S08.4: Sex allocation in relation to parental quality
Staffan Bensch
Department of Animal Ecology, Ecology Building, Lund University, S-223 62 Lund, Sweden, fax 46 46 222 4716, e-mail staffan.bensch@zooekol.lu.se
Bensch, S. 1999. Sex allocation in relation to parental quality. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
451-466. Johannesburg: BirdLife South Africa.Studies of whether birds manipulate offspring sex ratios in response to parental quality have examined two categories of variables: (1) phenotypic qualities that may influence the parent as a provider and (2) qualities associated with sexual selection. The phenotypic quality of parents may influence breeding time and the amount of resources allocated to offspring. When the relative value of sons and daughters change seasonally or with fledging mass, a parent may gain increased fitness through facultative manipulation of offspring sex ratio. There is strong support for adaptive manipulation of sex ratios in relation to breeding time among birds of prey. Birds in good condition generally breed at an early date which suggests a link between sex allocation and parental quality. Theories of sexual selection predict that females mated to attractive males may increase their fitness by producing more male offspring if these inherit the father’s attractiveness. A few studies have provided results compatible with either or both of the good provider or sexy mate models. The list of studies producing negative results is extensive and together with an unknown (probably large) number of unpublished studies (which are probably biased towards negative results) make it premature to accept sex allocation in relation to parental quality as an important evolutionary adaptation in birds.
INTRODUCTION
Given that it would increase an individual’s life time fitness to manipulate the sex ratio of its offspring, we would expect evolution to make such manipulations possible (Trivers & Willard 1973). Several authors have, however, questioned the existence of adaptive sex ratio adjustments for various reasons. For example, the ability to manipulate sex ratios might be severely constrained in animals with chromosomal sex determination (e.g. in birds and mammals), and the cost of later manipulations might be excessively large (Williams 1979). Because sexually reproducing organisms need to produce both males and females at a frequency that for most species is close to 50:50 (Fisher 1930), it might thus be better to rear an offspring of the wrong sex than sacrificing it (Maynard Smith 1980).
Trivers and Willard’s (1973) hypothesis suggests that females should produce an excess of the gender that would benefit most in the prevailing conditions (Leimar 1996). For example, if the fitness of sons are more sensitive to the feeding conditions than is the fitness of daughters, we would expect females of above average condition to rear sons and females below average condition to rear daughters. The population sex ratio, on the other hand, is still expected to be close to the equilibrium identified by Fisher (1930), with an investment in the two sexes corresponding to their value. Within populations, some individuals might favour sons and others daughters, depending on the relative value of males and females in each situation. Support for Trivers and Willard’s (1973) hypothesis has been obtained in mammals. For example, in Red Deer Cervus elaphus, dominant mothers give birth to an excess of sons which profit more from their mothers’ better condition than do daughters (Clutton-Brock et al. 1984). This is because only the best quality males will be able to defend harems and reproduce whereas quality is less decisive for female reproduction. In Savannah Baboons Papio cynocephalus, on the other hand, dominant females produce daughters, because these inherit their mother’s social rank which positively influences their survival and reproduction (Altmann et al. 1988).
Adaptive sex ratio manipulations in birds might occur already before laying, resulting in an adjustment in the primary sex ratio. However, parents could also manipulate the sex ratio throughout the period of parental care by means of selective feeding or direct infanticide, provided that they can identify the sex of their offspring. Adjustment of the primary sex ratio is the better strategy, because parents thereby avoid investing resources in offspring that will later be sacrificed. For the question examined here, however, both primary and secondary manipulation mechanisms might play a role, though examples of the former are more controversial as it requires an unknown physiological mechanism (Krackow 1995). However, whether secondary sex ratio adjustments are adaptive or just a consequence of one sex (the costly sex) being more vulnerable than the other is an open question in many studies (Weatherhead & Teather 1991).
To date, there are few convincing examples of adaptive sex ratio adjustment in birds (Frank 1990; Ellegren & Sheldon 1997). One exception is the spectacular case of sex ratio adjustment recently reported for the Seychelles Warbler Acrocephalus sechellensis (Komdeur et al. 1997). Parents breeding on high quality territories can increase their reproductive success by having helpers, which in these warblers are daughters. As predicted from the ‘production of helpers’ hypothesis’ (Gowaty & Lennartz 1985), pairs without helpers on high quality territories produce daughters to sons in a ratio of 87:13 (Komdeur et al. 1997). Conversely, pairs that occupy high quality territories and already have two helpers, and pairs on low quality territories, both favour sons which disperse from the territory and hence do not utilise scarce resources. Hence, social and ecological circumstances have a dramatic effect on the offspring sex ratio in the Seychelles warbler.
Another exception is the seasonal adjustment of offspring sex ratios in birds of prey (Daan et al. 1996). Early in the season, parents in several raptor species favour the sex for which a good start in life has the largest effect on the offspring’s prospect of breeding at one year old. For small species, such as the Kestrel Falco tinnunculus, this skew is towards sons early in the season (Dijkstra et al. 1990). In larger species, e.g. the Marsh Harrier Circus aeruginosus, males rarely breed at one year old whereas females in good condition may initiate breeding at that age. Consistent with the hypothesis, early broods are often female biased (Zijlstra et al. 1992). In raptor species with seasonal sex ratio adjustments the skew is relatively moderate, with the proportion of one sex of offspring varying from 60% to 40% during the course of the season (Daan et al. 1996). The sex ratio adjustments observed in raptors are thus not as prominent as in the Seychelles Warbler, but the consistent relationship for a range of species with age of first breeding (Daan et al. 1996) makes the raptors a convincing case of sex ratio adjustment in birds.
