S09.1: Anatomy of the song system
J. Martin Wild
Department of Anatomy, School of Medicine and Health Science, University of Auckland, Auckland, New Zealand,
fax 64 9 3737484, e-mail jm.wild@auckland.ac.nz.J. M. Wild. 1999. Anatomy of the song system
. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 484-490. Johannesburg: BirdLife South Africa.Our knowledge of the anatomy of the vocal control system in birds may be said to have a dual origin, one branch originating in the midbrain, as it were, (e.g., Brown 1965; 1971; Wild et al. 1997) and another in the telencephalon (Nottebohm et al. 1976). These two origins reflect our present concerns with the distribution of the capacity for song learning and performance across the range of aves. That is, although all birds are able to call and have a nucleus in the midbrain that is in some way related to the production of calls, only some birds sing. Of these, only the true oscines learn their songs, a facility that is perfectly correlated with the presence within the telencephalon of a group of nuclei that have come to be known as the song control nuclei. Song itself, however, is not necessarily learned, as the suboscines remind us, but these species do not possess the telencephalic song control nuclei that are present in oscines (Kroodsma & Konishi, 1991). Detailed reviews of the anatomy, physiology and endocrinology of the song control nuclei and their role in song learning and production have recently been published (Brenowitz et al. 1997; Wild 1997a; b). The purpose of the present talk is simply to lay out the basic connectional anatomy of the song control pathways for those who may be less familiar with the song system and as a basis for subsequent talks in this symposium. The major song control nuclei and pathways in the oscine brain are shown in Fig. 1 and Fig. 2.
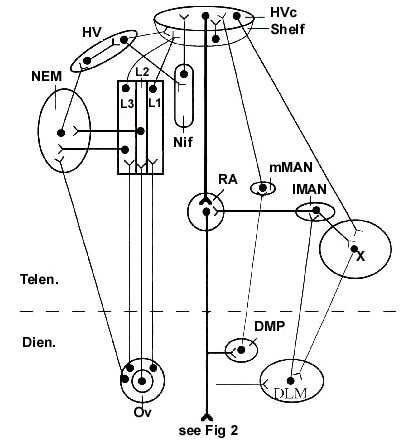
It is common to divide the telencephalic nuclei and their connections into two groups or circuits, although the two are themselves interconnected. In the more anterior parts of the brain are two nuclei, one called lMAN (lateral magnocellular nucleus of the anterior neostriatum) and another called 'area X'. lMAN lies within what avian neuroanatomists mistakenly call the neostriatum, while X lies within a part of the true basal ganglia. Certain cells in X project upon a nucleus in the dorsal thalamus called DLM (dorsolateralis anterior, pars medialis) and these DLM cells project upon lMAN. This circuit has recently been completed by the discovery that cells in lMAN project upon X, and the same cells also project upon nucleus robustus archistriatalis (RA), the pre-eminent premotor nucleus of the song system (Vates & Nottebohm, 1995; Perera et al. 1995; Nixdorf-Bergweiler et al. 1995). This anterior forebrain circuit, as it is called, appears to be particularly concerned with song learning. That is, lMAN is necessary for young birds to be able to learn their song from conspecifics, but does not appear to be involved in song production once the song is learned (Bottjer et al. 1984; Scharff & Nottebohm 1989; Shorabji et al. 1990). The role of lMAN in adult song therefore remains something of a mystery.
