S10.1: Energetics and nutrition of moulting
Mary E. Murphy
Department of Zoology, Washington State University, Pullman WA 99164 USA, correspondence to 80 High Gate Lane, Blue Bell, Pennsylvania,19422 USA, email murp287@ibm.net
Murphy, M.E. 1999. Energetics and nutrition of moulting. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 527-535. Johannesburg: BirdLife South Africa.During their annual moults, birds expend energy and allocate substrate to support plumage replacement and concomitant adjustments in whole body tissue metabolism. Comparisons of energy expenditure and amino acid allocation by naturally moulting sparrows Zonotrichia leucophrys gambelii and by wintering sparrows induced by plucking to replace as much as one-third their plumage revealed that the prepotent determinants of the energy and nutrient costs of annual moult are the concomitant adjustments in whole body tissue metabolism. Moreover, changes in whole body tissue metabolism that are characteristic of the natural annual moult do not appear to be universally nor obligatorily linked with feather synthesis, as they were not evident in wintering birds replacing simultaneously as many as one-third the total number of feathers in each feather tract. The cumulative data from Z. l. gambelii imply that the annual moult is more than simply a period of plumage replacement: this period may be described better as a metabolic transition phase in the annual cycle of birds, that involves widespread tissue renovation
INTRODUCTION
The annual moult refers generally to the phase of a bird’s annual cycle when the plumage and other epidermal structures are replaced. The process of moulting results in deposition of protein, mainly as keratins, in amounts equalling or even exceeding one-quarter of a bird’s total protein mass (Murphy 1996). Moulting is an essential self maintenance process in birds that, at least in some species, proceeds even at the expense of other body tissues and functions, when such a response is necessitated by time, nutrient, or energy constraints (Murphy et al. 1988).
The physiology, metabolism, nutrition, and energetics of avian moult are poorly understood despite the fundamental importance of this process in the lives of birds. Considerable speculation surrounds the impact of the nutrient and energy demands imposed by this process on the individual and ultimately on species life histories and ecology. A full appreciation of this impact awaits a clearer characterisation of the process itself.
For at least two decades biologists have recognised that the annual moult in birds entrains widespread metabolic adjustments (Murphy 1996; Murphy & King 1992; King 1981). But the extent and character of these adjustments are poorly defined. The intent of this report is to review what is known about adjustments in metabolism during the annual moult in a well studied bird, the White-crowned Sparrow Zonotrichia leucophrys gambelii (henceforth Gambel’s Sparrow), and to present evidence to suggest that the annual moult is more than simply a period of plumage replacement.
No bird has been studied as extensively during its annual moult as Gambel’s Sparrow (Murphy 1996). The pattern and timing of moult in this species is typical of that of most passerines and its energy requirements for moult are like those reported for other small passerines (Murphy 1996; Murphy & King 1992; King 1981). Other than undergoing a comparatively rapid moult (ca. 54 days), Gambel’s Sparrow appears representative of at least the passerine species.
ADJUSTMENTS IN TISSUE METABOLISM OF MOULTING SPARROWS
Although the annual moult is characterised mainly as a period of feather replacement, significant changes in whole body tissue metabolism appear to occur concomitant with feather regeneration. In Gambel’s Sparrows, changes in bone metabolism and in whole body protein metabolism (synthesis and degradation) accompany changes in the metabolism and organization of the integument during the annual moult.
Adjustments in the integument
Because moult is periodic rather than continuous and results in formation of a metabolically inert product, synthesis of feathers requires metabolic and organisational processes beyond simply accelerating or decelerating local anabolic or catabolic processes. Initiation of feather synthesis requires recrudescence of various components of the integument, including increased vascularization of the active feather follicle and pulp formation (Lillie 1940). When the follicle is active, feather growth proceeds by the process of keratinization (Fraser et al. 1972).
Adjustments in bone metabolism
A sudden change in bone metabolism appears coincident with the annual moult in well-nourished Gambel’s Sparrows. This change is best described as a cyclic osteoporosis and it is not unique to passerines. Moult osteoporosis has been reported for 16 species of birds in 7 families (Meister 1951; Zahnd 1954). But the phasing of this cyclic osteoporosis in relation to the intensity of feather synthesis appears to vary among species. In Gambel’s Sparrows, moult osteoporosis was mild during the most intensive phase of feather replacement when compared with some species (cf. Meister 1951) but was nonetheless evident as indicated by (1) the appearance of the bone in cross-section, (2) significant decreases in bone density (g/ml), and (3) significant increases during the moult in both the ratio of marrow-cavity area to total bone area and in the void area per unit area of cortical bone (Murphy et al. 1992).