Gowaty (1991) pointed out four reasons why sex ratio studies in birds are particularly difficult to carry out. Briefly, this was because (1) sexing of young birds is difficult, (2) the magnitude of sex ratio variation is likely to be small, (3) field experiments are difficult to carry out and (4) ‘unrealistic statistical constraints are inhibiting’. At present, only seven years later, the prospects for avian sex ratio studies look much brighter. The recently developed molecular sexing techniques allow us to sex large number of young of presumably any species (Griffiths & Tiwari 1993; Griffiths et al. 1996; Quinn 1999). The introduction of generalised linear models (Crawley 1993) into sex ratio studies (Lessells et al. 1996), facilitates tests of brood sex ratio variation, both with regard to deviations from binomial distributions and effects of one or more independent variables, as this approach eliminates the problem of non-normal and non-constant variances in proportional data. Remaining problems are however the limited variation of sex ratios which requires large data sets to be collected and the difficulties in carrying out field experiments.
In the present paper I evaluate existing data on sex ratio adjustment in birds to see whether females manipulate the sex of their offspring in relation to their own and/or their partner’s quality. It is possible, but not necessary, that the sex ratio manipulation in the two systems described above (the birds of prey and Seychelles Warblers) is related to parental quality (via timing of breeding and territory quality, respectively).
HYPOTHESES
Fisher (1930) was the first to explain why the population sex ratio should be close to unity, or more precisely, be at a frequency which equalises the total investment in each of the two sexes. However, his model does not make predictions about the sex ratios of individual when a population has reached the equilibrium. If adaptive sex ratio adjustments are employed by individuals, the population sex ratio may still be close to the frequency predicted by Fisher (1930), or biased towards the sex produced under the poorer condition (Frank & Swingland 1988), but most markedly, the variation among broods will be larger than expected from a binomial distribution. Hence, if adaptive sex ratio adjustments are at work, this should result in an exaggerated variance of sex ratios among broods (Williams 1979). However, low power of tests, due to small samples sizes, may strongly reduce the ability to detect exaggerated brood sex ratios statistically. Thus, the failure to find a deviation from the binomial distribution cannot be used to reject the occurrence of sex ratio adjustments (Gowaty 1991).
Studies of sex allocation in relation to parental quality have examined two categories of variables: (1) phenotypic qualities that may influence the parent as a provider and (2) qualities associated with sexual selection.
The good provider model
The condition of a parent may set an upper limit to how much care it can invest in the offspring. Parents in good condition might be able to breed earlier than parents in poor condition, and/or because of higher feeding effort, fledge well-nourished young. If the relative reproductive value of sons and daughters changes seasonally or with fledging mass, a parent may increase its fitness by adjusting the sex ratio of its offspring. Accordingly, we would expect to find a correlation between measures of sex ratio of offspring and the phenotypic qualities of their parents. I will refer to this explanation of sex ratio adjustments as the good provider model. In species with sexual size dimorphism, the larger gender consumes more food during development (Anderson et al. 1993) and is more likely to starve (Cronmiller & Thompson 1981; Røskaft & Slagsvold 1985). Unless otherwise stated, I assume below that the heavier sex is the most demanding for parents to raise (the costly sex) and according to the good provider model we would predict parents with above average resources to bias the production towards the heavier gender. For example, the feeding situation might be more favourable for high quality phenotypes, for females with high level of male assistance, or in certain years or times in the season when food supply is unusually abundant. It should be noted however, that the prediction of a skew towards the costly sex under favourable conditions is straightforward only when brood size is fixed (Myers 1978; McGinley 1984), otherwise parents may trade numbers of offspring against sex allocation.
The sexy mate model
There are numerous examples showing that females choose males according to the size or degree of ornamentation of phenotypic traits that do not correlate with how likely the males are to invest in parental care (Andersson 1994). Theories of sexual selection predict that females mated to attractive (ornamented) males can increase their fitness by producing more male offspring if these inherit their father’s attractiveness (Weatherhead & Robertson 1979; Burley 1981; Pomiankowski 1988). Similarly, females that for some reason must accept less than average ornamented males should do better by producing daughters. Moreover, females which are socially mated with poor quality males might seek extra-pair copulations from high quality males (e.g. Hasselquist et al. 1996), and in these cases we would expect such females to adjust their sex ratios towards sons. I will refer to this reason for sex ratio adjustment as the sexy mate model.
A special case of the sexy mate model is when sexually antagonistic selection pressures might be a reason for a female to adjust her offspring’s sex ratio in relation to the genotype of her partner (Merilä et al. 1997). For example, in species where large males and small females have higher fitness than small males and large females, and parent’s size is heritable, the combination of size and sex of the breeding pair may influence their offspring sex ratio. Large male - large female pairs are expected to produce sons and small male - small female pairs to produce daughters, in both situations the preferred offspring sex corresponds to the most fit parent (presumably being the most attractive). However, the adaptive sex ratios of pairs of dissimilar sizes is more complicated to calculate.
