S09.3: Bilateral hemispheric co-ordination of birdsong
Marc Schmidt & Masakazu Konishi
Division of Biology 216-76, California Institute of Technology, 1200 E. California Blvd, Pasadena, CA 91125, USA, fax 626 449 0679, e-mail konishim@cco.caltech.edu
Schmidt, M.F. & Konishi, M. 1999. Bilateral hemispheric co-ordination of birdsong. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
509-523. Johannesburg: BirdLife South Africa.Song production in passerines is controlled by a network of interconnected forebrain nuclei known as the song system. These nuclei are found in both the left and right side of the brain and are anatomically indistinguishable between hemispheres. We present evidence that vocal premotor activity is highly synchronised and co-ordinated across hemispheres. Specifically, recordings from vocalising adult Zebra Finches Taeniopygia guttat reveal that the onset of premotor neural activity in HVc, the hierarchically highest centre in the vocal motor pathway, is tightly synchronised between the left and right side. Furthermore, once synchronously initiated, song-related neural activity appears to be continuously monitored across hemispheres with premotor activity becoming resynchronised whenever a mismatch occurs between both sides. Details of this interhemispheric co-ordination are likely to be quite complex since there exist at least three feedback pathways capable of providing feedback and feedforward signals. Given the precision with which muscles from the left and right syringes must be co-ordinated during syllable production, the observed co-ordination between forebrain motor commands may play an important role in the high degree of motor co-ordination observed in the periphery.
INTRODUCTION
Simultaneous recordings of air flow and sound in the left and right bronchi show that songbirds are able to produce different sounds from the left and right halves of their syrinx (Hartley, 1990; Suthers, 1990; Suthers, et al., 1994; Suthers, 1997). These different sounds can either be generated simultaneously or in sequence within a syllable. In a few cases, both sides of the syrinx produce the same sound. Coordination between the two sides of the syrinx is thought to be exquisitely precise as birds can often rapidly switch from one side to the other between syllables and within a syllable (Allan & Suthers, 1994; Suthers & Goller, 1996). This coordination between both sides of the syrinx is thought to involve mainly the action of syringeal muscles which control the opening and closing of the syringeal aperture (Goller & Suthers, 1996; Goller & Suthers, 1996). Respiratory muscles, which also play a crucial role in song production (Wild, 1997), are less likely to be involved in bilateral coordination of syringeal output since air can pass from one side of the air sac system to the other.
A network of discrete nuclei collectively known as the song system (Fig. 1) controls both syringeal and respiratory muscles involved in song production (Nottebohm, et al., 1976; Wild, 1997). These nuclei are found on both the right and left side of the brain and are anatomically indistinguishable between hemispheres. The present article focuses on the general organization of the vocal motor pathway and, in particular, on recent findings showing that the song system does not act as two independent ipsilateral pathways. Rather, song motor commands appear to be precisely coordinated across hemispheres and may be a necessary prerequisite for the coordination observed in the periphery.
METHODS
Animals and surgery.
Adult (>120 d posthatch) male Zebra Finches Taeniopygia guttata were obtained from our breeding colony or from a commercial breeder (Magnolia Bird Farms, Anaheim, CA). In order to increase the frequency of singing behavior, these birds were implanted subcutaneously with small pellets of testosterone proprionate in Silastic (Gurney, 1981) several days before surgery. For chronic implantation of recording and stimulating electrodes, birds were anaesthetised with a mixture of ketamine (25 mg/kg) and xylazine (5 mg/kg, i.m.) and placed in a stereotaxic device with a custom-built bill bar to hold the upper mandible at 30° below horizontal. Body temperature was maintained at 38° C throughout the surgery. Two electrodes were placed into each HVc of each experimental bird. All electrodes for each bird were connected to a nanoconnector (Ultimate Inc., Orange, CA) and the whole assembly was cemented (GripCement, Milford, DE) onto the bird’s skull with the nanoconnector placed several mm away from any implanted electrode. A stainless steel wire was also inserted under the scalp and partly cemented to the skull to ground the animal.
Electrodes.
Both stimulating and recording electrodes were fabricated from Formvar-insulated nichrome wires (25 m m bare diameter; AM-Systems, Seattle, WA) with tips that were electroplated with gold or rhodium to lower the tip impedance. Typical impedances of electrodes ranged from 100 kW to 1 MW measured at 1 kHz). Stimulation electrodes were always implanted in pairs that were 300-400 m m apart; stimulation currents were applied across such electrode pairs and were isolated from ground.
Experimental sessions.
