S09.2: Peripheral mechanisms for singing: Motor strategies for vocal diversity
Roderick A. Suthers
Medical Sciences, Indiana University, Bloomington, IN 47405, USA., fax 812, 855 4436, e-mail suthers@indiana.edu
Suthers, R.A. 1999. Peripheral mechanisms for singing: Motor strategies for vocal diversity. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
491-508. Johannesburg: BirdLife South Africa.Oscine songbirds achieve their diverse vocal performances through the coordinated activity of respiratory, syringeal and other vocal tract muscles, in ways that take maximum advantage of the acoustic diversity made possible by the presence of two, independently controlled, sound sources in their bipartite syrinx. Recordings of respiratory and syringeal muscle activity during spontaneous song in several species, show that each side of the syrinx receives separate motor programs that determine the timing and acoustic properties of ipsilaterally generated sound. The proportion of song contributed by each half-syrinx ranges from unilateral syringeal dominance to bilateral equality, depending on the species. This syringeal activity is coordinated with special motor programs to respiratory muscles that alter the pattern of ventilation in order to maintain a supply of respiratory air and oxygen and permit songs of long duration, high syllable repetition rates, or maximum spectral complexity. Sound generated in the syrinx is further modulated by muscles that control the shape of the vocal tract. The varied properties of oscine song reflect different motor strategies in which the two sides of the syrinx phonate in a unilateral, bilateral, sequential or alternating pattern to achieve specific kinds of vocal complexity.
INTRODUCTION
The song of oscine birds is an important form of communication that depends on demanding motor tasks involving diverse vocal subsystems and many different groups of muscles. In this paper I consider some of the important physiological and motor limitations on song production and describe various ways different species have coped with these limitations. Recent comparative studies of song production by songbirds provide new information on the relationship between motor flexibility, motor constraints and vocal diversity in songbirds. In the light of this information, it is worth revisiting questions such as: What motor strategies have oscine songbirds adopted to achieve the diverse kinds of vocal output for which they are noted? Do interspecific differences in the temporal or spectral complexity of songs reflect the use of fundamentally different motor strategies for producing song or do they arise from differences in the proficiency with which various species are able to execute basically similar motor actions? To what extent do interspecific differences in song production appear to have been shaped by motor constraints? Although such questions involve all levels of the vocal system, the discussion here will focus on its two most important peripheral components, the respiratory system and the syrinx.
Recent approaches to the study of song production
The syrinx of songbirds is located in the interclavicular air sac at the confluence of the primary bronchi and the trachea. It is formed by modified bronchial and tracheal cartilages enveloped externally by about 6 bilaterally paired muscles that act on the cartilaginous framework to control sound production (King 1989). The muscles on each side of the syrinx are innervated by the ipsilateral tracheosyringeal branch of the hypoglossal nerve (NXIIts). The cranial end of each bronchus contains sound generating structures, making the oscine syrinx a double vocal organ that is potentially capable of producing two acoustically unrelated sounds at the same time (Fig. 1). This duplex structure of the oscine syrinx has important implications for the production and variety of song (e.g., Nowicki & Capranica 1986; Nowicki, et al. 1992).
The relatively inaccessible position of the syrinx deep within the thorax, close to the heart, has until recently severely limited our knowledge of its activity during song. This has changed within the last decade through the use of techniques that make it possible to monitor syringeal motor function and respiratory parameters during spontaneous song by behaving birds with a fully innervated and functionally intact vocal system (Suthers 1990). A temperature sensitive micobead thermistor placed in the lumen of each primary bronchus provides a record of the airflow through the ipsilateral side of the syrinx (Fig 1). A miniature piezoresistive pressure transducer, mounted on a backpack and connected to a small cannula that is inserted into the cranial thoracic air sac, measures air sac pressure. Since the air sacs are connected to other ipsilateral sacs and also, by way of the interclavicular air sac, to sacs on the opposite side of the body, only small pressure differences normally exist between them (Brackenbury 1971; Brackenbury 1972; Brackenbury 1973; Scheid & Piper 1989). The respiratory pressure in the cranial thoracic sac should closely approximate that in each of the primary bronchi. Muscle activity in the vocal tract and respiratory system can be measured by recording electromyograms with chronically implanted bipolar wire electrodes (Goller & Suthers 1996a).
The bronchial thermistors also provide a measure of the sound that is generated in the ipsilateral side of the syrinx. The thermistor's location within a few mm of the vibratory medial and lateral labia make it possible to detect fluctuations in the rate of bronchial expiratory airflow caused by these vibrating sound generators. Thus bronchial sound up to about 3 or 4 kHz, limited by the response time of the thermistor, can also be recorded. During an experiment, the bird is free to move about its cage and live normally except that it wears a small backpack from which fine wires carry physiological data out through the top of the cage to signal conditioning and recording instruments.
SONG DIVERSITY: MOTOR RESPONSES TO MOTOR CONSTRAINTS
Singing and breathing.
Respiratory air for sustained song.
Song production requires expiratory airflow through the syrinx. This simple fact places special demands on the respiratory system, which has the primary function of providing pulmonary ventilation. During song the respiratory needs must continue to be met while also coordinating expiration with phonatory activity of the syrinx and maintaining a supply of air for vocalisation. Connections between the respiratory and song control systems have recently been identified in the brain that likely play an important role in respiratory-syringeal coordination (Vicario 1993; Wild 1993a; Wild 1993b).