The two scenarios of sex ratio manipulations outlined above, i.e. the good provider and the sexy mate model, are not mutually exclusive and may act in concert. The two models propose a causal relationship between offspring sex ratios and parental quality, i.e. females manipulate their offspring sex ratio in relation to their own or their partner’s quality because this maximises their fitness. However, correlations between offspring sex ratios and parental quality might arise from non-causal relationships. For example, in many bird species, parents of high quality generally breed earlier than parents of poor quality (Price et al. 1988; Daan et al. 1988) and seasonal changes in offspring sex ratios have been detected in at least eight studies (Howe 1977; Dijkstra et al. 1990; Olsen & Cockburn 1991; Zijlstra et al. 1992; Tella et al. 1996; Daan et al. 1996; Lessells et al. 1996). Assuming that breeding time is determined by parental quality, and sex ratio is associated with breeding time because of a third variable that changes seasonally, the correlation between offspring sex ratio and parental quality might not be causal. In order to prove that there is an independent effect of parental quality in these systems, we have to (experimentally or statistically) examine the offspring sex ratios of simultaneously breeding parents of different quality. This has so far not been done.
CORRELATES OF PARENTAL QUALITY
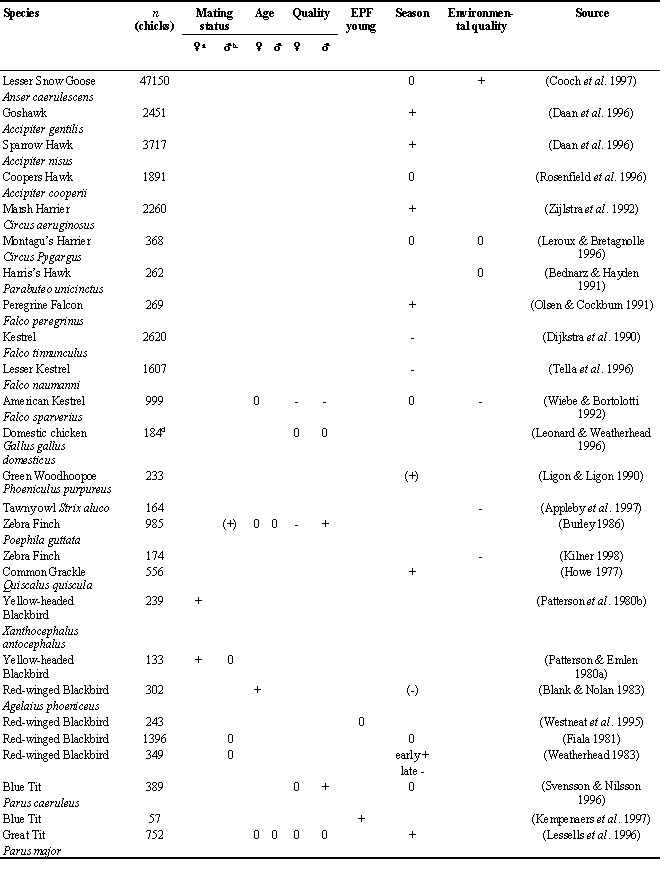
For the majority of published studies, offspring sex ratio was not the primary motivation for data collection, still less its possible relationship with parental quality. Hence, we have at hand several different measures of parental quality and in most studies only one or a few have been examined for relationships with offspring sex ratios. In the following I will examine different variables and categories of variables that should be correlated with parental quality and to what extent these have been found to associate with sex ratio adjustments. A complete list of the reviewed studies can be found in Appendix 1.
Parental age
Among females, age should be a good predictor of phenotypic quality as a provider because old females have gained experience from previous breeding (Yasukawa et al. 1990). In monogamous species, the same should hold for males. However, in polygynous species, old males might be poorer parents (in terms of nestling feeding) than young males, because they have larger harems and therefore spend more time courting females than caring for offspring (Smith 1995) or share the parental care that they do give between more broods. Hence, from the good provider model we would predict the production of the most costly sex to be positively associated with female age. Because males in many monogamous species take an equal share in parental care, we expect a similar association between offspring sex ratios and male age in these species. In contrast, the good provider model does not make any prediction regarding the association between offspring sex ratios and male age in polygynous species. In those species, the sexy mate model predicts that females mated to old males should skew their broods towards sons, because females would gain by producing sons if these inherit their father’s attractiveness.
Of nine studies examining offspring sex ratios in relation to the age of the mother only that of Red-winged Blackbirds Agelaius phoeniceus (Blank & Nolan 1983) found that old females produced a significantly higher proportion of sons than daughters. The other eight studies failed to find the suggested association (Burley 1986; Wiebe & Bortolotti 1992; Nishiumi et al. 1996; Koenig & Dickinson 1996; Lessells et al. 1996; Ellegren et al. 1996; Westerdahl et al. 1997; Bradbury et al. 1997). In males, five studies are available, none of which found any association between offspring sex ratios and male age (Burley 1986; Lessells et al. 1996; Ellegren et al. 1996; Westerdahl et al. 1997; Bradbury et al. 1997). Hence, with the exception of the Red-winged Blackbird study, parental age does not induce females to manipulate the sex ratio of their offspring.
