).
(3) A range of daylengths, within which shortening of daylength causes higher rates of moult, is specific for species and its populations. We named this range ‘photoperiodic interval of moult’ (PhPI).
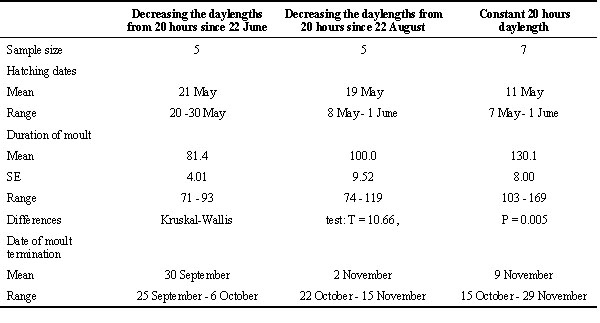
(4) Daylengths longer than the upper threshold of PhPI do not influence moult. In this case, moult is realized with rates determined by endogenous rhythm. In species in which photoperiod plays the leading role in control of moult, endogenous rhythm is considerably late with regard to environmental rhythms and can not ensure an adaptive timing of finishing moult. To such species belongs the Greenfinch Carduelis chloris. Under constant long daylength conditions, even in early-hatched birds, moult finished only in November-December, while in nature, the last moulting birds can be found in the given population in late October (Table 1).
(5) Daylengths shorter than the low threshold of PhPI inhibit the shedding of old feathers and cause an interruption in the moult process (Fig. 5).
From the standpoint of the existence of PhPI and the inhibiting effect of daylengths which are shorter than the low threshold, an additional way of decreasing moult duration by interrupting the moult process can be explained. This way of decreasing moult duration begins to function in very late-hatched birds when it is impossible to finish moult in time by changing the age of moult onset and rates of feather replacement. In such conditions, daylength which is too short blocks the replacement of feathers usually moulting during the last stages of moult. This phenomenon of ‘interrupted’ moult is found in many species from families Motacillidae, Turdidae, Sylviidae, Muscicapidae, Paridae, Emberizidae, Fringillidae, Corvidae and was analyzed by us under experimental conditions in 17 species (Rymkevich 1974, 1976, 1977; Noskov 1977; Noskov & Rymkevich 1985, 1988a,b; Noskov & Smirnov 1986;Iovchenko & Smirnov 1987; Rymkevich & Pravosudova 1987).
Thus, a decrease of moult duration in late-hatched birds is achieved, first, by moulting more feathers simultaneously, and second, by reducing the extent of the moult.
Extent of feather replacement
Many studies on free-living birds show that the extent of feather replacement can vary in connection with their dates of hatching (e.g. Newton 1966; Baggott 1970; Dhondt 1973; Hawthorn 1974; Yakovleva et al. 1987; Rymkevich 1990; Gosler 1991). Among passerines, differences in the extent of postjuvenile moult are especially expressed in Turdidae, Paridae and Fringillidae families. Late-hatched birds of these groups, as a rule, have more unmoulted feathers among wing coverts, upper and lower tail coverts, and sometimes, peripheral feather rows of the spinal and ventral tracts. The same evidence of the moult extent decrease is found in Columbidae family. Late-hatched sedentary birds of northern parts of the distribution range, Blue Hill Pigeon Columba rupestris, Rock-Dove C. livia and Laughing Dove Streptopelia senegalensis as well as the migratory Rufous Turtle Dove Streptopelia orientalis and Turtle Dove Streptopelia turtur always realise a lesser extent of moult, including a lesser number of renewed remiges, in breeding area (Noskov & Kotov 1976; Kotov & Noskov 1978).
Experimental studies showed that in the Chaffinch Fringilla coelebs, the Common Redpoll Carduelis flammea, the Siskin Carduelis spinus, and the Great Tit Parus major (Noskov 1977; Noskov & Smirnov 1986; Iovchenko & Smirnov 1987; Rymkevich & Bojarinova 1996), the early-hatched birds, kept under photoperiodic conditions, imitating natural daylengths for late-hatched birds, had a considerably lesser extent of moult than birds kept under photoperiodic conditions, imitating natural daylengths for early-hatched birds.
As it was mentioned above, one of the mechanisms of changing moult extent is direct control by inhibiting it under daylengths which are too short. Another mechanism is a natural increase of the extent of partial postjuvenile moult with an increase of age, because certain follicles begin to respond to the hormonal factor of moult in a certain age. This is well demonstrated on flight feathers and especially on primaries. At present, partial renewal of the primaries during the postjuvenile moult is found in many passerines (e.g. Westphal 1976; Mester & Prü nte 1982; Ginn & Melville 1983; Winkler & Jenni 1987; Jenni & Winkler 1994).In the Sardinian Warbler Sylvia melanocephala, early-hatched birds perform complete, or almost complete, postjuvenile moult, while late-hatched birds show a partial postjuvenile moult which includes a few primaries at most (Gauci & Sultana 1979). Extent of flight feathers moult in the House Finch Carpodacus mexicanus (Michener & Michener 1940) and in the Pinon Jay Gymnorhinus cyanocephalus (Ligon & White 1974) is also connected with hatching dates. Our experimental data confirm that partial primary moult depends on hatching dates and, consequently, on age. For example, unlike other species of the Emberizidae family, in the Yellow-breasted Bunting Emberiza aureola, in which postjuvenile moult starts only in September-October, not only body feathers moult, but also 1-4 distal primaries. For birds in which moult was experimentally prolonged up to January-March, 7 distal primaries moulted. The same was found in the Scarlet Rosefinch Carpodacus erithrinus (Noskov 1978). In the Greenfinch, in nature on the North-West of Russia, moult of one primary was found only once. In experiments among 7 birds of the same population, which began moulting at the age of 45-55 days, only 1 bird replaced 1 (9th) primary, while among 11 birds, which began moulting at the age of 60-110 days, 7 birds replaced from 1 to 7 distal primaries.