It is not known whether changes in bone metabolism during the annual moult are linked to feather synthesis, or if they proceed simultaneously with, but independent of, feather synthesis. The variation among species in phasing the relative intensities of changes in bone and integument metabolism suggest that the processes occur concomitantly but are probably not linked metabolically or nutritionally. Independence between these processes is further supported by the observation that feathers and bones do not significantly share any common substrate. Bone is primarily ash. Feathers typically contain less than 3% ash and much of the mineral content of feathers is deposited fortuitously rather than as structural components (Murphy 1996). Moreover, this cyclic osteoporosis was observed in well-nourished sparrows that were meeting their nutritional demands for feather synthesis by dietary intake. These observations suggest that a renovation of the skeleton may accompany the renovation of the plumage during the annual moult.
Adjustments in protein metabolism
During the annual moult, whole body protein turnover (synthesis and degradation) accelerates in well-nourished Gambel’s Sparrows (Murphy & Taruscio 1995; Taruscio & Murphy 1995). In absolute terms, during the most intensive phase of feather regeneration moulting sparrows synthesised and degraded daily 260 mg of body proteins (excluding keratins) above that synthesised by non-moulting sparrows. In relative terms, whole body protein turnover rates accelerated an average 35% during moult as compared with non-moult. This daily increase in whole body protein turnover equalled at least 3.5-fold the amount of protein synthesised and deposited as feather keratin per day (~ 75 mg).
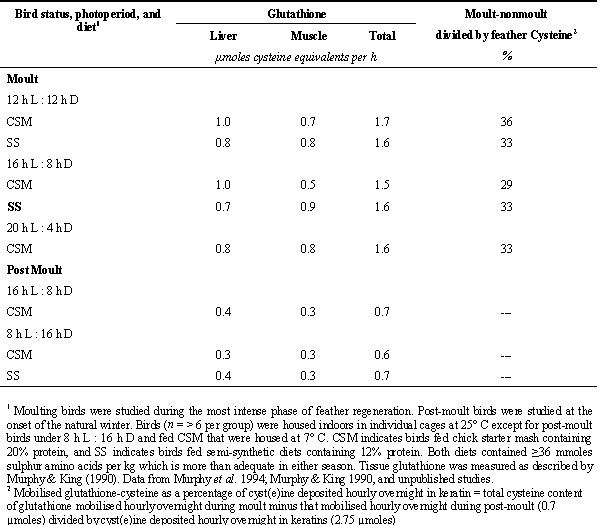
It is not known why whole body protein turnover accelerates during moult. It has been hypothesised that the annual moult may involve more extensive renovation of body tissues than previously recognised (Murphy & Taruscio 1995). The acceleration in whole body protein turnover during moult cannot be explained simply by a need for substrate, particularly cyst(e)ine, for keratin synthesis. Increased rates of whole body protein synthesis by moulting sparrows as compared with non-moulting ones were evident throughout the diurnal cycle and were independent of diurnal changes in body protein contents (i.e. mainly variation in degradation rates regulated diurnal fluxes in body protein content). Moreover, the low sulphur amino acid (methionine plus cysteine) content of tissue protein that would presumably supply amino acids for synthesis of cysteine-rich keratins overnight was compensated by contribution of cyst(e)ine from glutathione (Table 1). In well nourished Gambel’s Sparrows the tripeptide gamma-glutamylcysteinylglycine (glutathione) was stored in tissue by day during moult in amounts adequate to supplement cysteine derived by degradation of tissue protein overnight to support keratin synthesis (Murphy 1994; Murphy & King 1990). This adaptive adjustment in glutathione metabolism enhances the economy of tissue protein metabolism during moult and minimises waste of amino acid substrate by efficiently delivering the rate limiting amino acid for keratin synthesis. Regardless of the duration of the overnight fast, Gambel’s Sparrows stored amounts of glutathione by day above that stored by their non-moulting counterparts equal to about one-third the overnight need for cysteine for keratin synthesis.
Further evidence that substrate need contributes little to the significant changes in protein metabolism during moult is provided from responses of moulting sparrows to malnutrition. In response to a one-day fast or protein deficiency, moulting Gambel’s Sparrows reduced the naturally accelerated rates of muscle protein degradation that are characteristic of moult in well nourished sparrows (Pearcy & Murphy 1997a). Only when the sulphur amino acids were deficient were these sparrows unable to recruit compensatory mechanisms to forestall malnutrition. In moulting sparrows, but not in non-moulting sparrows, sulphur amino acid deficiency resulted in a profound increase in muscle protein degradation above moulting levels largely as a consequence of the inability to store glutathione during the feeding day. Similarly, moulting sparrows fed diets low in protein reduced their normally elevated rates of oxidation of essential amino acids, and increased retention (reutilization) of these amino acids in tissues proteins (Pearcy & Murphy 1997b). These results demonstrate that acceleration of whole body protein and amino acid metabolism is an integral feature of the annual moult in the well nourished bird and not a result of substrate (nutrient) shortages.