An important insight into how songbirds deal with this problem was gained by Calder (1970) who wondered how small birds with small vital capacities were able to sing long songs. He obtained experimental evidence that Canaries Serinus canaria expand their thorax slightly between song syllables, suggesting that they take small breaths, which he termed 'minibreaths', between the syllables of their song. Subsequent measures of tracheal airflow in singing Canaries (Hartley & Suthers 1989) have confirmed Calder's hypothesis (Fig 2). Electromyograms of abdominal respiratory muscles in Canaries (Hartley 1990) Brown Thrashers Toxostoma rufum (Goller & Suthers 1995a) Brown-headed Cowbirds Molothrus ater and Zebra Finches Taeniopygia gutta (Wild, et al. 1998) show that expiratory muscles are active during sound production. Expiratory muscle activity ceases at the end of expiration, when inspiratory muscle activity increases to generate the minibreath. Both phases of the respiratory rhythm during song are thus active processes under direct motor control (Wild, et al. 1998). By shortening the duration of inspiration, songbirds can achieve higher syllable repetition rates and songs of longer duration. Measurements of minibreath volume in Canaries show that it is approximately equal to the volume of air that must be exhaled to produce the syllable (Hartley & Suthers 1989). Since syllables of short duration require less air than long syllables, the volume, and therefore the duration, of the minibreath can be shorter in phrases composed of short syllables. This increases the maximum repetition rate at which such phrases can be produced. The minibreath thus links maximum repetition rate to syllable duration and presumably explains why young Swamp Sparrows Melospiza georgiana were unable to learn to sing syllables of normal duration at a faster repetition rate without omitting notes or inserting pauses for respiration (Podos 1996; Suthers & Goller 1997). In terms of the respiratory air available for phonation, there is theoretically no limit on how long a song can be. There is however a limit on the rate at which syllables can be produced.
Another way to increase song duration is to conserve air during phonation by vocalising on only one side of the syrinx, while keeping the other side closed. Unilateral sound production is common in some species. It does not come without a cost, however. The spectral and temporal complexity (see below) that is potentially available when singing with 'two-voices' is lost and it is possible that vocal intensity may be affected since there is only one sound source instead of two (Suthers & Goller 1997). By reducing the volume of air exhaled during a syllable, unilateral sound production also reduces the duration of the minibreath needed to refill the air sacs. Shorter minibreaths permit higher syllable repetition rates.
Pulmonary ventilation during song.
Data on the energy cost of avian vocalisation are scarce and conflicting (Chappell, et al. 1995; Eberhardt 1994; Eberhardt 1996; Gaunt, et al. 1996; Horn, et al. 1995; Vehrencamp, et al. 1989). Although the inspiration immediately before song is often deeper than normal inspirations of the quietly resting bird, during song birds must rely primarily on minibreaths to provide oxygen and remove carbon dioxide, as well as to replenish the volume of air available for phonation. The extent to which minibreaths provide adequate oxygen for pulmonary gas exchange depends on their volume relative to that of the respiratory deadspace. Minibreath volume is inversely related to the syllable repetition rate (Hartley & Suthers 1989; Suthers 1997; Suthers & Goller 1997). The relationship between volume and ventilation is complicated, however, by the possibility that rapid ventilation during minibreaths may provide oxygen even when the tidal volume is considerably smaller than the deadspace, as is the case for panting in pigeons (Bech, et al. 1988). The correlation between minibreath volume and syllable duration, shown in Canaries (Hartley & Suthers 1989), raises the possibility that singing Canaries may increase song duration and avoid hypoxia by periodically switching to phrases with long duration syllables. It would be very interesting to know if song syntax is dictated in part by the respiratory constraints on sound production.
Achieving high syllable repetition rates.
Fulfilling the needs of both respiration and song production are easier if the song's tempo is slow, since more time is available between syllables for inspiration. Achieving a fast tempo increases the conflicting demands on the respiratory system. The time required for minibreaths limits the maximum syllable repetition rate that can be attained. It is not surprising that the highest repetition rates are achieved by eliminating minibreaths. Waterslager Canaries omit minibreaths in trilled phrases with syllable repetition rates above about 30 s-1. The same is true of Northern Cardinals Cardinalis cardinalis for syllable repetition rates greater than about 16 s–1.
At these high repetition rates these birds switch to an alternative respiratory strategy of pulsatile expiration (Hartley & Suthers 1989). During pulsatile expiration respiratory pressure remains positive during the entire phrase and each syllable is produced by briefly opening the syrinx to allow a little air to escape (Fig 2). Since no air is inhaled during a trill, its maximum duration is limited by the singer's vital capacity. The maximum tempo at which minibreaths can be employed is likely determined by the mass and other physical properties of the respiratory (e.g., abdominal wall) and syringeal structures (e.g., dorsal muscles and labia) that must rhythmically oscillate at the respiratory frequency. Such a relationship predicts an inverse relationship between syllable repetition rate and body mass. Whether or not songbirds actually sustain a trill until they use their full vital capacity or whether the trill's duration is normally limited by some other parameter--such as hypoxia, hypercapnia or declining motivation--is not known.
Temporal versus spectral vocal expertise.
Another factor that has probably influenced the evolution of vocal diversity in songbirds is the conflict between attaining maximum levels of both temporal and spectral virtuosity in song. By temporal and spectral expertise I mean the ability to achieve very high note or syllable repetition rates and generate precisely timed, complex temporal patterns or to produce well regulated, complex spectral patterns involving simultaneous or sequential frequency modulated (FM) or constant frequency elements, respectively. Attaining vocal prowess in one of these areas tends to limit what is possible in the other area. Short notes or syllables associated with high repetition rates do not provide time for developing much spectral complexity. In the Emberizidae, for example, frequency bandwidth is inversely related to trill rate (Podos 1997). The acoustic attributes of song must often reflect a balance between temporal vs spectral prowess. The nature of this balance between these song attributes may have special behavioural significance (e.g., Suthers & Goller 1997; Vallet, et al. 1998).
Motor control of vocal timing.