Following electrode implantation, birds were kept isolated in sound-attenuating chambers (Industrial Acoustics Company, New York, NY) for several days before being subjected to a single recording session. They were provided with food, gravel, and water ad libitum. At the beginning of the experimental session, the bird was placed in a cage within a sound-attenuated recording chamber and attached to a commutator via a flexible cable made from a bundle of nine Teflon-coated stainless steel wires (200 m m bare diameter). This cable was attached to an ultra-lightweight LinCMOS precision operational amplifier (TLC27L4B, Texas Instruments, Dallas, TX) which was connected to the bird’s head. The amplifier served as a unity-gain voltage follower to eliminate movement artifacts from the signal. The flexible cable and commutator allowed full freedom of movement while providing electrical connections between the bird and the recording and stimulating circuitry. Neural signals and sounds recorded by a microphone placed 30 cm from the recording cage were digitised and stored with a computer (SPARC Station, Sun Microsystems, Palo Alto, CA) equipped with a data acquisition system (Proport Model 656, Ariel). Both sound and neural records were digitised at 32 kHz sampling rate and 16-bit resolution and bandpass filtered between 400 Hz and 10 kHz.
The duration of each recording session was between 30 min and 3 hours. Typically, a female Zebra Finch was placed in an adjacent cage at the start of the session in order to induce directed singing (Sossinka and Böhner, 1980). In experiments requiring stimulation, electrical stimuli to the brain were generated and isolated from ground using an AM-Systems 2100 stimulator (Seattle, WA). Stimuli were manually triggered during singing and consisted of a train of 2-7 biphasic pulses at 400 Hz with a pulse duration of 0.4 msec/phase.
Histology.
To locate the sites of implanted electrode tips, birds were deeply anaesthetised with a mixture of ketamine and xylazine (same dosage as for surgery) and perfused transcardially with 0.9% saline followed by 2% (vol./vol.) formaldehyde. The chronic electrodes were postfixed inside the brain in 2% formaldehyde for 48 hours, then placed in 30% sucrose in phosphate buffer (PB) for 5-7 days for cryoprotection. The electrodes were carefully removed from the brain before the brain was removed from the skull. Parasagittal sections of 40 m m thickness were cut on a freezing microtome, mounted, and stained with cresyl violet. The locations of individual recording or stimulating electrodes were identified based on the significant amount of gliosis present around the tip of the electrode.
Data Analysis.
Digitised data were analyzed off-line using the commercial programs Matlab (The MathWorks, Natick, MA) and Origin (Microcal, Northampton, MA). Following the conventions of Sossinka and Böhner (1980), a Zebra Finch song (or 'strophe') was characterised as a group of vocalisations that is preceded by at least 2 sec of silence and that begins with a set of three or more introductory notes. For quantification of the stereotypy of the pattern of neural activity in HVc during singing, song and neural records were segmented into their component 'motifs' or phrases, which are the minimal sequences of syllables that are repeated within the songs. Neural records were full-wave rectified and subsequently smoothed by convolving the waveform with a Gaussian window of 12 msec duration. Statistical analyses employed were paired t tests; all mean data are expressed with their corresponding standard errors of the mean. Analysis of correlations between waveforms employed the Pearson’s correlation coefficient.
RESULTS AND DISCUSSION
Organization of the song motor pathway
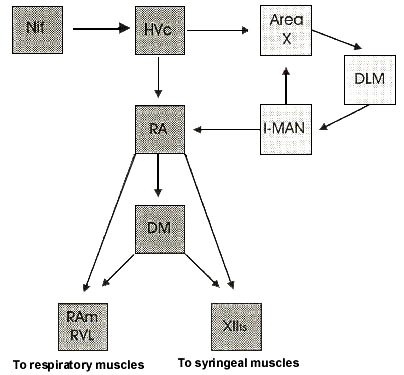
The motor pathway within the song control system is thought to include at least NIf (nucleus interfacialis), HVc (nucleus hyperstriatum ventrale, pars caudale also known as high vocal center), RA (nucleus robustus archistriatalis), DM (dorsomedial nucleus of the intercollicular complex), and XIIts (tracheosyringeal part of hypoglossal nucleus (Nottebohm, et al., 1976; McCasland, 1987). During singing, and production of other vocalisations (e.g. calls, chirps), premotor neural activity is observed in NIf, HVc, and RA (McCasland, 1987; Yu & Margoliash, 1996; Vu, et al., 1998) and precedes the onset of vocalisation by 40-80 msec (McCasland, 1987; Fig. 3). Premotor activity occurs first in NIf, followed by HVc, RA and XIIts suggesting that these brain areas are sequentially activated during vocal production (McCasland, 1987). Although NIf has been suggested to be at the top of the hierarchy of vocal commands, too little is known about this structure and it is difficult to assess its exact role in song production. This is compounded by the fact that lesioning NIf only produces transient motor deficits (Vu, et al., 1995) whereas lesioning of either HVc or RA causes severe and permanent disruption of song production (Nottebohm, et al., 1976; Simpson & Vicario, 1990; Williams, et al., 1992).