Mating status
The mating status of females (primary, secondary, tertiary, etc.) often has a dramatic effect on their reproductive success (e.g. Alatalo et al. 1981). In polygynous mating systems, females can expect different amount of male assistance in relation to mating status, i.e. their rank position within the harem. In most species in which males provide at least some parental care, the primary females receive more help from the male than later-mated females (Alatalo et al. 1981; Urano 1990; Veiga 1990). According to the good provider model, females of primary status are expected to manipulate their brood sex ratios towards the most demanding sex, whereas secondary females are expected to bias their offspring sex ratio towards the less demanding sex. In contrast, the sexy mate model does not predict that primary and secondary females should produce different offspring sex ratios because these females are mated to the same male.
The association between sex ratios and female mating status has been examined in three polygynous species. In the Yellow-headed Blackbird Xanthocephalus xanthocephalus, primary females raised more sons than did lower ranked females (Patterson & Emlen 1980; Patterson et al. 1980). Recently, similar findings have been made in the Great Reed Warbler Acrocephalus arundinaceus (Westerdahl et al. unpubl.) and the Oriental Reed Warbler Acrocephalus orientalis (Nishiumi 1998). Hence, consistent with the good provider model, primary females seem to produce more sons than do lower-ranked (secondary) females. In these three studies, however, we cannot exclude higher mortality of male offspring in the nests of secondary females as an explanation for the skew. Since males are larger, they might need more nutrients and thus suffer more from lack of paternal assistance than their sisters (Weatherhead & Teather 1991; Slagsvold & Amundsen 1992).
The social mating status of males (monogamous, bigynous, trigynous, etc.) is a direct measure of the male’s success in obtaining females. In some systems, most of the variation in male mating success is explained by the resources that the male holds (territory quality) or are willing to give (parental quality), whereas in others, it reflects the males’ attractiveness as sires. Many systems probably involve a little of both. When polygyny is predominantly resource-driven, females of males with the largest harems will be nesting on better quality territories and might therefore have more food to give their nestlings (Orians 1969). Hence, according to the good provider model, we predict females mated to males with the largest harems to produce male-biased broods. In systems where male attractiveness per se is the cause of his mating success, the sexy mate model makes us predict that the proportion of sons in broods should be positively related to harem size. Therefore, irrespective of the cause of variation in mating success, (environmental quality or male quality) we would expect to find a positive relationship between offspring sex ratios and harem size. This has been examined in seven studies of six species; in five no relationship was reported (Patterson & Emlen 1980; Fiala 1981; Weatherhead 1983; Nishiumi et al. 1996; Hartley et al. 1998). In the Zebra Finch Poephila guttata ‘polygynous red-banded males produced a somewhat higher sex ratio than monogamous red-banded males’ (Burley 1986), but the relationship was not significant (P>0.3). In the Collared Flycatcher Ficedula albicollis, females mated with polygynous males did indeed produce more sons than females of monogamous males (P<0.01, Ellegren et al. 1996). In this species, polygynous and monogamous males do not differ in how much they feed nestlings at primary nests. Therefore, the result of Ellegren et al. (1996) could be interpreted as support for the sexy mate model. However, it could still be that females mated with polygynous males biased their offspring’s sex ratio towards the costly sex because of some unknown resource on these territories. To conclude, data on the relation between offspring sex ratios and male mating status provide conflicting results of whether sex ratio adjustments is operating in relation to paternal quality. In females, on the other hand, the observation of male-biased broods in nests of primary compared to secondary females is in support of the good provider model and speaks against the sexy mate model.
Phenotypic quality
Various measures of phenotypic quality have been used to explain variation in offspring sex ratios. The different measures of quality are either indirect measures, such as future survival rate (Svensson & Nilsson 1996), or direct measures, such as plumage characters (Ellegren et al. 1996) or body condition (Wiebe & Bortolotti 1992). According to the good provider model, we expect parents with high scores for these measures to manipulate their offspring sex ratio towards the costly sex and parents with low scores towards the less costly sex. The sexy mate model would make us predict that high quality fathers should be associated with sons and in species where sexual selection is equally intense in males and females, that high quality mothers should be associated with daughters. Because the costly sex is in most cases the male, the same relationship is expected for fathers from the good provider model and the sexy mate model, i.e. no matter the mechanism (sexually selected good genes or feeding) we would expect to find a positive relationship between offspring sex ratios and their fathers’ quality. On the other hand, high quality mothers should invest in males (the costly sex) if quality makes them good providers, but in daughters if they would inherit their mothers attractiveness as partners. However, because high quality males are often mated to high-quality females, conclusive predictions are not easily derived in such systems where the sexy mate model is operating equally strongly in both sexes.
Sex ratio adjustments in relation to sexually antagonistic selection pressures is not a separate hypothesis as it can be included under one of the two models outlined above, depending on whether the phenotypic variable is environmentally (good provider model) or genetically (sexy mate model) controlled. Olsen and Cockburn (1991), studying Peregrine Falcons Falco peregrinus (in which it is assumed that large females and small males are most fit), suggested antagonistic selection as the reason why early breeders (large females) produced daughters (the costly sex). If variation in body size within gender has a genetic basis, we should predict that small females would produce sons, particularly if mated with relatively small males and that, conversely, large females should favour daughters, especially if mated with large males. Size-assortative mating was not examined in the peregrine study but, in line with this argument, Wiebe & Bortolotti (1992) found that structurally (genetically) small American Kestrel F. sparverius females produced more sons.