The motor program sent to the respiratory muscles defines the song's overall temporal pattern (Vicario 1991a; Vicario 1991b), but the temporal 'fine structure' of song is determined by respiratory modulation of expiratory effort (Goller & Suthers 1996a; Hartley 1990) in concert with syringeal motor activity that determines the timing of sound production within the expiratory periods set by the respiratory muscles. The bipartite syrinx increases the possibilities for generating more complex temporal patterns within songs. Each side of the syrinx can potentially produce its own temporal pattern of notes during phonation, through the ability to separately control the aperture, or syringeal valve at the cranial end of the ipsilateral bronchus (Fig. 3). The ipsilateral adductor muscles (the dorsal syringeal and dorsal tracheobronchialis) control the timing of sound production by gating the temporal pattern of airflow through the internal and external labia (Goller & Suthers 1995b) (Fig 1). These muscles regulate airflow during sound production and can also silence the ipsilateral syrinx by preventing airflow. The timing or patterning of sound production on each side of the syrinx and the proportion of the song that each side contributes (i.e., lateral dominance) is determined by the separate motor programs sent to the dorsal muscles. The temporal complexity of the song is increased by the ability to independently control the timing of phonation on each side of the syrinx.
Cyclical patterns of amplitude modulation (AM), prominent in the songs of some species such as Brown Thrashers and Grey Catbirds Dumetella carolinensis, can also affect the temporal properties of song. The prominence of AM components in mimid song and the often-complex motor patterns required for its production, suggest that they may have special behavioural significance in communication. The generation of AM is complex, with different mechanisms involved depending in part on the frequency of the modulation (Goller & Suthers 1996a; Suthers, et al. 1994). Some of these mechanisms take advantage of the separate sound generators in each side of the syrinx. High frequency AM is sometimes produced as a beat note between two high intensity sounds generated in the left and right syrinx at slightly different frequencies. Other kinds of AM can be produced by using the syringeal muscles, including the dorsal adductor muscles, to rapidly vary the aperture of the right syrinx, thus modulating sound amplitude by varying the rate of airflow (Fig 4). It is not known if this right dominance in the production of some kinds of AM reflects peripheral or central specialisations. Sometimes there is no obvious relationship between AM and airflow.
Spectral vocal expertise.
Three features of the oscine syrinx are especially important in controlling the spectral composition of song. The first of these is its duplex structure, which provides independent control of the sound frequency generated on each side. Greenewalt (1968) and Stein (1968) pointed out the potentially important role of the duplex syrinx in song production. Greenewalt in particular provided strong circumstantial evidence for two independent sound generators. His careful analysis of the songs showed that a number of species can simultaneously sing two different frequencies that are independently modulated or lack an harmonic relationship with each other (Fig. 3). Direct recordings of bronchial sound in several species confirm Greenewalt's and Stein's hypothesis (Suthers 1997; Suthers & Goller 1997). This ability of many songbirds to produce separate sounds simultaneously, i.e., to sing with 'two voices', greatly increases the spectral complexity of their song. The limited comparative data on oscine song production clearly demonstrate that songbirds have taken advantage of the added motor flexibility in their duplex syrinx to increase the diversity of their songs. It is surprising that no behavioural or psychophysical data on the biological significance of two voices in avian communication have been obtained during the 30 years since Greenewalt (1968) and Stein (1968) brought them to the attention of the scientific community.
A second feature favouring increased phonetic complexity is the large number of intrinsic syringeal muscles compared to non-passerines (Gaunt 1983). The key role of the dorsal syringeal muscles in gating song production was described above. The ventral syringeal muscles, have an important role in controlling fundamental frequency. Patterns of muscle activity during song show that the paired, intrinsic ventral syringeal muscles regulate the fundamental frequency on the ipsilateral side of the syrinx . The amplitude of the electromyogram (EMG) is positively correlated with this frequency (Fig. 5). The importance of syringeal muscles in controlling fundamental frequency is apparent from the sound produced after these muscles are denervated. If one side of the syrinx is paralysed by cutting the ipsilateral NXIIts, airflow through it can still cause it to vibrate and produce sound, but the fundamental frequency is reduced and is directly proportional to the air sac pressure and the rate of syringeal airflow (Suthers & Hartley 1990). Sound from the denervated side of the syrinx also looses its tonal quality and contains multiple harmonics. The syringeal muscles thus allow the bird to regulate frequency independently from pressure or airflow.
Exactly how the ventral muscles control fundamental frequency is not known. They may vary the tension of the vibratory sound generators. Until recently, it was widely assumed that song is produced by vibration of the thin medial tympaniform membranes (e.g., Miskimen 1951). However, recent endoscopic observations of the syrinx during sound production, as well as surgical destruction of the medial tympaniform membranes, implicate the medial and lateral labia as the vibratory sound source (Goller & Larsen 1997).
A third feature of the syrinx increases the frequency range or bandwidth of the vocalisation. This is due to the fact that the two sides of the syrinx have different vocal registers. The extent of this difference and the amount of frequency overlap between sides varies considerably between species. Nevertheless, in each of the species we have studied, high frequency notes tend to be generated on the right side and the lower notes are generated on the left (Suthers 1997; Suthers & Goller 1997). The basis of this lateral difference in frequency range has not been determined. Electromyographic data from Brown Thrashers suggest it is more likely due to subtle anatomical asymmetries than centrally generated differences in the intensity of motor activation of the two ventral syringeal muscles. In Brown Thrashers at any given amplitude of EMG in the ventral muscles, the fundamental frequency on the right side is higher than on the left (Fig 5).
LEARNING TO SING: THE DEVELOPMENT OF MOTOR COORDINATION
Achieving precision in vocal performance, whatever motor strategy is employed, requires a high degree of coordination between the different muscle groups, e.g., respiratory, syringeal and suprasyringeal, that contribute to vocalisation. The important role of learning in oscine song development enables juvenile songbirds to acquire this precision through motor practice. Juvenile songbirds go through a sensitive sensory period during which they hear the song of their own species if they are to sing a normal song as an adult (Konishi 1965; Konishi & Nottebohm 1969). It is assumed that during this period the adult song is stored in the brain as a template. The sensory period of song learning is followed by a sensorimotor phase during which the juvenile bird gradually learns, apparently through trial and error motor practice, to produce vocalisations that match its auditory template. The initial low intensity and highly variable vocalisations of subsong develop into identifiable syllables in plastic song, which becomes crystallised as the adult song.