Thus, in addition to direct photoperiodic control by interruption of moult, there is indirect control by means of changing the age of moult onset. As a result, some feathers, which are usually replaced in early-hatched birds, are not ready to moult in late-hatched birds, which begin to replace feathering at an earlier age. These mechanisms of moult extent control find their extreme expression in very late-hatched Siskins and Common Crossbills Loxia curvirostra when short daylengths, combined with age limitation of moult onset, interrupt moult in the beginning of the process or even inhibit it completely (Iovchenko & Smirnov 1987; Noskov & Smirnov in Rymkevich 1990). The adaptive significance of the decrease of moult extent undoubtedly consists of a decrease in duration by reducing the number of moulting feathers. This gives to birds the possibility to finish moult at an earlier age, to migratory species the possibility to begin migratory activity earlier, thereby receiving a chance to escape unfavorable environmental conditions, and to sedentary species the possibility to finish moult before unfavorable wintering conditions set in.
SEASONAL VARIATION OF PARAMETERS OF POSTNUPTIAL MOULT
Postnuptial moult begins either after finishing postnuptial (summer) migration, or, if the latter is absent in the annual cycle, immediately after finishing sexual activity. In connection with this, temporal parameters of postnuptial moult, in many respects, depends on the dates of the onset and duration of the preceding events.
Time of onset of postnuptial moult
When postnuptial moult begins immediately after finishing sexual activity, a duration of gonad activity is the main factor determining the time of the onset of postnuptial feather replacement. Results of experiments undertaken on many species, with the purpose of reducing the duration of sexual activity by shortening daylength and, thereby, shifting the postnuptial moult dates, are different. For the majority of species, experimental daylength shortening does not lead to a reduction in gonad activity (Farner 1964; Dolnik & Gavrilov 1972; Gavrilov & Dolnik 1974; Dolnik 1975; Noskov 1977).
For example, in the Chaffinch, shortening of daylength during sexual activity (by different dates after its beginning) did not reveal any differences in dates of postnuptial moult onset. The same results were obtained for the Pied and Yellow Wagtails Motacilla alba and M. flava, the Willow Warbler Phylloscopus trochilus, the Whitethroat Sylvia communis, the Garden Warbler Sylvia borin, the Blackcap Sylvia atricapilla, the Pied Flycatcher Ficedula hypoleuca, the Rustic Bunting Emberiza rustica, the Ortolan Bunting Emberiza hortulana and some species of the Fringillidae family (Rymkevich 1976; Noskov 1977; Noskov & Smirnov 1978; Rymkevich & Pravosudova 1987).
On the other hand, the shortening of daylength during sexual activity led to an earlier beginning of postnuptial moult in the Rock Dove, the Blue Hill Dove and the Rusos Turtle Dove (Noskov & Kotov 1976). Most likely, in these species, there is a direct photoperiodic control of gonad activity, and consequently, the time of the postnuptial moult onset can be shifted by shortening daylength. Some passerine species in which postnuptial moult is separated from sexual activity by a period of postnuptial migration, can also shift the dates of their moult by changing the daylength. In experiments, such dependence was shown for the Scarlet Rosefinch (Noskov 1978). Moreover, this species has a narrow PhPI and their moult does not start until daylength reaches its upper threshold. Finally, in the Siskin, a capacity to change the duration of sexual activity in response to shortening or lengthening daylength and, as a consequence, to shift the dates of postnuptial moult onset, was found (Iovchenko 1988).
Rates, duration and extent of postnuptial moult
The previously given conception of PhPI and its thresholds for postjuvenile moult is, likewise, applicable for postnuptial moult. As the rates and duration of postjuvenile moult, rates and duration of postnuptial moult are usually under photoperiodic control. The necessary condition for moult, which is controlled by the photoperiod, is shortening of daylength. The moult prolongs unnaturally under constant, even sufficiently short daylength (Fig. 6). Owing to photoperiodic control, moult in adults moulting later under shorter and quickly shortening daylength, is shorter than in birds moulting in the beginning of the moult season (Noskov & Rymkevich 1978, 1985, 1988a,b). In experiments, the dependence of the duration of postnuptial moult on daylength was shown for about 70 species. On the basis of the conception of PhPI, it is possible to understand the widespread phenomenon of moult interruption in the last stages. The moult interruption is observed when, in spite of the high rates of moult, its last stages fall on the dates in which daylength is shorter than the low threshold of PhPI (Noskov & Rymkevich 1978, 1985).
Besides photoperiodically regulated variation, the first stages of moult in some species can sharply slow down and even interrupt when the beginning of moult overlaps with egg-laying, incubating and feeding of nestlings or fledglings (e.g. Ashmole 1968; Harris 1971; Payne, 1972). As was shown in some passerine species (Lapshin 1981, 1986; Iovchenko 1990, 1993a), regularly overlapping moult and breeding, the next primary often shed only after the previous primary was full grown. Sometimes they have asymmetric moult and body feathers begin to renew in later stages of moult than in non-breeding birds. The phenomenon of prolongation of sexual activity or full gonad recrudescence in moulting birds, in response to a high seed crop of the main food trees in late summer and early autumn , in some years, can postpone the moult onset or slow down its rates in the White-winged Grosbeak Mycerobas carnipes (Iovchenko 1990) or, arrest it in the Pinon Jay Gymnorhinus cyanocephalus (Ligon 1971; Ligon & White 1974). Moult recovers its normal rates after the young fledge.
Unlike the changes of duration and extent of moult, regulated by photoperiodic reaction, postponement of the moult onset, connected with the prolongation of sexual activity by food abundance, has not such a regular character.
VARIATION OF PARAMETERS OF PRENUPTIAL MOULT
It is necessary to keep in view that not every seasonal moult taking place in the wintering range can be considered as prenuptial moult. The fundamental characteristic of prenuptial moult is its stimulation by the increasing of daylength. This characteristic allows us to distinguish between prenuptial moult and postnuptial/postjuvenile moult, when the last two take place in wintering areas.