The relationship between offspring sex ratios and one or more measurements of phenotypic quality has been investigated in eight species for both parents (Burley 1986; Wiebe & Bortolotti 1992; Nishiumi et al. 1996; Lessells et al. 1996; Ellegren et al. 1996; Svensson & Nilsson 1996; Leonard & Weatherhead 1996; Westerdahl et al. 1997) and in one additional species for mothers (Koenig & Dickinson 1996). If we score each study and sex for presence or absence of evidence for offspring sex ratio manipulation (17 examined relationships), in three cases high quality parents produced daughters and in three cases they produced sons, whereas the remaining eleven did not find any relationship. Below, I comment on some of the relationships found in these studies between offspring sex ratios and parental phenotypic quality.
In a study of the American Kestrel, Wiebe and Bortolotti (1992) found that the production of daughters was associated with high body condition of both parents. As in most raptors, female kestrels are larger than males and hence should be the costly sex. The mortality of eggs and young did not appear to have been responsible for the observed biased sex ratios.
In Blue Tits Parus caeruleus, males that survived the following winter had a higher proportion of sons in their broods than males that died (Svensson & Nilsson 1996). No such pattern was found in females. This observation of sex ratio adjustment in relation to male quality is strengthened by the finding of a larger variance in brood sex ratios than expected from a binomial distribution (Svensson & Nilsson 1996). In Blue Tits, a correlation between offspring sex ratios and male survival could come about either because males with high survival prospects were good providers, enticing females to adjust the offspring sex ratio towards males (males are 4% heavier than females), or because males with high survival sire sexually attractive sons. Unfortunately, no data are available of male nestling feeding effort in relation to their subsequent survival.
The male Collared Flycatcher has a white forehead patch that seems to function as a badge of status which is important for sexual selection (Gustafsson et al. 1995). Males with a large forehead patch have higher mating success and lifetime reproductive success than males with a small patch. In a set of analyses, Ellegren et al. (1996) found that females mated with males with large forehead patches biased the hatching sex ratio of their broods in favour of sons. To establish a causal link between male ornamentation and offspring sex ratios requires experimental manipulations. For this, Ellegren and coworkers made use of an experiment carried out in previous years which examined the effects of parental effort on viability and expression of secondary sexual characters (Gustafsson & Sutherland 1988; Gustafsson et al. 1995). Collared flycatcher parents were manipulated either to have two additional offspring or two offspring removed from their broods while a third group of birds acted as controls. This manipulation resulted in a change in the size of the male forehead patch in the following year with males experiencing a reduced work load developing larger white forehead patches (Gustafsson et al. 1995). Moreover, this manipulation also resulted in a difference in the proportion of sons among treatment groups, with a bias in favour of sons in males exposed to reduced work-loads (Ellegren et al. 1996). Manipulation of the work-load of females did not influence their subsequent sex ratios. That females adjusted brood sex ratios in relation to a male secondary sexual character, apparently not associated with any direct benefits, suggests that this sex ratio manipulation is best explained by the sexy mate model. However, because the forehead patch was not directly manipulated (only via work-load), the observed sex ratio adjustment might be in relation to a third character not measured, which was correlated to both work-load and forehead patch size.
One striking feature of studies of sex ratio adjustment is the relative paucity of experimental manipulations. One exception is the work of Burley (1981; 1986) of the Zebra Finch. These birds are easy to breed in aviaries and parental attractiveness can be manipulated by applying plastic rings of different colours. Burley (1986) showed that attractive males (ringed red) raised a higher proportion of sons than did unattractive males (ringed orange or green). Similarly, attractive females (ringed black) were associated with daughters (Burley 1986). In the case of the Zebra Finches, sex ratio adjustments seem to result from selective rejection of young during the first days after hatching rather than from primary sex ratio manipulations (Burley 1986 but see Clotfelter 1996).
Extra-pair paternity
In birds, with few exceptions (e.g. Hunter & Davis 1998), extra-pair copulations (EPCs) do not appear to bring direct benefits to females, in terms of care or access to additional resources (Birkhead & Møller 1992). Rather, evidence is accumulating that females seek extra-pair copulations from high quality males, presumably to acquire good genes for their offspring (Kempenaers et al. 1992; Hasselquist et al. 1996). Assuming that young with extra-pair paternity are sired by high quality males, the sexy mate model predicts that the sex ratio of such young should be biased in favour of sons. In contrast, the good provider model predicts that the sex ratio of young with extra-pair parents should not differ from that of their nest mates. In three studies of passerine species, there is no indication that extra-pair fertilized young were more male-biased than their half siblings, nor that broods containing young with extra-pair paternity were more male-biased than broods in which all young were legitimate (Westneat et al. 1995; Sheldon & Ellegren 1996; Westerdahl et al. 1997). In contrast, in Blue Tits (Kempenaers et al. 1997), extra-pair offspring were more likely to be males, consistent with the sexy mate model.