Studies we are conducting on the motor development of song in young birds indicate that developing motor coordination between diverse muscle groups is an important part of this process. The ability to accurately coordinate respiratory and syringeal motor patterns and motor activity on the two sides of the syrinx are important milestones in the motor development of song production by juvenile Northern Cardinals (Suthers & Goller 1998a; Suthers & Goller 1998b). During subsong, for example, expiration is not well coordinated with phonation, resulting in a very inefficient use of the respiratory air available for phonation. Whereas adult cardinals time each expiration to coincide with a song syllable (Fig. 6), this is not the case during subsong when expiration may begin well before and/or continue considerably after a vocalisation. Often a single expiration contains several vocalisations and sometimes a high respiratory pressure generates airflow without a detectable sound being produced. The two sides of the syrinx are also often poorly coordinated with each other. Sometimes both sides may be closed during part of an expiratory cycle or one side may open without producing sound. The fundamental frequency often fluctuates irregularly during a note, suggesting that mechanisms responsible for frequency control have not yet matured. Independent left-right motor control is apparent by mid to late subsong but is not coordinated into the sequential production of long FM sweeps until plastic song.
Patterns of song lateralization
The diversity of birdsong has been achieved to a significant extent through the evolution of different motor strategies in the use of the bipartite oscine syrinx. Although the basic structure of the syrinx is very similar throughout oscines, different species have taken advantage of the versatility of its duplex nature in different ways according to the properties of their songs (Suthers 1997; Suthers 1998; Suthers & Goller 1997).
Unilateral dominance.
Evidence that both sides of the syrinx do not necessarily make equal contributions to song was first obtained by Nottebohm (Nottebohm 1971; Nottebohm & Nottebohm 1976) who demonstrated that paralysis of the left syrinx, by unilateral section of the tracheosyringeal branch of the hypoglossal motor nerve, NXIIts, resulted in greater song deficits in Chaffinches and Waterslager Canaries than did paralysis of the right syrinx. The effect was most striking in Waterslager Canaries in which right nerve section eliminated only about 10% of the syllable types in the song repertoire whereas left nerve section eliminated about 90% of the bird’s repertoire. A similar result was later obtained by Hartley and Suthers (1990) who plugged one bronchus, preventing ipsilateral sound production by blocking airflow through that side of the syrinx. McCasland (1987), however, reported that bronchial plugs in his Canaries produced deficits that were only slightly greater after left plugs compared to right plugs. It is not clear why Hartley and Suthers' repetition of McCasland’s experiment gave different results.
Measures of syringeal airflow and pressure in Canaries (Suthers 1992; Suthers 1997; Suthers & Goller 1997) confirm the conclusions derived from the effects of unilateral syringeal neurotomy and provide additional insights into how they produce their songs. During sound production, the silent side of the syrinx is kept closed. Just as the left side of the syrinx dominates sound production, the right side has a special role in facilitating the minibreaths that are inserted between each syllable to refill the air sacs. At a syllable repetition rate of 30 s-1, the duration of each minibreath can be less than 20 ms (Hartley & Suthers 1989). This respiratory strategy enables a bird with a resting tidal volume of only about 0.5 ml to sing a seemingly continuous song that may last more that 30 s. During song, most syllables are generated as air flows out through the left syrinx. The right syrinx is closed during phonation on the left, but opens for a short inspiratory minibreath between each syllable unless the syllable repetition rate is so high (greater than about 30 s-1) that the interval between syllables is too short (Fig. 2). Phrases with syllable repetition rates greater than 30 s-1 are produced by pulsatile expiration. These respiratory adaptations, together with unilateral sound production, are well suited to sustain a fast tempo for relatively long periods of time. The spectral complexity possible in two-voice syllables is lacking in Waterslager Canaries but the tendency to lateralize inspiration to the right side may increase the vocal versatility of the phonating side by freeing it from the inspiratory motor program.
Waterslager Canaries are the most strongly lateralized songbird studied to date. Varying degrees of left dominance have been reported in several other species but they are not as pronounced as that in Waterslager Canaries (Suthers 1997). A similar degree of right dominance has not yet been reported for any species. Some aspects of Zebra Finch song (Williams, et al. 1992) are affected more by right than by left neurotomy (but see Floody & Arnold 1997). Both sides of the syrinx contribute to the note clusters in cowbird song but the final whistle is always produced by the right syrinx (Allan & Suthers 1994).
Sequential lateralization.
Northern Cardinal song is composed of one or more phrases, each consisting of repetition of a particular syllable type (Calder 1970; Lemon 1965; Lemon 1966; Lemon & Chatfield 1971; Lemon & Herzog 1969). Minibreaths occur between syllables up to a repetition rate of about 16 s-1. At higher repetition rates pulsatile expiration is used. Unlike the Canary, most cardinal syllables include contributions from both sides of the syrinx, but the vocal register differs on each side. The majority of cardinal syllables include an upward or downward frequency modulation. These sweeps are tonal, lacking prominent harmonics, and may extend over as much as two octaves. This unusually wide fundamental bandwidth is achieved through precisely coordinated sequential phonation on each side of the syrinx. Frequencies below about 3.5 kHz are sung with the left syrinx whereas higher frequencies are sung by the right syrinx (Fig 6). Thus when singing a note that sweeps from one vocal register into the other, sound production begins on one side and switches to the opposite side in the middle of the syllable. Some notes only contain frequencies above or below 3.5 kHz and are therefore generated entirely on one side of the syrinx (Suthers & Goller 1996; Suthers & Goller 1997). Cardinals thus achieve some of the advantages of unilateral phonation, i.e., conserving the air supply, while gaining the extended frequency range made possible by separate left and right vocal registers.
Alternating lateralization.