The majority of neurophysiological studies concerning the central control of vocalization have been performed in Zebra Finches (Taeniopygia guttata) and it is appropriate to provide a brief description of its song structure. Zebra finch song is typically composed of several introductory notes followed by one or more 'motifs', which consist of a sequence of syllables sung in a stereotyped order and tempo (Sossinka & Boehner, 1980). While syllables appear to represent the basic behavioural units of song production, (Cynx, 1990) they can be further subdivided into sub-syllabic vocal units, referred to as notes (Yu & Margoliash, 1996; Margoliash, 1997). Neural recordings from singing Zebra Finches show that premotor activity in both HVc and RA is temporally patterned rather than tonically active (McCasland, 1987; Yu & Margoliash, 1996; Vu, et al., 1998). These patterns suggest that premotor signals descending from these nuclei likely convey specific motor commands for the production of specific song elements. Recent work by Yu and Margoliash (1996) suggests that HVc and RA represent different levels in the overall hierarchy of song structure. HVc appears to code for the general sequence and structure of syllables whereas RA, by coding for note elements rather than syllables, represents song at a finer level of detail.
The stereotyped delivery of song elements by singing Zebra Finches suggests that song patterns may be controlled by 'central motor programs'. Because song production is severely disrupted by lesions to premotor song nuclei, Vu and his associates (Vu, et al., 1994) reasoned that such pattern generating networks might exist in the descending motor pathway. By adapting a strategy commonly used to identify pattern generating circuits in 'simpler' invertebrate motor networks, various song nuclei were electrically stimulated during singing and the degree to which the subsequent song pattern was disrupted was assessed. Delivery of a brief (16 ms) train of electrical stimuli to HVc, while a Zebra Finch was singing, caused the bird to suspend the ongoing motif and begin a new motif sooner than it normally would have occurred. This resetting of the bird’s song pattern could be achieved by stimulating either the left or right HVc. In a few cases, electrical stimulation also caused abnormal sequencing of syllables. In contrast to HVc, stimulation of RA only caused momentary distortion of the syllable during the stimulus burst and did not result in any shifts in the timing of song motifs. The resetting of the song following stimulation in HVc was not dependent on the bird's ability to hear himself sing because deafening did not change the effects of electrical stimulation of HVc. Successful resetting of the song pattern by the application of brief stimulus pulses in HVc suggests that song production is controlled by a hierarchically organised song motor pathway which derives its temporal pattern from HVc, possibly in combination with afferent sources such as NIf.
Comparison of song motor patterns across hemispheres
In many songbird species, cutting the left tracheosyringeal (ts) nerve, thus exclusively denervating left syringeal muscles, causes significantly more deficits in song output than cutting the right syringeal nerve. This asymmetry, or lateralization, can be quite dramatic in certain species. In canaries, for example, lesioning the left ts nerve eliminates greater than 90% of the syllables whereas lesioning the right nerve eliminates or distorts only a small minority of the syllables (Nottebohm & Notebohm, 1976). Because syringeal muscle innervation is exclusively ipsilateral (Wild, 1997), it has been proposed that this asymmetry may originate from asymmetries in the song control system (Nottebohm, 1977). Support for this hypothesis comes from studies showing that unilateral lesions of HVc or RA produce similar effects to those observed at the periphery (Nottebohm, et al., 1976).
The search for physiological and anatomical correlates for bilateral asymmetries in forebrain song structures has been difficult to come by. First, anatomical differences (e.g. larger song nuclei on dominant side) have not been found in the song system. Second, neural recordings from HVc, which may be expected to reflect peripheral asymmetries, appear quite similar across hemispheres. In Zebra Finches, for example, which have been suggested to be right side dominant (Price, 1977 ; Williams, et al., 1992; however, see Floody & Arnold, 1997), we have recorded neural activity simultaneously from left and right HVc (Schmidt, 1997) and observed that premotor output patterns are nearly identical across hemispheres (Fig. 2). Activity levels on both sides are elevated during all syllables and appear to be modulated similarly throughout the song although fine details of the neural patterns are clearly not identical across hemispheres. While these recordings show no gross differences between hemispheres, it should be emphasised that the differences in the fine temporal structure of neural activity indicate that song motor commands in the different hemispheres, while not lateralized in the same sense as the periphery, may still code for different aspects of song.
The choice of Zebra Finch to study lateralization of song motor circuits is probably not ideal since the degree of lateralization is much weaker than in other species (Suthers, 1997). Although neural activity from left and right HVc of canaries has been recorded by McCasland (McCasland, 1987), recordings from both hemispheres were collected separately during different singing episodes. While this technique does not allow the fine temporal comparison afforded by simultaneous recording, McCasland nevertheless concluded that left and right premotor activity were very similar between hemispheres since neurons appeared to fire for every syllable with similar timing on both sides.