The largest component of body protein is contained in the skeletal muscle which constitutes 45% of body mass in most endotherms and contains approximately 20% protein (Millward & Bates 1983; Maynard et al. 1979). Muscle protein includes a myofibrillar structural component and a more labile sarcoplasmic component. In skeletal and in cardiac muscle, the structural protein turnover rates appear to increase proportionately to increases in whole body protein turnover during the annual moult making this period a time when more rapid reorganization of the sarcomeres of muscle occurs.
Myofibrillar protein turnover involves the formation of ‘easily releasable myofilaments’ (ERM) as intermediates in protein degradation (Murphy et al. 1996; Dahlmann et al 1986; Van der Westhuysen et al. 1981). ERM can be separated from isolated myofibrils using an ATP-relaxing solution and assayed for protein content. During moult, but not during winter, amounts of ERM in skeletal and cardiac muscle vary diurnally in well nourished Gambel’s Sparrows (Table 2). In evening, amounts of ERM in moulting birds increased in both tissues by about 28% when compared with morning and were 35% more abundant in moulting sparrows than in non-moulting sparrows. Muscle protein contents do not decline through the period of moult in well nourished Gambel’s Sparrows (Chilgren 1977) indicating that structural renovation of muscle is normally enhanced during the annual moult.
ADJUSTMENTS IN THE ENERGY DEMANDS OF MOULTING SPARROWS
Measures of oxygen consumption by moulting birds of several species reveal increases above non-moulting levels that range broadly from about 9-111% (Lindström et al. 1993; King 1981). These results indicate (1) that the replacement of the plumage by birds often proceeds at comparatively low energetic efficiencies (2% - 30% of energy expended for moulting is deposited as product; (Murphy 1996; Lindström et al. 1993), and (2) that there is significant variation and uncertainty in current estimates of the cost of plumage regeneration. The apparent energetic inefficiency and some of the variation in measured costs of avian moult could be accounted for by the costs of concomitant adjustments in non-keratinous tissue metabolism.
Early theoretical estimates of the costs of plumage synthesis were as low as 7.6% existence energy levels (King & Farner 1961) based on a heat of combustion of keratin equalling 21.7 kJ and a partial (net) efficiency of keratin synthesis equalling 70%. This value has rarely been approximated in reports of the cost of avian moult. King (1981) concluded ‘that the costs of moult involve metabolic or nutritional processes in addition to simple synthesis of keratin.’ King did not speculate on whether these processes were obligatorily linked with feather synthesis.
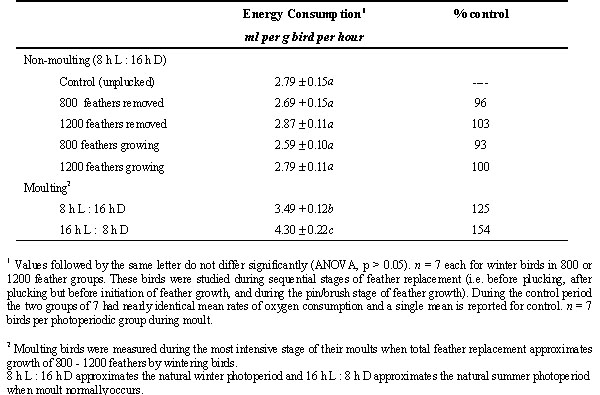
Comparisons of oxygen consumption rates by moulting Gambel’s Sparrows and their non-moulting conspecifics in varying plumage conditions reveals that the bulk of the energy costs of the annual moult and its apparent energy inefficiency do not result directly from feather synthesis and attendant processes (Table 3; Schieltz & Murphy 1997). Wintering sparrows replacing plumage masses equal to those replaced by sparrows during the most intensive phase of feather replacement during the natural annual moult (24% - 36% total plumage mass) expend an amount of energy corresponding to <3% of resting metabolic rate (RMR) compared with 25% to 54% for moulting sparrows (Schieltz & Murphy 1995; 1997).