Yet a different motor strategy is used by the Brown-headed Cowbird, which produces short songs that last less than one second and include only 3 or 4 expiratory cycles separated by minibreaths (Allan & Suthers 1994). Each song begins with two, or occasionally three, note clusters. Each of these clusters coincide with one expiration and contain alternating contributions from the left and right syrinx (Fig 7). The successive notes on the same side build in frequency and intensity with a frequency offset between sides that gives rise to a characteristic staggered frequency pattern. The two sides of the syrinx contribute about equally to the note clusters. Each song terminates with a loud frequency modulated whistle that is always produced on the right side during the last expiration (Allan & Suthers 1994). An adult male cowbird typically has a repertoire of about 5 or 6 different songs (West & King 1986), all following this basic pattern. Cowbirds and cardinals use their left and right vocal registers in quite different ways to achieve opposite acoustic effects. Cowbirds specialise in producing precisely controlled, abrupt left-right discontinuities in frequency, whereas cardinals have perfected a seamless continuity of frequency when switching sides. The cowbird's ability to make abrupt frequency changes between notes within the note clusters is likely facilitated by the left-right alternation of sound production which gives each side longer to reconfigure itself for its next contribution.
Independent bilateral phonation.
The songs of two closely related mimic thrushes, the Brown Thrasher and Grey Catbird, include about equal contributions from the two sides of the syrinx. Unlike other species we have studied, thrashers and catbirds often sing continuously for many minutes but at a slower, more deliberate repetition rate of a few syllables s-1. These mimic thrushes have very large song repertoires containing hundreds (catbird) (Boughey & Thompson 1976; Thompson & Jane 1969) or even a few thousand (thrasher) (Kroodsma & Parker 1977) different syllable types. The modest tempo permits longer syllables and larger minibreaths that replenish the respiratory volume and oxygen. Neither side of the syrinx dominates song production. Instead, both sides phonate independently to generate syllables that may be produced by one side or both, contain simultaneous components arising independently on opposite sides, and/or switch back and forth between sides (Goller & Suthers 1996a; Goller & Suthers 1996b; Suthers, et al. 1994; Suthers, et al. 1996; Suthers & Hartley 1990) (Fig 3). By singing slowly, these mimic thrushes are able to produce relatively long notes or syllables that provide time to demonstrate their competence at other kinds of vocal gymnastics, including FM and AM segments, with two independent voices. The intervals between syllables are long enough to replace the air used during bilateral phonation.
Strategies for song production may provide clues to its significance
Although the number of species sampled is still very small, these recent studies of song production reveal that various species have evolved different ways of coping with the motor constraints of song production. Each has evolved a distinctive motor strategy for singing that maximises their ability to achieve certain kinds of acoustic effects. This vocal specialisation comes at the cost of limiting the potential to excel in other acoustic features that require different, conflicting motor strategies. Northern Cardinals, for example, have perfected a smooth and seamless transition between the two sides of the syrinx needed to produce an extended frequency sweep whereas Brown-headed Cowbirds display left-right spectral contrast with precisely timed, abrupt left-right switches between successive notes. Mimic thrushes have adopted a slower tempo with longer syllables that provide opportunities for more of the elaborate two-voice interactions. These interactions give rise to the temporal and spectral complexity that characterises their large song repertoires. Unilateral phonation by Waterslager Canaries is well suited for producing long songs with contrasting phrases that may include high syllable repetition rates. Each of these species has become a different kind of vocal specialist, pushing the envelope of vocal performance in a different direction in order to achieve its special brand of vocal virtuosity. The evolution of various motor patterns to increase the vocal diversity between species is perhaps not surprising since it could be useful in various aspects of acoustic communication, including species or individual recognition. But why push the envelope of vocal performance? In demonstrating the limits of his unique vocal prowess, might the singer provide his audience with a diagnostic check of his sensory, learning, memory and motor competence? Studies of the behavioural significance of the particular acoustic features associated with specific motor strategies may help provide an answer.
REFERENCES
Allan, S. E. & Suthers, R. A. 1994. Lateralization and motor stereotypy of song production in the brown-headed cowbird. Journal of Neurobiology 25: 1154-1166.
Bech, C., Johansen, K. & Nicol, S. 1988. Gas exchange during high-frequency ventilation in the pigeon (Columba livia). Acta Physiologica Scandinavica 132: 217-221.
Boughey, M. J. & Thompson, N. S. 1976. Species specificity and individual variation in the songs of the brown thrasher (Toxostoma rufum) and catbird (Dumetella carolinensis). Behavior 57: 64-90.
Brackenbury, J. H. 1971. Airflow dynamics in the avian lung as determined by direct and indirect methods. Respiration Physiology 13: 319-329.
Brackenbury, J. H. 1972. Physical determinants of air flow pattern within the avian lung. Respiration Physiology 15: 384-397.
Brackenbury, J. H. 1973. Respiratory mechanics in the bird. Comparative Biochemistry and Physiology 44A: 599-611.
Calder, W. A. 1970. Respiration during song in the canary (Serinus canaria). Comparative Biochemistry and Physiology 32: 251-258.
Chappell, M. A., Zuk, M., Kwan, T. H. & Johnsen, T. S. 1995. Energy cost of an avian vocal display: crowing in red junglefowl. Animal Behaviour 49: 255-257.
Eberhardt, L. S. 1994. Oxygen consumption during singing by male carolina wrens (Thryothorus ludovicianus). The Auk 111: 124-130.
Eberhardt, L. S. 1996. Energy expenditure during singing: A reply to Gaunt et al. The Auk 113: 721-723.
Floody, O. R. & Arnold, A. P. 1997. Song lateralization in the zebra finch. Hormones and Behavior 31: 25-34.
Gaunt, A. S. 1983. An hypothesis concerning the relationship of syringeal structure to vocal abilities. The Auk 100: 853-862.
Gaunt, A. S., Bucher, T. L., Gaunt, S. L. L. & Baptista, L. F. 1996. Is singing costly? The Auk 113: 718-721.
Goller, F. & Larsen, O. N. 1997. A new mechanism of sound generation in songbirds. Proceedings of the National Academy of Sciences. U.S.A. 94: 14787-14791.
Goller, F. & Suthers, R. A. 1995a. Contributions of expiratory muscles to song production in brown thrashers. In: Nervous Systems and Behaviour. Proceedings of the 4th International Congress of Neuroethology. Burrows, M., Matheson, T., Newland, P. &Schuppe, H. (Eds.). Stuttgart, Georg Thieme Verlag: 334.