The other group of nuclei and their connections is more directly involved in song production. HVc (High Vocal center) may be regarded as the keystone of the song system, in the sense that it receives auditory input from auditory fields in the telencephalon - albeit for the most part by a very circuitous set of pathways - and projects directly upon RA, which in turn plays a major role in vocal production. HVc also projects upon area X, although by a different set of neurons, and this projection is thought to be the way auditory information gains access to the anterior forebrain circuit for song learning to take place. Auditory input to the song system is, of course the sine qua non of song learning, and possibly also for continued song production (Konishi 1965; Nordeen & Nordeen 1992; Okanoya & Yamaguchi 1997), but its routes of access to HVc have been very difficult to determine (Fortune & Margoliash, 1995; Vates et al. 1996). They include not only the primary telencephalic auditory field (L2) and its associated laminae, but also the caudomedial neostriatum (Ncm), caudal regions of the hyperstriatum ventrale, a region immediately ventral to HVc known as the 'shelf', and another nucleus called interface (NIf). Most of the direct access to HVc from the auditory telencephalon seems to be via either NIf or the shelf underlying HVc. The circuitous nature of these routes of access to HVc, and hence the rest of the song system, probably accounts for the highly processed nature of the auditory signals that finally enter, via multisynaptic pathways, what is essentially a pemotor pathway for song production.
HVc sends bundles of axons ventrally to synapse on neurons that make up the remarkable and perhaps unique premotor nucleus called robustus archistriatalis (RA). While HVc appears to take care of the production of song syllables, RA is concerned with the production of individual notes (Yu & Margoliash 1995). It does this via its monosynaptic connections with vocal motoneurons in the tracheosyringeal nucleus (XIIts) situated in the middle and caudal regions of the dorsal medulla. Vicario (1991) has shown that RA is topographically organised with respect to the functional groups of XIIts motoneurons, such that middle and ventral regions of RA project upon motoneurons that innervate either dorsal or ventral syringeal muscles. The precise function of these different sets of syringeal muscles in song may vary between different species, but in mimic thrushes the dorsal syringeal muscles are thought to be concerned directly with the sound producing mechanism, i.e., with phonation - and hence their activity varies from side to side on a momentary basis as the left and/or the right side of the syrinx is opened or closed to produce or prevent sound, respectively. The ventral syringeal muscles, the activity of which is not lateralised, appear to be concerned with the acoustic frequency of song elements (Goller & Suthers 1996). RA is thus concerned with the control of both the production of sounds and with the acoustic frequency of those sounds.
But this is not all that RA is concerned with. To produce sound at all requires that air be driven past the vibratable syringeal membranes and labia, and the pressures required and the timing of the pulses of air have to be precisely coordinated with the activity in syringeal muscles so that one expiration is correlated with one or a small cluster of notes (Hartley 1989). Despite the frequently used analogy, the air sac system does not function simply as a pair of bellows, at least not one that operates independently of the syrinx. The respiratory muscles have an extraordinary degree of fine control over the amount and timing of the pulses of air and this control is coordinated with syringeal activity with a high degree of temporal precision. It appears that one way the songbird nervous system caters for this precise coordination between these widely separated groups of muscles is to provide an input to the syringeal and respiratory systems that is derived from a common source, namely RA (Wild 1993a; b; Vicario 1993).
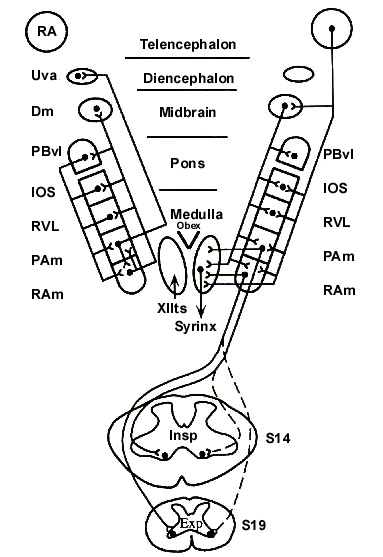
When Nottebohm et al. (1976) discovered the direct relations of RA to the syringeal motoneurons, they recognised RA as a most remarkable nucleus, perhaps one with no parallel in any other vocal animal, including ourselves. Now, RA can be considered doubly remarkable because it is now seen to provide direct projections not only to the vocal motoneurons but also to expiratory (RAm) and inspiratory (PAm) groups of premotor neurons that drive respiratory muscles during singing (Fig. 2; Wild, 1993a; b; Reinke and Wild, 1998). In this way, RA produces a precisely timed set of signals that ensures the necessary coordination of individual syringeal and respiratory muscles during song.