Timing of onset of prenuptial moult
Prenuptial moult of an overwhelming majority of palearctic and nearctic species occurs in wintering areas. The timing of its onset is determined, on one hand, by endogenous rhythm and, on the other hand, by photoperiodic reaction to increasing daylength. Experiments on some species, wintering in the tropics and subtropics of Africa, showed that increasing the daylength before certain calendar dates could not stimulate the onset of feather replacement. For example, in experiments with the Pied Flycatcher (Rymkevich & Pravosudova 1987), birds did not start moult earlier than in January, even if the daylength was sufficient for the activating of their moult. Since the beginning of January the Flycatchers became receptive to a photoperiod and the daylengths longer than 11 hours stimulated the onset of moult (Fig. 7). Photoperiodic control of prenuptial moult onset was found also in the Yellow Wagtail Motacilla flava, the Spotted Flycatcher Muscicapa striata, the Whitethroat, the Lesser Whitethroat Sylvia curruca, the Rustic Bunting Emberiza rustica, the Yellow-breasted Bunting and some other species.
At the same time, the periods of prenuptial moult in wintering areas vary within greater limits than periods of postjuvenile and postnuptial moult in breeding areas. In the Ortolan Bunting, the dates of prenuptial moult onset vary between 20 November and 30 March, that is to say, within 130 days (Rymkevich 1976). In the Whitethroat, the onset of postnuptial moult is recorded during the greater part of winter (Stresenann & Stresemann 1968 ; Muzaev & Noskov 1985). These differences can not be explained by the common wintering of different populations alone. In captivity, the dates of moult onset in birds taken from the nests in the same region were scattered about 90 days (Stolbova 1987).
The existence of such differences may be explained by the weak influence of natural selection on the timing of prenuptial moult. It is considered that moult can pass in a great range of calendar dates without detriment to an individual. Concerning species wintering in Africa, we assume that a sufficient variety of prenuptial moult timing may be maintained by natural selection in accordance with variations in timing for dry periods from year to year. Unpredictable timing of optimal food conditions, on one hand, and high energy requirements of the moulting process on the other hand, make intrapopulation variability of moult timing an adaptive feature which determines the successful survival of the entire population during the prenuptial moult period.
Duration, rates and extent of prenuptial moult
The principles of the regulation of duration, rates and extent of prenuptial moult by photoperiodic reaction are similar to those of postjuvenile and postnuptial moults. They are based on the existence of PhPI, within which moult is regulated. The differences are, first, in that the prenuptial moult, as other seasonal events of the winter-spring period, is activated not by shortening of daylength but by increasing it.
For example, in the experiments with the Pied Flycatcher, the moult was slower and its duration was longer under relatively short daylengths of PhPI (Fig. 7 A,D). Under relatively long daylengths, the moult was more rapid and its duration was shorter (Fig. 7 B,C). When daylength achieved the upper threshold of PhPI, prenuptial moult was interrupted. As a result, the extent of moult and also duration were decreased (Fig. 7 E). Revealed differences of moult under experimental photoperiodic conditions have shown that in the Pied Flycatcher prenuptial moult could not pass naturally and in natural timing without photoperiodic control. The birds of the studied population probably find the optimal photoperiodic conditions in range between 5 and 20° N.
The second peculiarity of photoperiodic control of prenuptial moult is significantly narrow PhPI. In the Pied Flycatcher from the North-West of Russia, the PhPI is within the daylength range of 11 - 13 hours.
Thus, in some species, such as the Pied Flycatcher, the prenuptial moult are entirely under the control of photoperiodic reaction in spite of a narrowness of PhPI and small daylength changes in low latitudes. This allows birds to leave a wintering area and begin spring migration in time. Owing to photoperiodic regulation, significant individual variations of the onset date, rates, duration and extent of their prenuptial moult may occur if the birds of the same population moult on different latitudes.
In other species, such as the Whitethroat, significant variation of the onset and rate occur in connection with individual genetic differences in endogenous rhythms. It is most likely that only moult which begins in the end of the prenuptial moult season are under photoperiodic control and the thresholds of PhPI are photoperiods with relatively long days.
In some species individual variation in prenuptial moult extent is very high. As the extent of postjuvenile moult, the extent of prenuptial moult also depends on the endogenous rhythms of certain follicles. For example, the outermost primaries and innermost secondaries are usually ready to renew earlier than the others. Such a pattern of prenuptial moult was revealed in few immatures (first year birds) and adults of some Sylviidae and Motacillidae species. Differences in endogenous rhythms of follicles combined with differences in timing of feather replacement determine, for a significant part of the season, the variation of prenuptial moult extent. In some species the extent of prenuptial moult depends on the extent of postnuptial moult. In experiments on the same individuals of the Ortolan Bunting (Rymkevich 1976) and the Whitethroat it was shown that during prenuptial moult they certainly replaced those remiges, which did not moult for postnuptial moult. Thereafter, prenuptial moult began, in order, from the next feather.
GEOGRAPHICAL VARIATION OF MOULT
Photoperiodic regulation of moult within the thresholds of PhPI is the ready mechanism for creating the geographical variation of moult. In birds which inhabit the temperate zone of the Northern Hemisphere (30° -65° N), the geographical variation of moult is conditioned by a single photoperiodic reaction to different local ranges of daylength, or the genetic differences of the thresholds of PhPI. Given this, the moult parameters of short- and especially long-distance migrants partly differ owing to genetic population differences of endogenous rhythms. Finally, in the populations of wide-spread species which inhabit the high latitudes of the Arctic and Subarctic regions, the photoperiodic control of moult is either relaxed or disappears entirely.