Goller, F. & Suthers, R. A. 1995b. Implications for lateralization of bird song from unilateral gating of bilateral motor patterns. Nature 373: 63-66.
Goller, F. & Suthers, R. A. 1996a. Role of syringeal muscles in controlling the phonology of bird song. Journal of Neurophysiology 76: 287-300.
Goller, F. & Suthers, R. A. 1996b. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. Journal of Neurophysiology 75: 867-876.
Greenewalt, C. H. 1968. Bird Song: Acoustics and Physiology. Washington, D.C., Smithsonian Institution Press.
Hartley, R. S. 1990. Expiratory muscle activity during song production in the canary. Respiratory Physiology 81: 177-187.
Hartley, R. S. & Suthers, R. A. 1989. Airflow and pressure during canary song: evidence for mini-breaths. Journal of Comparative Physiology A. 165: 15-26.
Hartley, R. S. & Suthers, R. A. 1990. Lateralization of syringeal function during song production in the canary. Journal of Neurobiology 21: 1236-1248.
Horn, A. G., Leonard, M. P. L. & Weary, D. M. 1995. Oxygen consumption during crowing by roosters: talk is cheap. Animal Behaviour 50: 1171-1175.
King, A. S. 1989. Functional anatomy of the syrinx. In: Form and Function in Birds. King, A. S. & McLelland, J. (Eds.). London, Academic Press. 4: 105-192.
Konishi, M. 1965. The role of auditory feedback in the control of vocalisation in the white-crowned sparrow. Zeitschrift für Tierpsychologie. 22: 770-783.
Konishi, M. & Nottebohm, F. 1969. Experimental studies in the ontogeny of avian vocalisations. In: Bird Vocalization. Hinde, R. A. (Ed.). Cambridge, Cambridge University Press.
Kroodsma, D. E. & Parker, L. D. 1977. Vocal virtuosity in the brown thrasher. The Auk 94: 783-785.
Lemon, R. E. 1965. The song repertoires of cardinals (Richmondena cardinalis) at London, Ontario. Canadian Journal of Zoology 43: 559-569.
Lemon, R. E. 1966. Geographic variation In the song of cardinals. Canadian Journal of Zoology 44: 413-428.
Lemon, R. E. & Chatfield, C. 1971. Organization of song in cardinals. Animal Behaviour 19: 1-17.
Lemon, R. E. & Herzog, A. 1969. The vocal behavior of cardinals and pyrrhuloxias in Texas. Condor 71: 1-15.
McCasland, J. S. 1987. Neuronal control of bird song production. Journal of Neuroscience 7: 23-39.
Miskimen, M. 1951. Sound production in passerine birds. The Auk 68: 493-504.
Nottebohm, F. 1971. Neural lateralization of vocal control in a passerine bird I. song. Journal of Experimental Zoology 177: 229-262.
Nottebohm, F. & Nottebohm, M. E. 1976. Left hypoglossal dominance in the control of canary and white-crowned sparrow song. Journal of Comparative Physiology 108: 171-192.
Nowicki, S. & Capranica, R. R. 1986. Bilateral syringeal coupling during phonation of a songbird. Journal of Neuroscience 6: 3595-3610.
Nowicki, S., Westneat, M. & Hoese, W. 1992. Birdsong: motor function and the evolution of communication. Seminars in the Neurosciences 4: 385-390.
Podos, J. 1996. Motor constraints on vocal development in a songbird. Animal Behaviour 51: 1061-1070.
Podos, J. 1997. A performance constraint on the evolution of trilled vocalisations in a songbird family (Passeriformes: Emberizidae). Evolution 51: 537-551.
Scheid, P. & Piiper, J. 1989. Respiratory mechanics and air flow in birds. In: Form and Function in Birds. King, A. S. & McLelland, J. (Eds.). London, Academic Press. 4: 369-392.
Stein, R. C. 1968. Modulation in bird sound. The Auk 94: 229-243.
Suthers, R. A. 1990. Contributions to birdsong from the left and right sides of the intact syrinx. Nature 347: 473-477.
Suthers, R. A. 1992. Lateralization of sound production and motor action on the left and right sides of the syrinx during bird song. 14th International Congress on Acoustics: I1-5.
Suthers, R. A. 1997. Peripheral control and lateralization of birdsong. Journal of Neurobiology 33: 632-652.
Suthers, R. A. 1998. The motor basis of vocal performance in songbirds. In: Neural Mechanisms of Communication. Konishi, M. & Hauser, M. (Eds.). Cambridge, MA, MIT Press.
Suthers, R. A. & Goller, F. 1996. Respiratory and syringeal dynamics of song production in northern cardinals. In: Nervous Systems and Behaviour. Proceedings of the 4th International Congress of Neuroethology. Burrows, M., Matheson, T., Newland, P. &Schuppe, H. (Eds.). Stuttgart, Georg Thieme Verlag: 333.
Suthers, R. A. & Goller, F. 1997. Motor correlates of vocal diversity in songbirds. In: Current Ornithology. Nolan Jr., V., Ketterson, E. &Thompson, C. F. (Eds.). New York, Plenum Press. 14: 235-288.
Suthers, R. A. & Goller, F. 1998a. Ontogeny of song lateralization in juvenile Northern Cardinals. Society for Neuroscience Abstracts 24.
Suthers, R. A. & Goller, F. 1998b. Respiratory-syringeal motor coordination during song learning in northern cardinals. Fifth International Congress of Neuroethology. Abstracts.
Suthers, R. A., Goller, F. & Hartley, R. S. 1994. Motor dynamics of song production by mimic thrushes. Journal of Neurobiology 25: 917-936.
Suthers, R. A., Goller, F. & Hartley, R. S. 1996. Motor stereotypy and diversity in songs of mimic thrushes. Journal of Neurobiology 30: 231-245.
Suthers, R. A. & Hartley, R. S. 1990. Effect of unilateral denervation on the acoustic output from each side of the syrinx in singing mimic thrushes. Society for Neuroscience. Abstracts. 16: 1249.