Precisely how it does this is another matter, for the RA neurons that project upon the respiratory premotor neurons are concentrated in dorsal regions of RA and hence are not the same ones that project upon the syringeal motor neurons (Vicario 1991; 1993; Reinke & Wild 1998). Perhaps the temporal coordination of syringeal and respiratory muscles is not organised within RA, but within HVc, for it appears as though single HVc axons can make contact with neurons in both dorsal and ventral parts of RA (Kittelberger & Mooney 1997).
The projections of RA also target several other nuclei on their way downstream Within the dorsal thalamus RA axons terminate to a small extent upon DLM, the thalamic component of the anterior forebrain pathway, and upon another nucleus (dorsomedialis posterior, DMP) that sends bilateral projections to the medial magnocellular nucleus of the anterior neostriatum (Vates et al. 1997; Wild 1993b). mMAN then projects upon HVc (Foster et al. 1997). So here we have two ways by which information related to motor commands for song can be fed back to the telencephalic song control system, one of which may contribute to the bilateral coordinaton of song. Verification of the precise function of these feedback pathways, however, is still required.
Within the midbrain RA also terminates upon the dorsomedial nucleus of the intercollicular complex (DM), as was shown by Nottebohm et al. (1976) in their original paper (see also Gurney 1981; Vicario 1991; Wild 1993b; Reinke & Wild 1998). In non-songbirds DM seems to be the principal nucleus for the control of vocalisation (i.e., for unlearned calls), and in this respect DM is comparable with the lateral periaqueductal grey of mammals. In other words there does not appear to be in non-songbirds a telencephalic nucleus comparable to RA in non-songbirds. Nevertheless, the descending projections of DM in both songbirds and non-songbirds are very similar to those of RA in songbirds (Wild et al. 1997). Both project upon two other nuclei in the ventrolateral parts of the brainstem, one called infra-olivaris superior (IOS, lying ventral to the superior olive), and another called the ventrolateral nucleus of the rostral medulla (RVL; Wild 1993b). Nothing is known about the functions of these nuclei in song, but they, like RA and DM, both project upon respiratory premotor neurons and syringeal motoneurons (Wild et al. 1997; Reinke and Wild, 1997; 1998). RA may also project upon laryngeal motoneurons (Wild 1993b), although the role of the avian larynx in vocalisation is unknown.
There is thus a cascade of descending projections from higher to lower centres concerned with vocal control in birds, although they may not all be involved in song as such (Wild 1994). All of them are predominantly ipsilateral projections, but all the smaller contralateral projections, even of RA, may not be functionally insignificant. This is presumably an important consideration because of the need to coordinate the output of the two sides of the brain during song.
A final nucleus with projections upon the respiratory premotor neurons and the syringeal motor neurons is the ventrolateral parabrachial nucleus (PBvl; Wild & Arends 1987; Reinke & Wild 1998). This nucleus receives projections from the lateral parasolitary nucleus, which in turn is the recipient of vagal pulmonary afferents that mediate information related to the gaseous composition of lung air. The afferent and efferent projections of PBvl thus seem to ensure its involvement in certain aspects of the overall control of vocalisation, including song, although what these aspects are has yet to be determined. The respiratory-vocal circuit of which PBvl is a part may be concerned with the regulation of song duration, and possibly with the temporal pattern of song, which is in part defined by the frequency and timing of inspiratory minibreaths (Hartley & Suthers 1990; Wild et al. 1998).
Finally, mention should be made of the fact that songbirds, like humans, cannot sing without opening their mouths, and the extent of beak opening during song is positively correlated with fundamental frequency, at least up to about 3-4 kHz (Westneat et al., 1993; Suthers et al., 1996). This implies that the neural control of the jaw and perhaps of other upper vocal tract structures, such as the tongue and larynx, is linked to that of the syrinx and respiratory apparatus. Investigations of these links are currently in progress (Wild & Suthers, unpublished).