Changes of moult parameters owing to differences of local photoperiodic conditions
Many species can use the same photoperiodic reaction in the entire distribution range. Such a type of photoperiodic control is more often found in nomadic species, which can change breeding areas in dependence of food supply from year to year or even during one season. For example, in the Siskin, the first cycle of breeding may take place in Central or Southern Europe. After that, adults and young usually migrate to taiga forests in northern and north-eastern parts of the continent. A similar pattern of changing of breeding place is demonstrated by the Common Redpoll, the Greenfinch and the Goldfinch Carduelis carduelis. Summer migrations in the northern direction from the hatching places are usually observed in the species of genus Turdus (T. pilaris, T. iliacus, T. philomelos, T. merula), the Robin Erithacus rubecula and some others. It leads to wide exchanges of individuals in a large part or the entire distribution area. With such character of territorial connections, it is necessary to have a universal pattern of photoperiodic control of autumn moult. Such a pattern arose because the low threshold of PhPI was shifted to 12 hours. Such daylength occurs on the same calendar dates everywhere. Owing to this universal pattern of photoperiodic control, the birds complete the moult at the same time utilizing all the space of their distribution area (Fig. 8).
Geographical variation of minimal and maximal age of postjuvenile moult onset
Analysis of postjuvenile moult onset in a species shows that inhabitants of northern regions or regions more distant from wintering quarters may have genetically determined differences in age of feather replacement onset. Thus, for example, the Reed Bunting Emberiza schoeniclus on the North-West of Russia have minimal and maximal ages of postjuvenile moult at 18 and 50 days, respectively (Rymkevich 1983), while birds in Polar Ural at only 23 and 29 days, respectively (Ryzhanovsky 1997). Young Chaffinches, in the North-West of Russia begin moulting at an age of 28-50 days, while the conspecifics in the Crimea at an age of 35-70 days (Noskov 1975). Such change of age limits of postjuvenile moult onset allow the conspecifics of the more northern population to begin moulting at an earlier age, in spite of longer daylengths in higher latitudes and, thereby, to decrease the period between the birth date and the onset of autumn migration.
Change of thresholds of PhPI of postjuvenile and postnuptial moult
In long- and some short-distance migrants utilizing large distribution areas, the differences in moult are most likely assured due to the existence of genetic differences in photoperiodic reactions. In the northern parts of the distribution ranges, the birds’ moult processes take place under considerably longer daylengths than in their counterparts in the southern populations (Fig 9). Nevertheless, the moult duration in the southern populations is longer and its extent is increased. Among such species may be mentioned many species of Sylviidae. We suppose that the more short and intensive moult in such species, under longer daylengths is ensured by higher PhPI values. In some species having two moults in the annual cycle (postnuptial and postjuvenile in breeding areas and prenuptial in the wintering areas), conspecifics breeding in regions most distant from wintering quarters, as a rule, decrease moult extent or completely exclude feather replacement in the breeding area. Such regularity is found in some species of genus Phylloscopus. The Spotted Flycatcher has no moult on the east side of the Urals. The physiological mechanism of moult reduction in the breeding area is very simple. It is contained in the changes of the thresholds of PhPI when those thresholds increase. This is easily proven when birds are kept under photoperiodic conditions with the daylength being longer than the maximal daylength in the breeding area of the studied populations. Under such experimental conditions, conspecifics which usually have no moult in the breeding area, can react to overly long daylengths by feather replacement. In the Indian Whitethroat S. c. rubicola in Tien Shan (43° N) adults either have no postnuptial moult at all or have partial moult excluding primaries, secondaries and some wing coverts (Iovchenko 1993b). Birds from the same population kept in outdoor aviaries in St. Petersburg Region (60° N) under natural photoperiodic conditions had complete postnuptial moult.
It can justifiably be proposed that changes in thresholds of PhPI for prenutptial moult is fairly widespread among migrants wintering in different parts of Africa due south and north from the equator. Without this mechanism, it is impossible for conspecifics of different geographical populations wintering in various regions with different photoperiodic conditions to begin and to finish prenuptial moult at similar dates.
Replacement of photoperiodic control with endogenous control
Not only during the wintering phase near the equator where there is a consistent 12-hour daylength, but also during the summer phase inhabiting the most northern Arctic Circle regions where daylength is 24 hours, the birds lose the opportunity to use the photoperiod as a ‘timing’ mechanism. For this reason, the expansion of breeding areas further across the Arctic Circle leads to the relaxation or disappearance of photoperiodic control of moult (Ryzhanovsky 1997). As he shown in experiments with some species which inhabit the area near the Polar Ural and the Yamal Peninsula, the parameters of moult did not depend on the photoperiodic condition. At the same time, endogenous control of moult ensured an early age and high renewal rate, which were similar to those in late moulting birds of more southern populations (Table 2). Our experiments with postjuvenile moult of the Common Redpoll (Noskov & Smirnov 1986) and postnuptial moult of the Snow Bunting Plectrophenax nivalis (unpubl. data) revealed that only the last stages of moult in these species were controlled by the photoperiod, while their first stages have similar high rates under both longer and shorter days alike.
EVOLUTIONARY SIGNIFICANCE OF VARIATION OF MOULT PARAMETERS IN ANNUAL CYCLE
E.Stresemann (1967) was most likely the first to pay close attention to the evolutionary aspect of changes of moult parameters. Absolutely, the solution of the problem regarding the deficit of time in the breeding areas is one of the most significant challenges facing the numerous species inhabiting temporate and high latitudes. Mainly, for this reason, the creation of a mechanism for regulating the timing of moult onset, rates and duration became the logical solution with regard to the birds’ environmental requirements. This mechanism makes it possible to decrease the duration of their stay in the breeding areas and, owing to this, to extend the borders of their distribution range to northern, dry and mountainous regions - habitations suitable for short periods of nesting.
Changes of moult parameters which correspond to environmental characteristics and the preservation of adaptive characteristics in the genefund of the population resulted in the creation of the premises for microevolutionary changes and intraspecific differentiation of ecolophysiological parameters. Hence, many subspecies very often differ with regards to moult characteristics. For example, a large number of species can be mentioned among the genera Sylvia , Phylloscopus, Motacilla, Emberiza and others. Closely related species also, as a rule, have differences in moult parameters and moult locations within their distribution ranges. In order to be fully convinced of this fact, we can compare such closely related species as Meadow-Pipit Anthus pratensis and Tree-Pipit A. trivialis, Pied Flycatcher Ficedula hypoleuca and Whitecollared Flycatcher F. albicollis, Whitethroat Sylvia communis and Menetrie’s Warbler S. mystacea, etc.