Although it is still unclear whether strongly peripherally lateralized birds, such as the canary, will show lateralization in forebrain song areas with the use of simultaneous recording techniques, it is likely that the differences will not be as dramatic as those observed at the periphery. Support for this idea comes from work by Suthers and colleagues who have shown that ventral syringeal muscles on both sides of the syrinx often are active even when phonation on one side is blocked because of the action of dorsal syringeal muscles (Goller & Suthers, 1995; Goller & Suthers, 1996; Goller & Suthers, 1996) . Thus what may sometimes appear as simple, all or none, lateralization at the periphery, really results from the differential activity of only one syringeal muscle group. Multi-unit recordings may thus prove inadequate for bilateral comparison since left and right HVc may code for song features which are too abstract to detect such small asymmetries.
Bilateral synchronisation of vocal premotor activity
Bilateral syringeal co-ordination could hypothetically be accomplished by the synchronous initiation of two distinct motor programs, which, once started, would ensure that syringeal motor output is coordinated bilaterally. To test the possibility that motor activity in each hemisphere may be initiated synchronously, we have carefully analyzed onset timing between simultaneously recorded vocal pre-motor neural activity from both left and right HVc of singing adult Zebra Finches (Schmidt, 1997). As shown in Fig. 2, not only do neural patterns between hemispheres show a high degree of similarity but individual bursts of activity associated with given vocalisations, such as introductory notes, are strikingly aligned with both onsets and offsets starting and stopping at the same time. Analysis of these different neural records reveals that onset times are almost perfectly aligned with near 0 msec time lag (Schmidt, 1997). In a few birds, we observed premotor activity that had a characteristically bursty appearance. These bursts, which lasted approximately 5 msec and occurred at a frequency of about 150 Hz., were precisely synchronised across hemispheres (Fig. 3). Although the significance of these bursts is unclear, the precise alignment between bursts serves to illustrate the high degree of synchrony between both motor pathways. During the song proper, motor activity in these multi-unit recordings does not show clear onset and offset responses during different syllables. Thus while the beginning and end of individual introductory notes and calls are clearly synchronised, it is less clear whether neural activity for specific song elements shows the same degree of alignment. The lack of obvious synchrony during song suggests the possibility that, while left and right HVc receive a common initial synchronising input, the remainder of the song is controlled by independent motor programs from each side. During the song proper then, once these different programs are initiated, one may expect that activity patterns would be sufficiently different that synchrony between hemispheres would no longer be observed.
Because of the lack of direct interhemispheric connections between left and right HVc, the observation of interhemispheric synchronisation of pre-vocal motor activity is strong evidence that both structures must receive common inputs and that these inputs act to initiate and synchronise HVc premotor activity. The origin and exact role of this input is presently unknown, however, because the earliest interhemispheric crossover between left and right song nuclei occurs in the midbrain (Wild, 1997), we hypothesise that song motor programs are synchronously initiated from bilaterally connected vocal centres situated in the midbrain.
Interhemispheric coordination of song premotor activity
Although the onset of premotor activity is synchronised between left and right HVc, activation by a single timing source of two independent motor programs is unlikely to be sufficient to coordinate syringeal motor output. Experiments described earlier demonstrated that brief stimulation to only one HVc (either left or right) is sufficient to reset the song pattern. Such interruption and resetting of the song pattern during singing suggests that individual motor programs are not completely independent of one another but rather interact in a dynamic fashion.
To test whether motor commands in each hemisphere are, in fact, dynamically coordinated in their ongoing output, in collaboration with Eric Vu (Vu et al, 1998), we recorded neural activity in one HVc (on either side) during singing and forced premotor activities in the two hemispheres out of synchrony by perturbing neural activity in the contralateral HVc with electrical stimulation. If both motor programs were truly independent from one another, such perturbation should only affect the stimulated side. As shown in Fig. 4, brief stimulation in one HVc had a rapid effect on the song as well as on neural activity in the contralateral, unstimulated HVc implying that motor activity between hemispheres is dynamically coordinated. The song motif became suspended and was followed by rapid resumption of singing with the start of a new motif. When the stimulus was applied late in the song (e.g. the second stimulus in Fig. 4), when the bird might have stopped singing under normal conditions, the suspended motif was not followed by a new motif. Stimulating HVc always caused neural activity in the contralateral HVc to become suppressed to baseline levels within 20-30 msec and activity generally remained suppressed for at least another 100 msec until a new motif was initiated. In such cases, activity rapidly resumed time locked (preceding by 50-70 msec) to the onset of the new motif. This resumed activity represented a true resetting of the motor pattern since it corresponded to the stereotyped activity pattern associated with the beginning of a new motif (Fig. 5).