Thompson, W. L. & Jane, P. L. 1969. An analysis of catbird song. Jack-Pine Warbler 47: 115-125.
Vallet, E., Beme, I. & Kreutzer, M. 1998. Two-note syllables in canary songs elicit high levels of sexual display. Animal Behaviour 55: 291-297.
Vehrencamp, S. L., Bradbury, J. W. & Gibson, R. M. 1989. The energetic cost of display in male sage grouse. Animal Behaviour 38: 885-896.
Vicario, D. S. 1991a. Contributions of syringeal muscles to respiration and vocalisation in the zebra finch. Journal of Neurobiology 22: 63-73.
Vicario, D. S. 1991b. Neural mechanisms of vocal production in songbirds. Current Opinion in Neurobiology 1: 595-600.
Vicario, D. S. 1993. A new brain stem pathway for vocal control in the zebra finch song system. NeuroReport 4: 983-986.
West, M. J. & King, A. P. 1986. Song repertoire development in male cowbirds (Molothrus ater): its relation to female assessment of song potency. Journal of Comparative Psychology. 100: 296-303.
Wild, J. M. 1993a. The avian nucleus retroambigualis: a nucleus for breathing, singing and calling. Brain Research 606: 119-124.
Wild, J. M. 1993b. Descending projections of the songbird nucleus Robustus Archistriatalis. Journal of Comparative Neurology 338: 225-241.
Wild, J. M., Goller, F. & Suthers, R. A. 1998. Inspiratory muscle activity during birdsong. Journal of Neurobiology 36: 441-453.
Williams, H., Crane, L. A., Hale, T. K., Esposito, M. A. & Nottebohm, F. 1992. Right-side dominance for song control in the Zebra Finch. Journal of Neurobiology 23: 1006-1020.
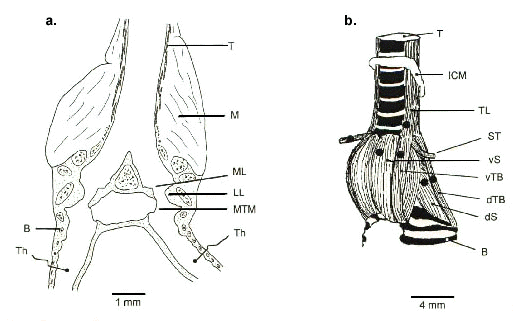
Fig. 1. The oscine syrinx is a bipartite structure containing two sound sources. a. Frontal section through a Brown Thrasher syrinx, showing the position of the microbead thermistor, Th, used to measure rate of airflow through each side of syrinx. b. Ventrolateral external view of a thrasher syrinx depicting syringeal muscles. Black dots indicate, for one side, the approximate location where bipolar wire electrodes were placed. Abbreviations: T, trachea; M, syringeal muscle; ML, medial labium; LL, lateral labium; MTM, medial tympaniform membrane; B, bronchus; ICM, membrane of the interclavicular air sac; TL, m. tracheolateralis; ST, m. sternotrachealis; vS, m. syringealis ventralis; vTB, m. tracheobronchialis ventralis; dTB, m. tracheobronchialis dorsalis; dS, m. syringealis dorsalis. (Modified from Goller & Suthers, 1996a. Reproduced with permission).

Fig. 2. Representative segment of Waterslager Canary song containing 4 phrases, a – d, each composed of repetitions of a different syllable type. Each syllable type is characterised by a distinct pattern of airflow through each side of the syrinx and subsyringeal air sac pressure. Minibreaths are used in the first 3 phrases. The fourth phrase is a trill produced by pulsatile expiration through the left syrinx while air sac pressure remains positive. Phrases a, c and d are produced in the left side of the syrinx. Phrase b is unusual in that expiration starts on the left side but switches sides in mid-expiration to produce the syllable on the right. The minibreath is still on the right side. Note the lateralization of minibreaths to the right syrinx. Direction of airflow is indicated by air sac pressure. For each syllable type, the first and second vertical lines indicate expiration and the second and third lines indicate inspiration. Air sac pressure (P) is positive during vocalisation. Zero line equals ambient pressure. Oscillogram of the vocalisation (V) at bottom is also shown spectrographically at top. The rate of airflow through the left (FL) and right (FR) sides of the syrinx is also shown. Both inspiration and expiration produce an upward deflection of the flow trace. Horizontal lines equal zero airflow. (From Suthers 1998)
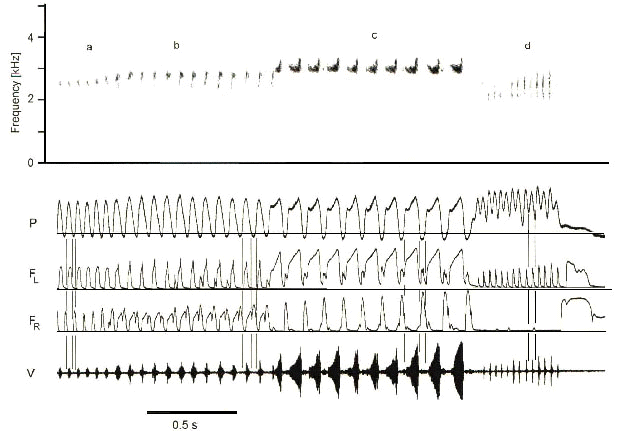