These low energy costs of plumage synthesis by wintering birds are consistent with early theoretical estimates of the costs of feather synthesis. The energy expended by Gambel’s Sparrows for the annual moult seems to be predominantly associated with metabolic changes beyond the integument. Moreover, these metabolic changes, including at least an accelerated turnover of tissue proteins and renovation of the skeleton do not appear to be obligatorily linked to feather production.
CONCLUSIONS
The cumulative data from Gambel’s Sparrows suggest that the annual moult is more than just a period of plumage replacement, but rather may involve widespread tissue renovation. If so, several important questions pertaining to the ecology and evolution of different groups of birds emerge. First, does the extent and phasing of non-keratinous tissue renovation during the annual moult vary with species and (or) life history patterns? Gambel’s Sparrow is a small, migratory, omnivore. The dynamics of muscle and bone metabolism have not been studied thoroughly in a non-migratory bird. Second, the apparent dissociation of plumage regeneration and non-keratinous tissue renovation in wintering Gambel’s Sparrows could also occur during the prenuptial moult. If so, are the added energy costs of pre-nuptial moult trivial in the energy budgets of birds? Third, what constraints, if any, are conferred on birds by tissue renovation during the annual moult? Moulting birds are known to significantly reduce their activity (e.g. Morton & Morton 1990; Bryant & Tatner 1988; Bailey 1985; Wijnandts 1984; Newton 1966; Eyster 1954). This could be accounted for simply on the basis of impaired flight and the susceptibility of growing flight feathers to damage. In that case, the annual moult would present an ideal time for widespread tissue renovation. Alternatively, widespread tissue renovation, particularly in the skeletomuscular system, could exacerbate impaired flight requiring limited mobility during the annual moult. Much attention has been given to the energy and nutrient demands of moulting as potential constraints in the life history of birds. Through selective feeding, metabolic adjustments such glutathione storage, and adjustments in the components of the energy budgets (e.g. activity level), birds seem to have minimised the nutritional challenges of moult (Murphy 1996; Murphy & King 1992). How they have coped with the physiological, metabolic, and ecological challenges remains to be discerned.
ACKNOWLEDGMENTS
This review is a product of the efforts of numerous graduate students, including John Franson, Kealy McCleery, Shawn Pearcy, Paul Schieltz, Jack Small, and Todd Taruscio. Charles M. Tatum provided a continuous source of thoughtful comments, queries, and encouragement. This work was supported by the U. S. National Science Foundation and The Program in Biology, Washington State University, Pullman, WA.
REFERENCES
Bailey, R.O. 1985. Protein reserve dynamics in postbreeding adult male Redheads. Condor 87: 23-32.
Bryant, D.M. & Tatner, P. 1988. Energetics of the annual cycle of Dippers Cinclus cinclus. Ibis 130: 17-38.
Chilgren, J.D. 1977. Body composition of captive White-crowned Sparrows during postnuptial molt. Auk 94: 677-688.
Dahlmann, B., Rutschmann, M. & Reinauer, H. 1986. Effect of starvation or treatment with corticosterone on the amount of easily releasable myofilaments in rat skeletal muscles. Biochem. J. 234: 659-664.
Eyster, M.B. 1954. Quantitative measurement of the influence of photoperiod, temperature, and season on the activity of captive songbirds. Ecol. Monogr. 24: 1-28.
Fraser, R.D.B., MacRae T.P. & Rogers, G.E. 1972. Keratins. Springfield, Illinois, C. C. Thomas.
King, J.R. 1981. Energetics of avian moult. Acta Int. Ornithol. Congr. 17: 312-317.
King, J.R. & Farner, D.S. 1961. Energy metabolism, thermoregulation, and body temperature. In: Marshall, A. J. (ed) Biology and comparative physiology of birds. New York; Academic Press: 215-288.
Lillie, F.R. 1940. Physiology of development of the feather. III. Growth of the mesodermal constituents and blood circulation in the pulp. Physiol. Zool. 13: 143-175.
Lindström, A., Visser, G.H., & Dann, S. 1993. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 66: 490-510.
Maynard, L.A., Loosli, J.K., Hintz, H.F. & Warner, R.G. 1979. Animal nutrition. New York, McGraw Hill.
Meister, W. 1951. Changes in histological structure of the long bones of birds during the molt. Anat. Rec. 111: 1-21.
Millward, D.J. & Bates, P.C. 1983. 3-Methylhistidine turnover in the whole body, and the contribution of skeletal muscle and intestine to urinary 3-methylhistidine excretion in the adult rat. Biochem. J. 214: 607-615.
Morton G.A. & Morton, M.L. 1990. Dynamics of the postnuptial molt in free-living mountain White-crowned Sparrows (Zonotrichia leucophrys oriantha). Condor 92: 813-828.