The most widespread forms of the variation of moult parameters in the annual cycle are the following: 1. shifting of the moult process to later or earlier dates; 2. changing of its duration; 3. changing of its extent; 3. complete reduction of postnuptial/postjuvenile or prenuptial moult; 4. shifting the location of moult from one part of the distribution range to another.
When the timing and location of moults, arranged for autumn and spring periods of the annual cycle, change, the character of the photoperiodic reaction, regulating this moult by shortening or lengthening daylength, does not change but, the thresholds of PhPI may change. Thus, using the characteristics of the photoperiodic reaction became a mechanism of adaptive variation of moult parameters. Deriving from this mechanism was the unique possibility to bring moult parameters into balance with environmental conditions.
All changes of moult parameters in the annual cycle of migratory birds lead, essentially, to the solution of the main problem - how to escape unfavorable environmental conditions in a timely manner and reach, in optimal time, an area with an abundant food supply. For this reason, the variation of moult parameters became the most widespread form of microevolutionary variation. The system of photoperiodic control of moult, combined with the pressure of natural selection, ensures the simplicity and rapidity of such variation. Therefore, the changes in moult parameters may become the first step towards intraspecific differentiation.
ACKNOWLEDGEMENTS
We are very grateful to all our colleagues from the Laboratory of Avian Biology and Bird Protection and everyone who at some time took part in investigations at the Ladoga Ornithological Station. Field and experimental studies on the Whitethroat and the Great Tit were supported by a grant from International Association for the Promotion of Cooperation with Scientists from the Independant States of the Former Soviet Union (INTAS-94-3169). The main investigations on geographical variation of moult were carried out in the framework of the project ‘Species in the distribution area’ of the UNESCO programme ‘Man and Biosphere’.
REFERENCES
Ashmole, N.Ph. 1968. Breeding and molt of the White Tern (Gygis alba) on Christmas Island, Pacific Ocean. Condor 70: 35-55.
Baggott, G.K. 1970. The timing of the moults of the Pied Wagtail. Bird Study 17: 45-46.
Dhondt, A.A. 1973. Postjuvenile and postnuptial moult in a Belgian population of Great Tits Parus major with some data on captive birds. Gerfaut 63: 187-209.
Dolnik V.R. 1975. Migracionnoe Sostojanie Ptic (in Russian). Moskau, Isdatelstwo Nauka: 398 pp.
Dolnik, V.R. & Gavrilov, V.M 1972. Photoperiodic control of annual cycles in the Chaffinch – a migrant within the temperate zone (in Russian). Zoological Journal 51: 1685-1696.
Evans, P.R. 1966. Autumn movements, moult and measurements of the Lesser Redpoll Carduelis flammea cabaret. Ibis 108: 183-216.
Farner, D.S. 1964. The photoperiodic control of reproductive cycles in birds. American Scientist 52: 137-156.
Gauci, C. & Sultana, J. 1979. Moult of the Sardinian Warbler. Il-Merill 20: 1-13.
Gavrilov, V.M. & Dolnik, V.R. 1974. Bioenergetics and regulation of postnuptial and postjuvenile moults in the Chaffinch (Fringilla coelebs) (in Russian). USSR Acad. Sci., Proc. Zool. Inst., Leningrad, Vol. 55: 14-61.
Ginn, H.B. & Melville, D.S. 1983. Moult in birds. BTO Guide 19. BTO, Tring, UK: 105pp.
Gosler, A.G. 1991. On the use of greater covert moult and pectoral muscle as measures of condition in passerines with data for the Great Tit Parus major. Bird Study 38: 1-9.
Gwinner, E. , Berthold, P. & Klein, H. 1971.Untersuchung zur Jahresperiodic von Laubsangern. Einfluss der Tageslichtdauer auf die Entwicklung des Gefieders, des Gewichts und der Zugunruhe bei Phylloscopus trochilus und Ph. collybita. Journal fur Ornithologie 112: 253 - 265.
Harris, M.P. 1971. Ecological adaptations of moult in some Brirish gulls. Bird Study 18: 113-118.
Hawthorn, I. 1974. Moult and dispersal of juvenile Wrens. Bird Study 21: 88-91.
Iovchenko, N.P. 1988. Comparative study of some seasonal events in Fringillidae family (Aves) (in Russian). PhD Thesis, Zoological Institute of Academy of Sciences of the USSR, Leningrad, USSR.
Iovchenko, N.P. 1990. Ecology and adaptive peculiarities in annual cycle of the White-winged Grosbeak (Mycerobas carnipes) (in Russian). In: Ornithology 24, Moscow: 84-94.
Iovchenko, N.P. 1993a. Ecology of the Greyheaded Goldfinch (Carduelis caniceps Vig.) in Tien-Shan (in Russian). In: USSR Acad. Sci., Proc. Zool. Inst, Leningrad, Vol. 252: 22-53.
Iovchenko, N.P. 1993b. Peculiarities of seasonal phenomena in the Whitethroat (Sylvia communis Lath.) in Tien-Shan. In: Proc. YI Meeting on the Project ‘Species and its Productivity in the Distribution Area’. St. Petersburg: 151-153.
Iovchenko, N.P. & Smirnov, Y.N. 1987. The postjuvenile moult of the Siskin Spinus spinus L. and the features of its photoperiodic regulation (in Russian). In: USSR Acad. Sci., Proc. Zool. Inst., Leningrad, Vol. 163: 22-53.
Jenni, L. & Winkler, R. 1994. Moult and ageing of European passerines. Academic Press, London: 225pp.
King, J.R. 1972. Postnuptial and postjuvenal molt in Rufous-collared Sparrows in northwestern Argentina. Condor 74: 5-16.
Kotov, A.A. & Noskov G.A. 1978. Comparative characterization of the moult in the Blue Hill Dove, the Rock Dove and domestic pigeons. Zoological Journal 57: 1202-1209.