These results demonstrate that outputs of forebrain song nuclei are continuously monitored and that active mechanisms exist for resynchronising the premotor activities in the two hemispheres whenever a mismatch in their timing signals occurs. Thus interhemispheric coordination is likely accomplished using both feedforward as well as feedback mechanisms that would allow premotor outputs from each hemisphere to be continuously compared. Based on the rapidity with which activity on the side contralateral to the stimulating electrode becomes suppressed, interhemispheric coordination is likely accomplished by a comparison of corollary discharges of premotor activities from the two hemispheres rather than through mechanisms involving proprioceptive, somatosensory or auditory feedback. Furthermore, the observation that hemispheres can reset following large temporal mismatches, such as those caused by electrical stimulation, suggest that this online monitoring, and comparing, plays an important role during normal vocalisation. Based on timing arguments, Vu et al. (1998) propose that interhemispheric comparison of premotor outputs occurs at the level of gross temporal patterning (syllable duration and spacing) rather than fine detail (syllable structure). This might be desirable because it would allow motor commands to the two sides of the syrinx to differ in fine detail but be sufficiently synchronised to allow for the coordination between syringeal muscles.
Anatomical substrates for interhemispheric coordination and synchronization.
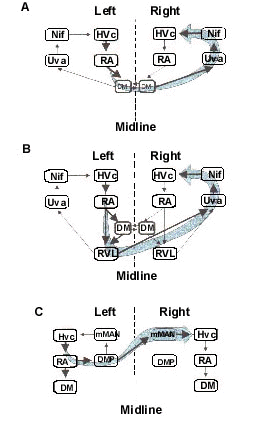
Recent anatomical evidence suggests that there may be as many as three separate bilateral feedback projections that could function to synchronize as well as coordinate hemispheres during singing (Fig. 6). All three pathways cross hemispheres at the level of the thalamus, or midbrain, and eventually project bilaterally back to HVc.
The first such loop, described by Vates et al. (Vates, et al., 1997), consists of a pathway from RA to the dorsomedialis posterior nucleus of the thalamus (DMP). DMP projects bilaterally to the medial magnocellular nucleus of the anterior neostriatum (mMAN) which then projects to HVc and a region adjacent to HVc known as paraHVc (Foster, et al., 1997; Foster & Bottjer, 1998). The output of RA to DMP appears to originate from the portion of RA that projects to respiratory regions of the brain stem and may, thus, preferentially relay signals back to HVc that relate to the temporal organization of song (Vicario, 1991; Wild, 1993). While this pathway may be involved in hemispheric coordination, its exact role is presently unclear since lesioning mMAN in adult Zebra Finches has little effect on song production (Foster & Bottjer, 1993).
The second and third loop both involve bilateral projections via the thalamic nucleus uvaeformis (Uva), which then projects to NIf and HVc. The first of these Uva-mediated feedback loops consists of a pathway from RA to a region in the ventrolateral part of the rostral medulla (RVL) which then projects bilaterally to Uva and then to NIf and HVc (Okuhata & Nottebohm, 1992; Reinke & Wild, 1998; Striedter & Vu, 1998). The second Uva-mediated pathway involves bilateral projections from the dorsal medial nucleus of the intercollicular region (DM), a midbrain vocal control region, to Uva, which then projects to NIf and HVc (Striedter & Vu, 1998).
Several studies support the notion that Uva-mediated pathways may play an important role in mediating interhemispheric synchronisation and coordination (Williams & Vicario, 1993). First, unilateral stimulation of Uva has been shown to cause bursts of activity in the contralateral motor pathway (Williams, 1985), presumably via antidromic activation of neurons in RVL and/or DM, and, second, stimulation of RA has been shown to elicit orthodromic activation of Uva (Okuhata & Nottebohm, 1992). In addition, behavioural studies indicate that bilateral lesions of Uva (Williams & Vicario, 1993) cause adult Zebra Finches to produce abnormal repetitions and sequences of song syllables. All of these results are consistent with the disruption of an inter-hemispheric coordination system.
CONCLUSIONS
The precision with which syringeal muscles from the left and right side must be synchronised, for example, during the production of syllables that require switching from one half of the syrinx to the other, is astounding. The mechanisms underlying this coordination are not understood. However, recent evidence presented in this article suggests that coordination between song motor commands originating from the forebrain may be necessary for coordination to occur at the periphery. The demonstration that HVc motor commands are both synchronised and co-ordinated across hemispheres provides evidence for the existence of both feedforward and feedback mechanisms in the song control system. The details of this interhemispheric coordination are likely to be quite complex since there exist at least three distinct anatomical pathways capable of providing feedback and feedforward signals.
ACKNOWLEDGMENTS
We wish to thank M. Kohwi, A. Leonardo and Dr. E. Vu for helpful comments on this manuscript. We also wish to thank E. Vu for many enlightening conversations. This research was supported by grants from National Institutes of Health (NRSA DC00125 and R03 DC03041 to M.F.S. and NH55984 to M. K. and M.F.S.).