REFERENCES
Brenowitz, E.A, Margoliash, D.& Nordeen, K.W. 1997. An introduction to birdsong and the avian song system. Journal of Neurobiology 33:495-500.
Brown, J.L. 1965. Vocalization evoked from the optic lobe of a songbird. Science 149:1002.
Brown, J.L. 1971. An exploratory study of vocalisation areas in the brain of the redwinged black-bird (Agelaius phoeniceus). Behavior 39:91-127.
Fortune, E.S. & Margoliash, D. 1995. Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata). Journal of Comparative Neurology 360:413-441.
Foster, E.F., Mehta, R.P. & Bottjer, S.W. 1997. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. Journal of Comparative Neurology. 382:364-81.
Goller, F. & Suthers, R.A. 1996. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. Journal of Neurophysiology 75:867-876.
Gurney, M. 1981. Hormonal control of cell form and number in the zebra finch song system. Journal of Neuroscience 1:658-673.
Hartley, R.S. 1990. Expiratory muscle activity during song production in the canary. Respiratory Physiology 81:177-187.
Hartley, R.S. & Suthers, R.A. 1989. Airflow and pressure during canary song: direct evidence for mini-breaths. Journal of Comparative Physiology A. 165:15-26.
Kittelberger, J.M. & Mooney, R. 1997. Individual HVc axons innervate RA subdomains that control temporal and spectral elements of learned song. Society for Neurocience Abstracts 23:245.
Konishi, M. 1965 The role of auditoy feedback in the control of vocalisation in the white-crowned sparrow. Zeitschrift für Tierpsychologie 22:770-783.
Kroodsma, D.E. & Konishi, M. 1991. A suboscine bird (Eastern Phoebe, Sayornis phoebe) develops normal song without auditory feedback. Animal Behaviour 42:477-487.
Nixdorf-Bergweiler B.E., Lips, M.B. & Heineman, U. 1995. Electrophysiological and morphological evidence for a new projection of LMAN-neurones towards area X. NeuroReport 6:45-48.
Nordeen, K.W. & Nordeen, E.J. 1992 Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behavioral & Neural Biology 57:58-66.
Nottebohm, F., Stokes, T.M. & Leonard, C.M. 1976. Central control of song in the canary (Serinus canaria). Journal of Comparative Neurology 165:457-486.
Nottebohm, F., Kelley, D.B. & Paton, J.A. 1982. Connections of the vocal control nuclei in the canary telencephalon. Journal of Comparative Neurology 207:344-357.
Okanoya K. & Yamaguchi, A. 1997. Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. Journal of Neurobiology. 33:343-56
Perera, A.D., Hunger, F., Gahr, M. & Wild, J.M. 1995. A parallel circuit linking lMAN and Area X in the avian song system. Society for Neuroscience Abstracts 21:962.
Reinke, H. & Wild, J.M. 1997. Distribution and connections of inspiratory premotor neurons in the brainstem of the pigeon (Columba livia). Journal of Comparative Neurology 379:347-362.
Reinke, H. & Wild, J.M. 1998. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. Journal of Comparative Neurology 391:147-163.
Scharff, C. & Nottebohm, F. 1989. Neural mechanisms of behavior. In: J. Erber, R. Menzel, H.-J. Pfüger & D. Todt (Eds), Proceedings of the 2nd International Congress of Neuroethology, Stuttgart, Georg Thieme, p122.
Suthers, R. A., Goller, F., Bermejo., R. Wild, J.M. & Zeigler, H.P. 1996. Relationship of beak gape to the lateralization, acoustics and motor dynamics of song in cardinals. Abstracts of the Nineteenth Midwinter Meeting. Assoc. for Research in Otolaryngology, Des Moines, p158.
Vates, G.E. & Nottebohm, F. 1995. Feedback circuitry within a song-learning pathway. Proceedings of the National Academy of Science USA 92:5139-5143.