Lapshin, N.V. 1981. Relationship between breeding and moulting in the Willow Warbler on the North-West of RSFSR (in Russian). In: Proc. X Baltic Ornithol. Conf. 1. Riga:100-109.
Lapshin, N.V. 1986. Relationship between breeding and moulting in the species of genus Phylloscopus in Karelia (in Russian). In: Ecology of the land vertebrates of the North-West of the USSR. Petrozavodsk: 26-35.
Lesher, S.W. & Kendeigh, S.Ch. 1941.Effect of photoperiod on molting of feathers. Wilson Bulletin 53: 169-180.
Ligon, J.D. 1971. Late summer-autumnal breeding of the Pin on Jay in New Mexico. Condor 73: 147-153.
Ligon, J.D. & White, J.L. 1974. Molt and its timing in the Pin on Jay Gymnorhinus cyanocephalus. Condor 76: 274-287.
Mester, H. & Prü nte, W. 1982. Die ‘sektorale’ postjuvenile Handschwingenmauser der Carduelinen in Sü deuropa. Journal fü r Ornithologie 123: 381-399.
Michener, H. & Michener, J.R.1940. The molt of House Finches of the Passadena Region, California. Condor 42: 140-153.
Miyazaki H. 1934 On the relation of the daily period to the sexual maturity and to the moulting of Zosterops palpebrosa japonica. Tohoku Imper. Univ. Science Reports, Biology 9: 183-203.
Mogilner, A.I.& Rymkevich, T.A. 1990. On quantitative regularities of fethering replacement (to tne method of studing the moult in birds) (in Russian). In: Proc. 20-th Meeting of Working Group on Project N 8b ‘Species and its Productivity in the Distribution Area. Vilnius: 33-37.
Muzaev, V.M. & Noskov, G.A. 1985. Prenuptial moult in the Whitethroat (Sylvia communis) from the southern shore of Ladoga Lake (in Russian). Zoological Journal 64: 889-896.
Newton, I. 1966. The moult of the Bullfinch Pyrrhula pyrrhula. Ibis 108: 41-67.
Noskov, G.A. 1970. On intraspecific variation of moult in birds (in Russian). In: Proc. YII Baltic Ornithol. Conf. 3. Riga: 52-54.
Noskov, G.A. 1975a. Moult in the Chaffinch (Fringilla coelebs L.) (in Russian). Zoological Journal 54: 413-424.
Noskov, G.A. 1975b. To the problem on the eco-physiological integrity of species in birds (in Russian). In: Studies of productivity in species in the distribution area. Vilnius: 106-116.
Noskov, G.A. 1977. Moult in the Chaffinch. II. Photoperiodic regulation and position in annual cycle (in Russian). Zoological Journal 56: 1676-1686.
Noskov, G.A. 1978. Molting of the Scarlet Rosefinch (Carpodacus erythrinus) and its photoperiodic regulation. Ecologia 1: 61-69.
Noskov, G.A. & Gaginskaya, A.R. 1969. Postjuvenile moult and migration in the Tree Sparrow in the conditions of Leningrad region (in Russian). In: Problems of ecology and biocenology 9, Leningrad: 48-58.
Noskov, G.A. & Gaginskaya, A.R. 1972. On the method of registration of moult in birds (in Russian). In: Proc. of the Baltic Commission for the Study of Bird Migration. 7: 154-163.
Noskov, G.A. & Kotov A.A. 1976. Some features of photoperiodic regulation of seasonal events in Doves (in Russian). Vestnik of Leningrad University 21: 39-46.
Noskov, G.A.& Rymkevich, T.A. 1977. The method for study of intraspecific variation of moult in birds (in Russian). In: The methods for study of productivity and structure of bird species in their distribution areas. Vilnius: 37-49.
Noskov, G.A.& Rymkevich, T.A. 1978. Mechanisms of photoperiodic control of moult in birds (in Russian). Vestnik of Leningrad University 9: 12-22.
Noskov, G.A.& Rymkevich, T.A. 1985. Photoperiodic control of postjuvenile and postnuptial moults in Passeriformes. In: Acta XYIII Congr. Int. Ornithol. Vol. II;Moscow: 930-934.
Noskov, G.A. & Rymkevich, T.A. 1988a. On regularities of variability of annual cycles of seasonal events in the distribution area (in Russian). In: Place of species among biological systems. Vilnius: 45-70.
Noskov, G.A.& Rymkevich, T.A. 1988b. On regularities of adaptive transformations of annual cycles in birds (in Russian). Dokl. AN SSSR 301: 505-508.
Noskov, G.A. & Smirnov Y.N. 1979. Experimental study of photoperiodic regulation of postnuptial moult in the Greenfinch (in Russian). Biological Sciences 3: 38-45.
Noskov, G.A. & Smirnov Y.N. 1986. Some features of photoperiodic control of timing and extent of postjuvenile moult in the Common Redpoll (in Russian). In: Actual problems of ornithology. Moscow: 70-80.
Payne, R. 1972. Mechanisms and control of moult. In: Farner, D.S. & King, J.R. (eds.). Avian biology. Vol. 2; New York, Academic Press:104-155.
Pearson, D.J. 1975. Moult and its relation to erruptive activity in the Bearded Reedling. Bird Study 22: 205-227.
Pinkovski, B.C. 1976. Photoperiodic effects on the postjuvenal molt of the Eastern Bluebird. Ohio Journal of Science 76: 268-273.
Rymkevich, T.A. 1974. Photoperiodic control of timing of postjuvenile moult in the Ortolan Bunting (in Russian). In: Proc. of YI Allunion Ornithol. Conf.. Moscow: 166-168.
Rymkevich, T.A. 1976a. Juvenile plumage development and postjuvenile moult in the Rustic Bunting (Emberiza rustica Pall.) in Leningrad region (in Russian). Zoological Journal 55: 1695-1703.
Rymkevich, T.A. 1976b. Photoperiodic regulation of timing of postjuvenile moult in the Ortolan Bunting (Emberiza hortulana) from the Leningrad region (in Russian). Vestnik of Leningrad University 15: 19-25.