REFERENCES
Allan, S. E. & Suthers, R. A. 1994. Lateralization and motor stereotypy of song production in the brown-headed cowbird. J. Neurobiol. 25: 1154-1166.
Cynx, J. 1990. Experimental determination of a unit of song production in the Zebra Finch (Taeniopygia guttata). J. Comp. Psychol. 104: 3-10.
Floody, O. R. & Arnold, A. P. 1997. Song lateralization in the Zebra Finch. Hormones and Behavior 31: 25-34.
Foster, E. F. & Bottjer, S. W. 1993. Lesions of mMAN produce slight disruptions in vocal behavior of juvenile male Zebra Finches. Soc. Neurosci. Abstr. 19: 1016.
Foster, E. F. & Bottjer, S. W. 1998. Axonal connections of the high vocal center and surrounding cortical regions in juvenile and adult male Zebra Finches. J. Comp. Neurol. 397: 118-138.
Foster, E. F., Mehta, R. P. & Bottjer, S. W. 1997. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in Zebra Finches. J. Comp. Neurol. 382: 364-381.
Goller, F. & Suthers, R. A. 1995. Implications for lateralization of bird song from unilateral gating of bilateral motor patterns. Nature 373: 63-66.
Goller, F. & Suthers, R. A. 1996. Role of syringeal muscles in controlling the phonology of bird song. J. Neurophysiol. 76: 287-300.
Goller, F. & Suthers, R. A. 1996. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. J. Neurophysiol. 75: 867-876.
Gurney, M. E. 1981. Hormonal control of cell form and number in the Zebra Finch song system. J Neurosci 1: 658-673.
Hartley, R. S. 1990. Expiratory muscle activity during song production in the canary. Respir. Physiol. 81: 177-188.
Margoliash, D. 1997. Functional organization of forebrain pathways for song production and perception. J. Neurobiol. 33: 671-693.
McCasland, J. S. 1987. Neuronal control of bird song production. J Neurosci 7: 23-39.
Nottebohm, F. 1977. Asymmetries in neural control of vocalization in the canary. Pages 23-44 in R. Harnad, R. W. Doty, L. Goldstein, J. Jaynes, and G. Krauthamer, Eds., In, Lateralization in the nervous system. Academic Press, New York.
Nottebohm, F. & Nottebohm, M. E. 1976. Left hypoglossal dominance in the control of canary and white-crowned sparrow song. J. Comp. Physiol. A 108: 171-192.
Nottebohm, F., Stokes, T. M. & Leonard, C. M. 1976. Central control of song in the canary, Serinus canarius. J Comp. Neurol 165: 457-486.
Okuhata, S. & Nottebohm, F. 1992. Nucleus Uva might be part of a feedback circuit for song processing. Soc. Neurosci. Abstr. 18: 527.
Price, P. H. 1977. Determinants of Zebra Finch song: Studies of species song uniformity, bases of physiological determination, and developmental plasticity. University of Pennsylvania. Ph. D. Thesis.
Reinke, H. & Wild, J. M. 1998. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. J. Comp. Neurol. 391: 147-163.
Schmidt, M. F. 1997. Interhemispheric synchronization of vocal premotor activity in songbirds. Soc. Neurosci. Abst. 23: 244.
Simpson, H. B. & Vicario, D. S. 1990. Brain pathways for learned and unlearned vocalizations differ in Zebra Finches. J. Neurosci. 10: 1541-1556.
Sossinka, R. & Boehner, J. 1980. Song types in the Zebra Finch Poephila guttata castanotis. Z. Tierpsychol. 53: 123-132.
Striedter, G. F. & Vu, E. T. 1998. Bilateral feedback projections to the forebrain in the premotor network for singing in Zebra Finches. J. Neurobiol. 34: 27-40.
Suthers, R. A. 1990. Contributions to birdsong from the left and right sides of the intact syrinx. Nature 347: 473-477.
Suthers, R. A. 1997. Peripheral control and lateralization of song. J. Neurobiol 33: 632-652.
Suthers, R. A. & Goller, F. Respiratory and syringeal dynamics of song production in northern cardinals. In, Nervous systems and behaviour: Proceedings of the 4th international congress on neuroethology, Stuttgart, 1996, pp. 333.
Suthers, R. A., Goller, F. & Hartley, R. S. 1994. Motor dynamics of song production by mimic thrushes. J. Neurobiol. 25: 917-936.
Vates, G. E., Vicario D. S. & Nottebohm, F. 1997. Reafferrent thalamo-'cortical' loops in the song system of oscine songbirds. J. Comp. Neurol 275-290.
Vicario, D. S. 1991. Organization of the Zebra Finch song control system: II. Functional organization of outputs from nucleus robustus archistriatalis. J Comp. Neurol 309: 486-494.
Vu, E. T., Kuo, Y. & Chance, F. S. 1995. Effects of lesioning nucleus interfacialis on adult Zebra Finch song. Soc. Neurosci. Abstr. 21: 964.