Vates, G.E., Broome, B.M., Mello, C.V. & Nottebohm, F. 1996. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taeniopygia guttata). Journal of Comparative Neurology 366:613-642.
Vates, G.E., Vicario, D.S. & Nottebohm, F. 1997. Reafferent thalamo-'cortical' loops in the song system of oscine songbirds. Journal of Comparative Neurology 380:275-290.
Vicario, D.S. 1991. Organization of the Zebra finch song control system: II. Functional organization of outputs from nucleus robustus archistriatalis. Journal of Comparative Neurology 309:486-494.
Vicario, D.S. 1993. A new brain stem pathway for vocal control in the Zebra finch song system. NeuroReport 4:983-986.
Westneat, M.W., Long, Jr., J.H., Hoese, W. & Nowicki, S. 1993. Kinematics of birdsong: functional correlation of cranial movements and acoustic features in sparrows. Journal of Experimental Biology 182:147-171.
Wild, J.M. 1993a. The avian nucleus retroambigualis: a nucleus for breathing, singing and calling. Brain Research 606:119-124.
Wild, J.M. 1993b. Descending projections of the songbird nucleus robustus archistriatalis. Journal of Comparative Neurology 338:225-241.
Wild, J.M. 1997a. Neural pathways for the control of birdsong production. Journal of Neurobiology 33:653-670.
Wild, J.M. 1997b. Functional anatomy of neural pathways contributing to the control of song production in birds. European Journal of Morphology 35:303-325.
Wild, J.M. 1994. The auditory-vocal-respiratory axis in birds. Brain, Behavior and Evolution 44:192-209.
Wild, J.M. & Arends, J.J.A. 1987. A respiratory-vocal pathway in the brainstem of the pigeon. Brain Research 407:191-194.
Wild, J.M., Li, D. & Eagleton, C. 1997. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in relation to respiratory-vocal nuclei in the brainstem of pigeon (Columba livia) and zebra finch (Taeniopygia guttata). Journal of Comparative Neurology 377:392-413..
Wild, J.M., Goller, F. & Suthers, R.A. 1998. Inspiratory muscle activity during singing. Journal of Neurobiology, in press.
Yu, A.C. & Margoliash, D. 1996. Temporal hierarchical control of singing in birds. Science 273:1871-1875.
Fig. 1. Schematic summary diagram of the nuclei and interconnections of the song control system at forebrain levels. Dots represent cell bodies. To the left of the main efferent pathway from HVc through RA (bold vertical lines) are shown structures associated with auditory input to the song system, originating in nucleus ovoidalis (Ov) of the dorsal thalamus (Dien.), which projects primarily to the Field L complex (L2, L1 and L3) in the caudomedial telencephalon. The anterior forebrain (song learning) circuit is composed of lMAN and area X in the telencephalon and DLM in the dorsal thalamus. Not all components are shown, e.g. nucleus uvaeformis (Uva - see Fig. 2) of the posterior thalamus, which is a major source of input to NIf and to HVc. Based on Nottebohm et al. 1976; 1982; Vates et al. 1995; 1996; 1997; et alia.

Fig. 2. Schematic summary diagram of the 'descending cascade' and interconnections of the nuclei making up the brainstem respiratory-vocal system in songbirds; based on Wild, 1993a; b; 1994; Wild et al. 1997; Reinke & Wild, 1997; 1998. Dots represent cell bodies. In the pons and medulla the nuclei make up a more or less continuous column in the ventrolateral tegmentum. Descending projections to these nuclei arising in RA and DM are shown on the right, as are the bulbospinal premotor projections arising in the expiratory-related nucleus retroambigualis (RAm) and inspiratory-related nucleus parambigualis (PAm). Reciprocal projections among the nuclei of the rhombencephalic column are shown on the left, as are the ascending projections to DM, and to the caudal thalamus (Uva) from or near PAm. Contralateral projections, of which there many among the brainstem nuclei, are not shown for reasons of clarity.