Fig. 3. Segment of Brown Thrasher song showing four two-voice syllables with independent frequency modulation of the left and right side contributions. These are separated from each other by shorter upward sweeping syllables produced by airflow through the right syrinx while the left side is closed (indicated by positive pressure but no airflow), and therefore silent. Sound production is frequently switched between sides of the syrinx. The vocal register of the right side is higher than that of the left although there is substantial overlap. R and L in spectrogram indicates contributions from right and left side of syrinx; inspiratory airflow (minibreaths) is stippled. Other abbreviations as in Fig. 2. (From Suthers et al. 1994. Reproduced by permission)
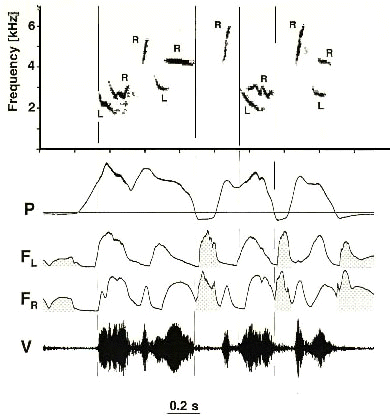
Fig. 4. Example of prominent rapid cyclical AM in the songs of mimic thrushes (Mimidae). Grey Catbird two-voice syllable in which AM is produced by repeatedly opening and closing the right side of the syrinx. Sound intensity increases with each increase in airflow through the right side. Since air sac pressure does not oscillate, this form of AM must be produced by activity in the dorsal syringeal muscles on the right side. (Modified after Suthers et al. 1994)
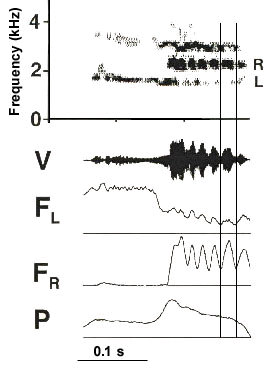
Fig. 5. Contraction of the ventral syringeal muscle in the Brown Thrasher is closely correlated with the fundamental frequency of the vocalisation. a. Bilateral EMG activity in the ventral muscles during syllables produced alternately on each side of the syrinx. Shaded areas indicate syllables generated on the right side, the left side being closed. High amplitude EMG's are associated with higher-frequency notes. This is not true for last burst in the EMG on the right side (arrow), since the right side is closed. b. Relationship between EMG activity in the ventral syringeal muscle and the mean fundamental frequency of the vocalisation. EMG strength increases exponentially with the rising fundamental frequency of both ipsilaterally (filled circles; contralateral side is closed) and contralaterally (open circles; ipsilateral side is closed) generated sound. This example is for the left ventral muscle. Note that the same level of EMG activity is associated with a higher fundamental frequency for sound generated on the right (contralateral) side than on the left side. c. An example of the close relationship between the amplitude pattern of EMG in the ventral syringeal muscles and the modulation of sound frequency. vSL and vSR denote the rectified EMG of the left and right ventral syringeal muscles, respectively. For explanation of other symbols see legend of Fig. 2. (From Goller & Suthers 1995b. Reproduced by permission)
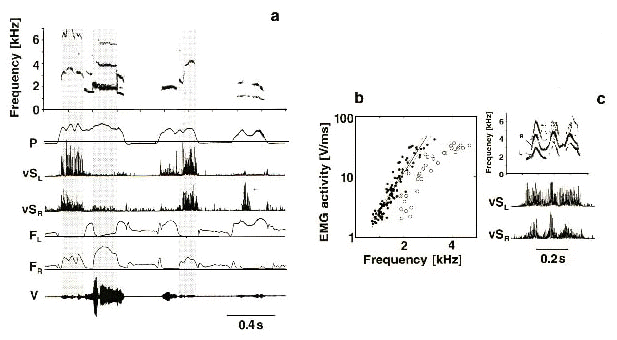
Fig. 6. Portion of a Northern Cardinal song. The first two syllables sweep upward from about 4.5 kHz and are generated on the right side with little airflow through the left. The initial high frequency portion of the last three syllables (first and second vertical lines) is produced by the right syrinx as the left side opens. Remainder of syllable (second and third vertical lines) is produced on the left side while the right side is closed. Note the minibreaths between syllables. See Fig. 2 for abbreviations. (From Suthers 1997)
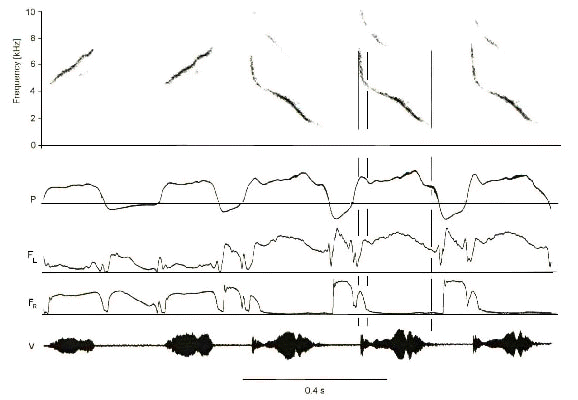
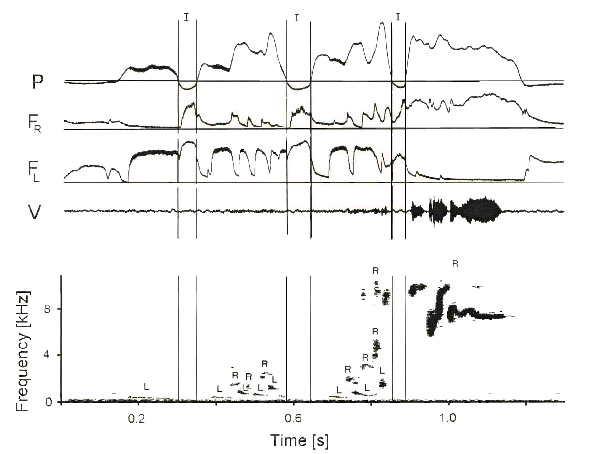
Fig. 7. Songs of the Brown-headed Cowbird are produced during four expirations separated by minibreaths (I). The first three expirations each produce a cluster of notes that increase gradually in frequency and intensity, beginning with a left side (L) note and alternating sides while the frequency increases in a staggered manner. In contrast to right side notes (R), most left side notes are lower in frequency and lack prominent frequency modulation. Some two-voice components accompany temporal overlap of left and right sounds. The final whistle during the last expiration is produced on the only on the right side. Note clusters are of a much lower intensity than the final whistle and are barely detectable in the oscillographic trace. See legend of Fig. 2 for explanation of symbols. (From Suthers 1998)