Vu, E. T., Mazurek, M. E. & Kuo, Y.-C. 1994. Identification of a forebrain motor programming network for the learned song of Zebra Finches. J. Neurosci. 14: 6924-6934.
Vu, E. T., Schmidt, M. F. & Mazurek, M. E. 1998. Interhemispheric coordination of premotor neural activity during singing in adult Zebra Finches. J. Neurosci. In press.
Wild, J. M. 1993. Descending projections of the songbird nucleus robustus archistriatalis. J. Comp. Neurol. 338: 225-241.
Wild, J. M. 1997. Neural pathways for the control of birdsong production. J. Neurobiol. 33: 653-670.
Williams. 1985. Interhemispheric coordination of bird song. Soc. Neurosci. Abstr. 11: 871.
Williams, H., Crane, L. A., Hale, T. K. & Esposito, M. A. 1992. Right-side dominance for song control in the Zebra Finch. J. Neurobiol. 23: 1006-1020.
Williams, H. & Vicario, D. S. 1993. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. J. Neurobiol. 24: 903-912.
Yu, A. C. & Margoliash, D. 1996. Temporal hierarchical control of singing in birds. Science 273: 1871-1875.
Fig. 1. Schematic diagram of the song system, highlighting the vocal motor pathway (dark gray). Nucleus HVc, which is innervated by NIf, sends projections to nucleus RA. RA then projects to the midbrain structure DM and both of these nuclei innervate brainstem motor and premotor neurons that control the syrinx (XIIts) and muscles of respiration (RAm and RVL). HVc also projects to area X, which forms part of the anterior forebrain loop (light gray), a circuit essential for song learning. Auditory inputs to HVc originate from NIf as well as from field L. HVc also receives other major inputs from the forebrain nucleus m-MAN and the thalamic nucleus Uva. These projections are not shown in this schematic. DLM = medial nucleus of the dorsolateral thalamus; DM = dorsal medial nucleus of the intercollicular region; HVc = hyperstriatum ventralis pars caudalis, also referred to as the High Vocal Center (HVC); l-MAN = lateral portion of the magnocellular nucleus of the anterior neostriatum; m-MAN = medial portion of the magnocellular nucleus of the anterior neostriatum; NIf = nucleus interfacialis; RA = nucleus robustus archistriatalis; RAm = nucleus retroambigualis; RVL = ventrolateral part of the rostral medulla.; Uva = nucleus uvaeformis; XIIts = tracheosyringeal branch of the hypoglossal nucleus.

Fig. 2. Comparison of premotor neural activity across hemispheres. Neural activity recorded simultaneously in left and right HVc from a singing adult Zebra Finch is shown together with the sonogram of the bird’s song (top panel). The Zebra Finch song, which typically consists of several stereotyped motifs, is generally preceded by several introductory notes. In this example, the bird sang four introductory notes followed by a short silence then sang five more introductory notes followed by the song. Neural activity in HVc, which precedes individual vocalisations by 50-70 msec, shows a high degree of similarity between hemispheres. This similarity is most striking during the first four introductory notes where neural patterns appear to share the same onset and offset times.
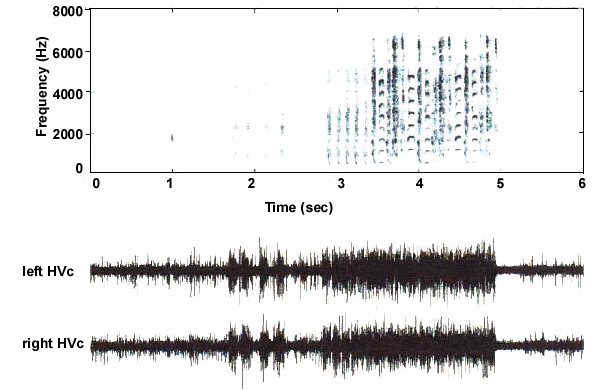
Fig. 3. High magnification of neural activity recorded simultaneously from left and right HVc preceding a song introductory note, shown here as an oscillogram (top trace). Neural activity in HVc, which typically precedes vocalisations by 50-70 msec, shows a high degree of burstiness. Bursts are approximately 5 msec in duration and occur with a periodicity of 150-200 Hz. As illustrated in the lower panel of this figure, there is considerable degree of interhemispheric synchronization between at least 5 bursts. The onset of individual bursts is illustrated by the dotted line. Neural activity does not remain bursty for the entire premotor episode as activity remains elevated and non-synchronised for at least another 50 msec following the period of synchronous bursting.