Environmental quality
According to the good provider model we would expect to find an excess production of the less costly sex during periods of food shortage whereas the reverse would be the case during favourable periods. This is not in conflict with Fisher’s (1930) predictions as long as the periods of good and poor conditions are short relative to individual life-span (Werren & Charnov 1978). Studies of Zebra Finches in the wild have shown that parents skew their offspring sex ratios towards sons when conditions are poor and towards daughters when conditions are favourable (Burley et al. 1989). This finding is nicely supported by an indoor experiment in which feeding conditions were manipulated. Parents biased their offspring sex ratio towards daughters when given excess food and towards sons when food was limited (Kilner 1998). In Zebra Finches, female fitness appears to be more sensitive to environmental quality than is male fitness (de Kogel 1997; Kilner 1998), thus making the observed sex ratio skew consistent with Trivers and Willard’s (1973) model. Similar results have recently been obtained for Tawny owls Strix aluco (Appleby et al. 1997). A higher proportion of daughters was born on territories with high prey abundance, and it was shown that prey abundance on natal territories later influenced female, but not male, reproductive success. Variation in food abundance between years seemed to influence the fledging sex ratio in lesser snow geese Anser caerulescens, with a slightly higher proportion of sons in good years (Cooch et al. 1997). In contrast, Leroux and Bretagnolle (1996), studying Montagu’s Harriers Circus pygargus, did not find a difference in sex ratios between years differing in prey abundance.
Green Woodhoopoes Phoeniculus purpureus with few helpers (low environmental potential) produce a bias of daughters, the less expensive sex (Ligon & Ligon 1990). However, daughters are better helpers so in this breeding system as in other co-operatively breeding birds, sex ratio manipulations may not be related to the fitness of offspring but to the future fitness of parents (Gowaty & Lennartz 1985). A study of the co-operatively breeding Harris’s Hawk Parabuteo unicinctus found no association between offspring sex ratios and prey abundance (Bednarz & Hayden 1991).
CONCLUSION AND PROSPECTS
Data accumulated so far on adaptive sex ratio manipulation in relation to parental quality are heterogeneous and sometimes contradictory. Another problem is the probable heavy bias towards negative results remaining unpublished. In order to attain a fuller understanding of this field the topic needs to be approached experimentally. Also, rather than testing the association between offspring sex ratios and a number of arbitrarily chosen variables, we need to know whether the relative success of sons and daughters varies with the candidate variable. For example, we have no reason to expect an association between offspring sex ratio and female age if the relative fitness of sons and daughters is the same for differently aged mothers.
Overall, offspring sex ratios seem to be related to female mating status in polygynous species, with females getting much male assistance producing more of the costly sex. On the other hand, offspring sex ratios seem not to be influenced by the age of either parent (irrespectively of mating system), which is surprising in the light of the results for female mating status, because old parents should be better providers. Similarly, direct and indirect measurements of phenotypic quality add only limited support for the hypothesis that sex ratio skew is associated with the parents’ quality as providers.
The relatively poor support for the good provider model might result from the fact that many supposed reasons to adjust offspring sex ratios may not be as adaptive as assumed. For example, it has been shown that individuals are more willing to invest in parental care if their mate is of high quality (Burley 1988). On the other hand, high quality individuals might lower their feeding effort because of their partner’s high feeding effort. Hence, observed feeding effort may not reveal the true quality or feeding capability of an individual but rather its motivation to invest. Because of this investment battle between the partners we may not be able to predict how sex ratios should be related to parental quality per se, only that we should expect offspring sex ratio manipulation towards the costly sex in situations where the total amount of food brought to the nest is higher than average. Furthermore, a parent’s optimal reproductive effort (RE) may not always equalise full numbers of young (Ricklefs 1968), e.g. a parent with a RE of 1.8 times the cost of producing one offspring, can only produce one offspring but would have extra resources (0.8) to invest in that offspring. One way to make use of this ‘fractional offspring’ is to invest in the more costly sex (McGinley 1984). Because the fractional problem increases with decreasing clutch size and poor quality parents have smaller clutches than high quality parents, it has been predicted that a larger fraction of the low quality parents should produce the costly sex (McGinley 1984), i.e. the opposite to the prevailing ideas. However, although it is clear that both the resources per offspring and sex ratios of individual broods, would be expected to vary more in small than in large broods, a systematic variation of either with brood size is not necessarily expected. There are no direct tests of the hypothesis outlined by McGinley (1984). One prediction is that offspring sex ratio should be correlated with clutch size. This has been found in Red-cockaded Woodpeckers Picoides borealis (Gowaty 1991) but not in other species (Lessells et al. 1996; Svensson & Nilsson 1996; Rosenfield et al. 1996; Westerdahl et al. 1997; Bradbury et al. 1997). By providing Great Tits with small and large nest-boxes, Slagsvold and Amundsen (1992) forced parents in small nest-boxes to reduce their clutch size and predicted that these should compensate by producing more (costly) sons. They found a non-significant difference in the expected direction.
Existing data on offspring sex ratio manipulation in relation to female mating status go against the predictions derived from the sexy mate model. If male quality was a reason to manipulate sex ratios, we would predict that primary and secondary females would produce similar sex ratios because these females are mated to the same male. Perhaps the most compelling evidence against the sexy mate model is that three out of four studies have found no indication that extra-pair young were biased in favour of males. One can argue that the reason why extra-pair young are not more often males than their half sibs, could be that females are incapable of separating the sperm from their social and extra-pair partner (Sheldon & Ellegren 1996). However, if females still tried to adjust sex ratios in such situations, it would result in broods containing extra-pair young (females that had engaged in extra-pair copulations) being more male biased than broods with only legitimate young.