Rymkevich, T.A. 1977. Photoperiodic regulation of postjuvenile and postnuptial moult in the Yellowhammer (in Russian). In: Proc. of YII Allunion Ornithol. Conf.. Kiev: 154-155.
Rymkevich, T.A. 1983. Comparative characterization of moult in Buntings (g. Emberiza) of Leningrad Region (in Russian). In: Proc. of the Baltic Commission for the Study of Bird Migration. 14: 83-112.
Rymkevich, T.A. (ed.) 1990. Moult of passerines of northwestern USSR (in Russian). Leningrad. Izd. Leningradskogo Universiteta: 302pp.
Rymkevich, T.A. & Bojarinova, J. G. 1996. Variation in the extent of postjuvenile moult in the Great Tit near Lake Ladoga (Russia). Bird Study 43: 47-59.
Rymkevich, T.A., Mogilner, A.I., Noskov ,G.A. & Yakovleva, G.A. 1987. New indices for characterizing the moult of passerine birds (in Russian). Zoological Journal 66: 444-452.
Rymkevich, T.A. & Pravosudova E.V. 1987. The moult in the annual cycle of Pied Flycatcher (Ficedula hypoleuca Pall.) (in Russian). USSR Acad. Sci., Proc. Zool. Inst., Leningrad, Vol. 163: 95-111.
Ryzhanovsky, V.N. 1997. Ecology of postnesting period in passerine birds of Subarctic. Ekaterinburg, Izdatelstwo Uralskogo Universiteta: 288pp.
Savinich, I.B. 1984. Forming of plumage in the Robin (Erithacus rubecula) in the fist year of life and photoperiodic regulation of postjuvenile moult (in Russian). Vestnik of Leningrad University 9: 27-31.
Stolbova, F. S. 1987. Analysis of geographical variation of annual cycles in migratory birds with using as model some species of genus Sylvia (in Russian). PhD Thesis, Leningrad University, Russia.
Stresenann, E. & Stresemann, V. 1967. Inheritance of the adaptation in moult. In: Proc. 14th Int. Ornithol. Congr., Oxford: 75-80.
Stresenann, E. & Stresemann, V. 1968. Winterquartier und Mauser der Dorngrasmü cke, Sylvia communis. Journal fü r Ornithologie 109: 303-314.
Westphal, D. 1976. Ü ber die postjuvenile Mauser beim Grü nfink Carduelis chloris. Journal für Ornithologie 117: 70-74.
Winkler, R. & Jenni L. 1987. Weitere Indizien fur ‘sektorale’ Handschwingenmauser bei jungen Singvö geln. Journal fü r Ornithologie 128: 243-246.
Yakovleva, G.A., Noskov, G.A. & Rymkevich T.A. 1987. Comparative characterization of postembrionic development and postjuvenile moult in the Pied Wagtails (Motacilla alba L.) from early and late broods. Vestnik of Leningrad University 2: 12-20.
Table 1. Timing, age of onset and duration of postjuvenile moult in early broods of the Greenfinch kept under the natural decreasing (for 61° N) and long daylengths

Table 2 . Age of moult onset (days) under short , natural and long light days in some birds of Subarctic. ( From Ryzhanovsky 1997)
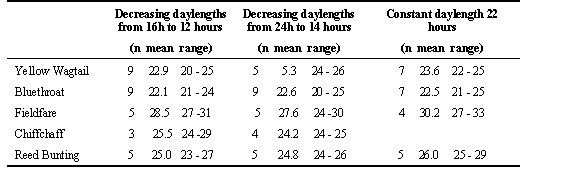
Fig. 1. The relationship between the age of moult onset and the daylength in the Common Redpoll Carduelis flammea. Means are represented by black circlets. Ranges are represented by vertical lines. (From Noskov & Smirnov 1986).
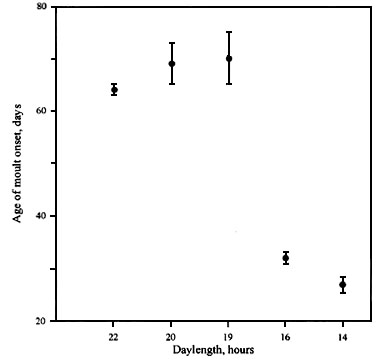
Fig. 2. Minimal and maximal ages of postjuvenile moult onset for the different passerines on the Northwest of Russia. 1,2, 3, from Stolbova 1987; 4, from Rymkevich and Pravosudova 1987; 5, from Rymkevich 1976b; 6, from Rymkevich 1976a; 7, from Rymkevich 1977; 8, from Savinich 1984; 9, from Rymkevich & Bojarinova 1996; 10, from Iovchenko & Smirnov 1987; 11, from Noskov & Smirnov 1986; 12, 13, from Noskov & Smirnov in Rymkevich 1990.
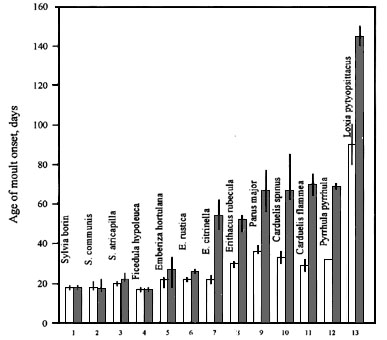
Fig. 3. Dynamics of postjuvenile moult under constant long (1) and natural decreasing (2) daylengths in experimental groups of the Greenfinch Carduelis chloris.
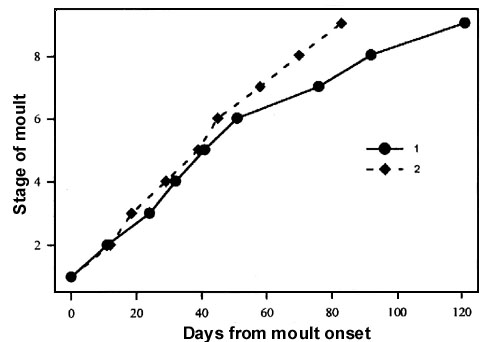
Fig. 4. The rates of the postjuvenile moult in the Great Tit under the decreasing daylengths simulated the natural photoperiodic conditions at 56° N: 1, late timing of moult; 2, early timing of moult. Moult is represented by filled symbols and solid lines. Photoperiodic conditions are represented by open symbols and broken lines.