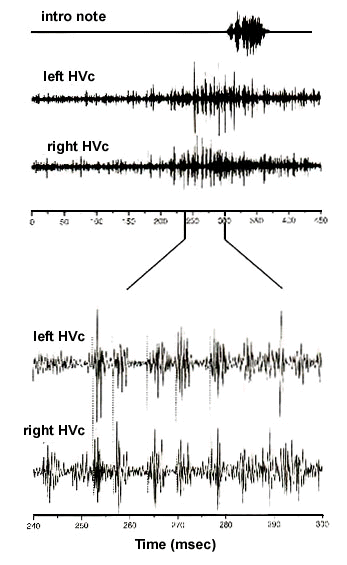
Fig. 4. Interhemispheric resynchronisation of premotor activity in HVc. A. Schematic diagram of the bilateral neural network for song production (arrows, anterograde projections) and the experimental paradigm. Note that no connection across the midline exists between any of the song control nuclei in the forebrain (top two lobes in the brain diagram), whereas at least one bilateral projection exists from a song nucleus below the forebrain to one in the forebrain (DM to Uva). Other bilateral projections have been omitted for clarity. A pair of stimulating electrodes were implanted chronically in one HVc on either side and a recording electrode was implanted chronically in the contralateral HVc. See text for acronym definitions. B. Perturbing neural activity in one HVc leads to a rapid change in neural activity in the contralateral HVc. A brief electrical stimulus (5 pulses at 400 Hz; 40 m A) applied to the left HVc during the second motif was sufficient to cause the bird to suspend the ongoing motif as well as interrupt premotor activity in the contralateral HVc. Both neural activity and song resumed after less than 100 msec. A second brief stimulus applied during the fourth motif caused the bird to suspend the ongoing motif and terminate song. Stim, stimulation; m, song motif; m*, suspended song motif. (Modified from Vu et al. (In Press). Reproduced with permission).
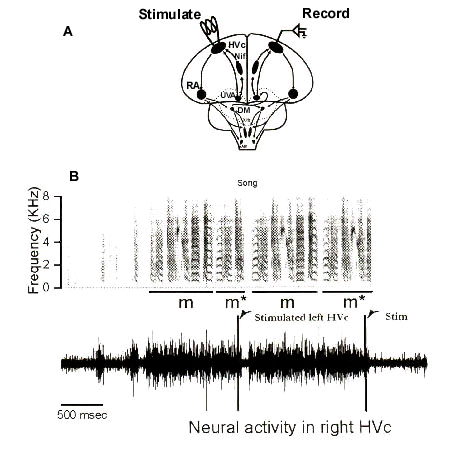
Fig. 5. Resumed neural activity pattern represents the beginning of a new motif. A. Diagram of the period following each stimulus from which the pattern of neural activity (Stimul(1) trace) is compared to the control neural pattern (Control trace) observed in unstimulated motifs at the same recording site. The comparison was made by calculating the linear correlation coefficient between a trace consisting of the average of control traces (Average trace) and either of the stimul(1) or Control traces. S, stimulus artifact. B. Correlation coefficients comparing traces containing a stimulus which did not cause song termination (filled bars) and paired control traces (open bars). Resynchronised activity following stimulation is clearly uncorrelated with activity patterns that would normally have occurred during that period. C. Diagram of the realignment of "stimulated" traces. Onset of the first syllable in the resumed motif is aligned with the start of the motif in the Average record. D. Segments of neural activity from the same set of traces as in B are realigned with the Average trace. Results indicate that alignment of neural activity with respect to vocal output results in a high degree of correlation between neural activity patterns. (Modified from Vu et al. (In Press). Reproduced with permission).
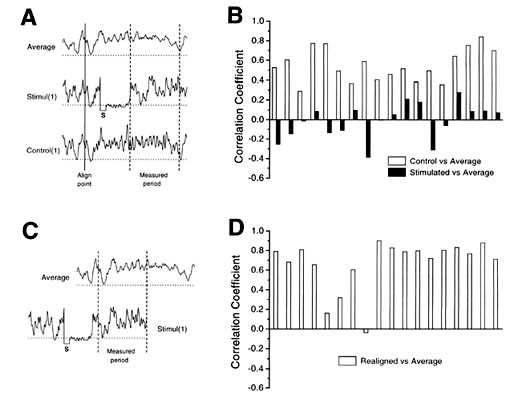
Fig. 6. Schematic illustration of three separate bilateral feedback projections that could function to synchronise as well as coordinate hemispheres during singing. A. Feedback loop consisting of a pathway from RA to a region in the ventrolateral part of the rostral medulla (RVL) which then projects bilaterally to Uva and then to NIf and HVc. B. Bilateral projections from the dorsal medial nucleus of the intercollicular region (DM) to Uva, which then projects to NIf and HVc. C. Feedback loop consisting of projections from RA to the dorsomedialis posterior nucleus of the thalamus (DMP). DMP then projects bilaterally to the medial magnocellular nucleus of the anterior neostriatum (mMAN) which then projects to HVc. A, B are adapted from Striedter and Vu (1998); C is adapted from Vates et al. (1997).