Because quite a few studies have examined various aspects of proposed relationships between offspring sex ratios and parental quality (Appendix 1) one must consider the frequency of type one statistical errors, i.e. that some of the significant results stem from chance events. To answer this satisfactorily more data are needed. Rather than exploring new systems and variables, I hope that in the years to come, researchers will return to the species and variables for which the existing data indeed suggest that females are manipulating their offspring sex ratios in relation to their own or their partners quality.
One candidate variable is the relation between offspring sex ratios and female mating status reported in three studies of polygynous passerines. Repeat studies in other populations of the same or other polygynous species will show whether the pattern is general. Second, data on Blue Tits show both that the variance in sex ratio among broods is larger than predicted from a binomial distribution and that male future survival may explain this skew (Svensson & Nilsson 1996). In line with this evidence of sex ratio bias in relation to paternal quality are observations from another Blue Tit population; males surviving the subsequent winter obtain more extra-pair copulations (Kempenaers et al. 1992) and extra-pair young are more often males than females (Kempenaers et al. 1997). Because blue tits breed in nest-boxes, it would be relatively easy to repeat these studies. The third example is also a nest-box breeder, the Collared Flycatcher, for which there exists both correlative and semi-experimental support for sex ratio adjustments in relation to male quality. However, variance among broods was no larger than expected from a binomial distribution and extra-pair young were no more likely to be males (Sheldon & Ellegren 1996). Finally, studies of the Zebra Finch have provided consistent results of sex ratio modification to the female’s and her partner’s attractiveness (Burley 1986). On the other hand, the study involves a captive strain of birds, leaving the worry that the finding might be an idiosyncrasy of that breeding stock. Zebra Finches can be bred in aviaries in relatively large numbers and feeding conditions seem to influence sex allocation (Kilner 1998). Hence, further experiments on Zebra Finches, manipulating parental quality, has a potential to establish whether or not adaptive sex ratio manipulations are related to the quality of the parents as providers and/or to their attractiveness as mates.
ACKNOWLEDGMENTS
Thanks to I. Hartley, B. Kilner, and I. Nishiumi for letting me use papers in press. H. Källander, B. Hansson, C. M. Lessells, J. Quinn and H. Westerdahl provided useful comments on the manuscript. While writing this review I was economically supported by the Swedish Natural Science Research Council (NFR) and Lund University Travel Grants (Hans Emil Hansson’s and Seved Ribbings foundations) enabled me to attend the congress in Durban.
REFERENCES
Alatalo, R.V., Carlson, A., Lundberg, A. & Ulfstrand, S. 1981. The conflict between male polygamy and female monogamy: the case of the Pied Flycatcher Ficedula hypoleuca. American Naturalist 117: 738-753.
Altmann, J., Hausfater, G. & Altmann, S.A. 1988. Determinants of reproductive success in Savannah Baboons, Papio cynocephalus; Clutton-Brock TH, editors. Reproductive success. Chicago and London: The University of Chicago Press, p. 403-18.
Appleby, B.M., Petty, S.J., Blakey, J.K., Rainey, P. & MacDonald, D.W. 1997. Does variation of sex ratio enhance reproductive success of offspring in Tawny Owls (Strix aluco)? Proceedings of the Royal Society of London B, Biological Sciences 264: 1111-1116.
Anderson, D.J., Reeve, J., Martinez-Gomez, J.E., Weathers, W.W., Hutson, S., Cunningham, H.V. & Bird D.M. 1993. Sexual size dimorphism and food requirements of nestling birds. Canadian Journal of Zoology 71: 2541-2545.
Andersson, M. 1994. Sexual selection. Princeton, New Jersey: Princeton University Press.
Bednarz, J.C. & Hayden, T.J. 1991. Skewed brood sex ratio and sex-biased hatching sequence in Harris's Hawks. American Naturalist 137: 116-132.
Birkhead, T.R. & Møller, A.P. 1992. Sperm competition in birds: Evolutionary causes and consequences. London: Academic Press.
Blank, J. L. & Nolan, V. Jr. 1983. Offspring sex ratio in Red-winged Blackbirds is dependent on maternal age. Proceedings of the National Academy of Sciences of the USA 80: 6141-6145.
Bradbury, R.B., Cotton, P.A., Wright, J. & Griffiths, R. 1997. Nestling sex ratio in the European Starling Sturnus vulgaris. Journal of Avian Biology 28: 255-258.
Burley, N. 1981. Sex ratio manipulation and selection for attractiveness. Science 211: 721-722.
Burley, N. 1986. Sex-ratio manipulation in color-banded populations of Zebra Finches. Evolution 40: 1191-1206.
Burley, N. 1988. The differential-allocation hypothesis: an experimental test. American Naturalist 132: 611-628.
Burley, N., Zann, R. A., Tidemann, S.C. & Male, E.B. 1989. Sex-ratios of Zebra Finches. Emu 89: 83-92.
Clotfelter, E.D. 1996. Mechanisms of facultative sex-ratio variation in Zebra Finches (Taeniopygia guttata). Auk 113: 441-449.
Clutton-Brock, T.H., Albon, S.D. & Guinness, F.E. 1984. Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308: 358-360.
Cooch, E., Lank, D., Robertson, R. & Cooke, F. 1997. Effects of parental age and environmental change on offspring sex ratio in a precocial bird. Journal of Animal Ecology 66: 189-202.
Crawley, M.J. 1993. GLIM for ecologists. Oxford: Blackwell Scientific Publications.