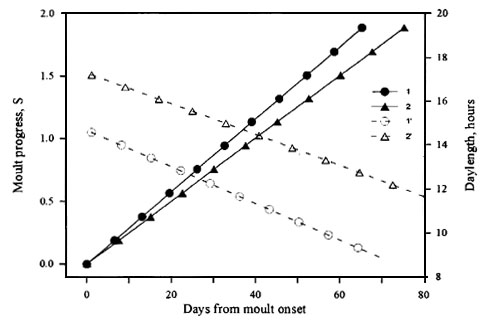
Fig. 5. The change of duration of postjuvenile moult in the Whitethroat by decreasing of moult extent. (A) the dynamics of moult progress under the photoperiodic conditions corresponding the late (1) and early (2) hatching dates on the Northwest of Russia; (B) the dynamics of portion of old body feathers for the same birds.
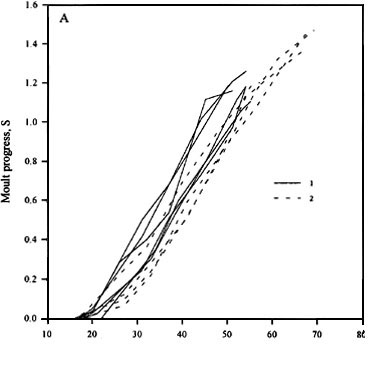
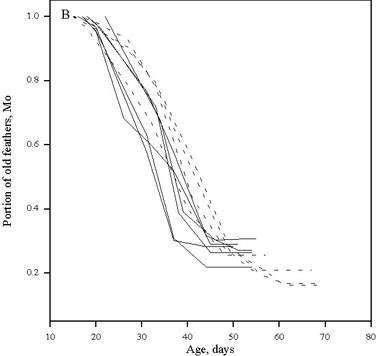
Fig. 6. The rates of postnuptial moult in experimental groups of the Woodlark Lullula arborea kept under the different photoperiodic conditions: (A) the natural decreasing daylengths (60° N) for the late (1) and early (2) timing of moult; (B) the rapid (
3) and slow (4) decreasing daylengths; (C) the 20h- constant (5) and natural decreasing daylengths; (D) the 14h- constant (6) and natural decreasing daylengths. Moult are represented by solid lines and filled symbols. The photoperiodic conditions are represented by broken lines and open symbols.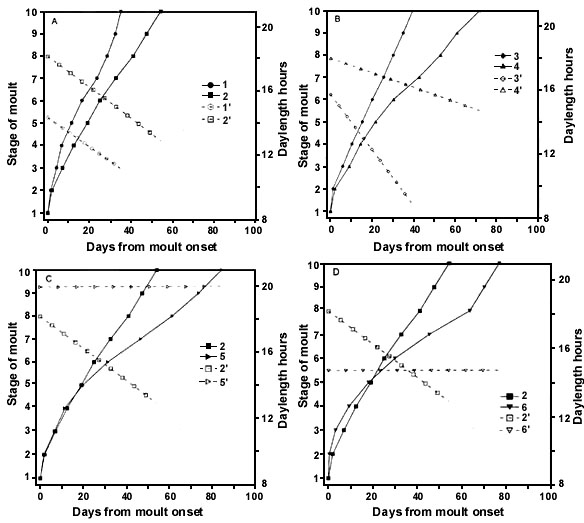
Fig. 7. Timing, duration and extent of prenuptial moult in five groups of the Pied Flycatcher kept under the different photoperiodic conditions (A, n = 6; B, n = 5; C, n = 5; D, n = 5; E, n = 2) for a winter. Periods of moult are represented by black bars. Daylengths are represented by broken lines. Du - individual duration of moult. C - completeness of body feather moult.
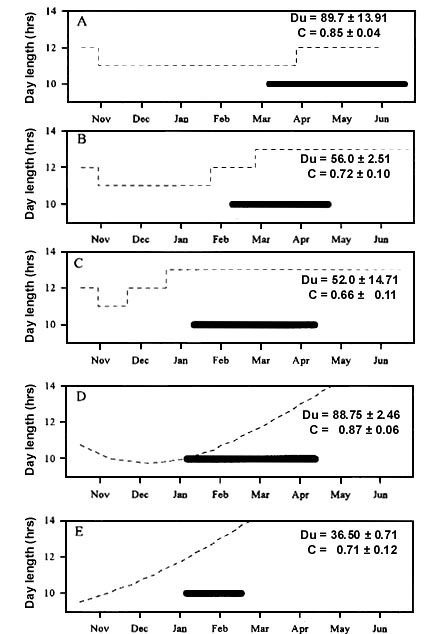
Fig. 8. The photoperiodic conditions and parameters of postjuvenile moult in the Siskins in which young birds hatched in the same region can renew the plumage in the different parts of distribution area. Moult timing are represented by black bars. Changes of daylength are represented by solid lines.
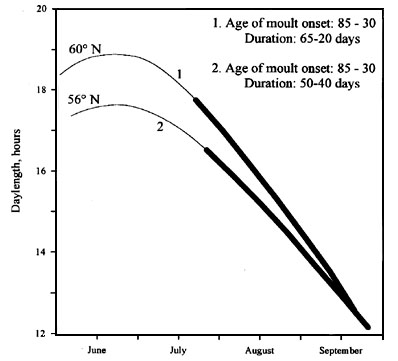
Fig. 9. The photoperiodic conditions and parameters of postjuvenile moult in the Blackcap in the different parts of the distribution area (1, Sankt-Petersburg region, n = 8; 2, Belgorod region, n = 9; 3, Black Sea region, n = 8). Moult timing are represented by black bars. Changes of daylength are represented by solid lines.