Murphy, M.E. 1996. Energetics and nutrition of molt. In:Carey, C., (ed) Avian energetics and nutritional ecology. New York; Chapman & Hall: 158-198.
Murphy, M.E. 1994. Glutathione as a reservoir of cysteine in well nourished animals. FASEB J. 8: 546.
Murphy, M.E. & Taruscio, T.G. 1995. Sparrows increase their rates of tissue and whole-body protein synthesis during the annual molt. Comp. Biochem. Physiol. 111A: 385-396.
Murphy, M.E. & King, J.R. 1992. Energy and nutrient use during moult by White-crowned Sparrows Zonotrichia leucophrys gambelii. Ornis Scand. 23: 304-313.
Murphy, M. E. & King J. R. 1990. Diurnal changes in tissue glutathione and protein pools of molting White-crowned Sparrows: The influence of photoperiod and feeding schedule. Physiol. Zool. 63: 1118-1140.
Murphy, M.E., Pearcy, S.D. & Hopkins, P. 1996. Easily releasable myofilaments (ERM) pools in skeletal and cardiac muscle of growing Coturnix quail. FASEB J. 10: A737.
Murphy, M.E., Taruscio, T.G., King, J.R. & Truitt, S.G. 1992. Do molting birds renovate their skeletons as well as their plumages? Osteoporosis during the annual molt in sparrows. Can. J. Zool. 70: 1109-1113.
Murphy, M.E., King, J.R. & Lu, J. 1988. Malnutrition during the postnuptial molt of White-crowned Sparrows: feather growth and quality. Can. J. Zool. 66: 1403-1413.
Newton, I. 1966. The moult of the Bullfinch Pyrrhula pyrrhula. Ibis 108: 41-67.
Pearcy, S.D. & Murphy, M.E. 1997a. 3-Methylhistidine excretion as an index of muscle protein breakdown in birds in different states of malnutrition. Comp. Biochem. Physiol. 116A: 267-272.
Pearcy, S.D. & Murphy, M.E. 1997b. Essential amino acid metabolism in molting and non-molting sparrows in different nutritional states. Comp. Biochem. Physiol. 118A: 1157-1163.
Schieltz, P.C. & Murphy, M. E. 1995. Diurnal variation in oxygen consumption by molting and nonmolting sparrows. Comp. Biochem. Physiol. 112A: 265-272.
Schieltz, P.C. & Murphy, M.E. 1997. The contribution of insulation changes to the energy cost of avian molt. Can. J. Zool. 75: 396-400.
Taruscio, T.G. & Murphy, M.E. 1995. 3-Methylhistidine excretion by molting and non-molting sparrows. Comp. Biochem. Physiol. 111A: 397-403.
Van der Westhuyzen, D.R., Matsumoto, K. & Etlinger, J.D. 1981. Easily releasable myofilaments from skeletal and cardiac muscles maintained in vitro. J. Biol. Chem. 256: 11791-11797.
Wijnandts, H. 1984. Ecological energetics of the Long-eared Owl (Asio otus). Ardea 72: 1-92.
Zahnd, J.P. 1954. Sur les modifications histologiques du squelette des Oiseaux pendant la mue. C. R. Seances Soc. Biol. Strasbourg 148: 1491-1493.
Table 1. Overnight mobilisation of glutathione from liver and muscle in moulting and non-moulting Gambel’s Sparrows.

Table 2. Diurnal and seasonal variation in the amounts of easily releasable myofilaments (ERM; mean + S. D.) in skeletal and cardiac muscle of moulting and non-moulting Gambel’s Sparrows.1
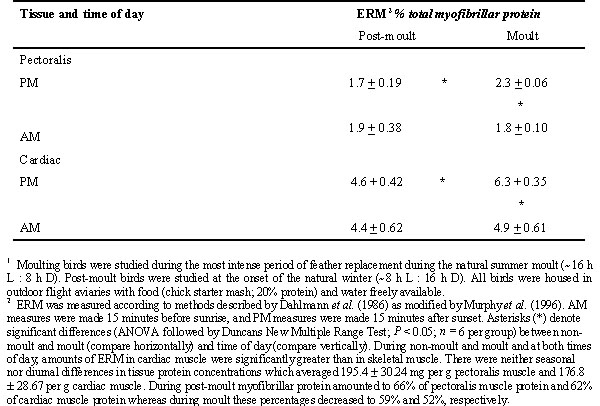
Table 3. Comparison of energy consumption (mean + S. E.) by moulting and non-moulting sparrows in differing plumage conditions at 25° C (Table adapted from Schieltz & Murphy 1997).