Cronmiller, J.R. & Thompson, C.F. 1981. Sex-ratio adjustment in malnourished Red-winged Blackbirds broods. Journal of Field Ornithology 52: 65-67.
Daan, S., Dijkstra, C., Drent R. & Meijer, T. 1988. Food supply and the annual timing of avian reproduction. Acta XIX Congressus Internationalis Ornithologici, Volume 2, p. 392-407.
Daan, S., Dijkstra, C. & Weissing, F.J. 1996. An evolutionary explanation for seasonal trends in avian sex ratios. Behavioral Ecology 7: 426-430.
de Kogel, C. H. 1997. Long-term effects of brood size manipulations on morphological development and sex-specific mortality. Journal of Animal Ecology 66: 167-178.
Dijkstra, C., Daan, S. & Buker, J.B. 1990. Adaptive seasonal variation in the sex ratio of kestrel broods. Functional Ecology 4: 143-147.
Ellegren, H., Gustafsson, L. & Sheldon, B.C. 1996. Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. Proceedings of the National Academy of Sciences of the USA 93: 11723-11728.
Ellegren, H. & Sheldon, B.C. 1997. New tools for sex identification and the study of sex allocation in birds. Trends in Ecology and Evolution 12: 255-259.
Fiala, K.L. 1981. Sex ratio constancy in the Red-winged Blackbird. Evolution 35: 898-910.
Fisher, R.A. 1930. The genetical theory of natural selection. London: Oxford University Press.
Frank, S. A. 1990. Sex allocation theory for birds and mammals. Annual Review of Ecology and Systematics 21: 13-55.
Frank, S.A. & Swingland, I.R. 1988. Sex ratio under conditional sex expression. Journal of theoretical Biology 135: 415-418.
Gowaty, P.A. 1991. Facultative manipulation of sex ratios in birds. Rare or rarely observed? Current Ornithology 8: 141-171.
Gowaty, P.A. & Lennartz, M.R. 1985. Sex ratios of nestling and fledging Red-cockaded Woodpeckers (Picoides borealis) favor males. American Naturalist 126: 347-353.
Griffiths, R., Daan, S. & Dijkstra, C. 1996. Sex identification in birds using two CHD genes. Proceedings of the Royal Society of London B, Biological Sciences 263: 1251-1256.
Griffiths, R. & Tiwari, B. 1993. The isolation of molecular genetic markers for the identification of sex. Proceedings of the National Academy of Sciences of the USA 90: 8324-8326.
Gustafsson, L., Qvarnström, A. & Sheldon, B.C. 1995. Trade-offs between life-history traits and secondary sexual character in male Collared Flycatchers. Nature 375: 311-313.
Gustafsson, L. & Sutherland, W.J. 1988. The cost of reproduction in the Collared Flycatcher. Nature 335: 813-817.
Hartley, I.R., Griffith, S., Wilson, K., Shepherd, M. & Burke, T.. 1998. Nestling sex ratios in the polygynously breeding Corn Bunting Miliaria calandra. Journal of Avian Biology [In Press]
Hasselquist, D., Bensch, S. & von Schantz, T. 1996. Correlation between male song repertoire, extra-pair paternity and offspring survival in the Great Reed Warbler. Nature 381: 229-232.
Howe, H.F. 1977. Sex ratio adjustment in the Common Grackle. Science 198: 744-747.
Hunter, F.M. & Davis, L.S. 1998. Female Adelie Penguins acquire nest material from extrapair males after engaging in extrapair copulations. Auk 115: 526-528.
Kempenaers, B., Verheyen, G.R. & Dhondt, A.A. 1997. Extrapair paternity in the Blue Tit (Parus caeruleus): female choice, male characteristics, and offspring quality. Behavioral Ecology 8: 481-492.
Kempenaers, B., Verheyen, G.R., Van den Broeck, M., Burke, T., Van Broeckhoven, C. & Dhondt A.A. 1992. Extra-pair paternity results from female preference for high-quality males in the Blue Tit. Nature 357: 494-496.
Kilner, R. 1998. Primary and secondary sex ratio manipulation by Zebra Finches. Animal Behaviour 56: 155-164.
Koenig, W.D. & Dickinson, J.L. 1996. Nestling sex-ratio variation in Western Bluebirds. Auk 113: 902-910.
Komdeur, J., Daan, S., Tinbergen, J. & Mateman, A.C. 1997. Extreme adaptive modification in sex ratio of the Seychelles Warbler's eggs. Nature 385: 522-525.
Krackow, S. 1995. Potential mechanisms for sex ratio adjustment in mammals and birds. Biological Reviews 70: 225-241.
Leimar, O. 1996. Life-history analysis of the Trivers and Willard sex-ratio problem. Behavioral Ecology 7: 316-325.
Leonard, M.L. & Weatherhead, P.J. 1996. Dominance rank and offspring sex ratios in domestic fowl. Animal Behaviour 51: 725-731.
Leroux, A. & Bretagnolle, V. 1996. Sex ratio variations in broods of Montagu's Harriers Circus pygargus. Journal of Avian Biology 27: 63-69.
Lessells, C.M., Mateman, A.C. & Visser, J. 1996. Great Tit hatchling sex ratios. Journal of Avian Biology 27: 135-142.
Ligon, J.D. & Ligon, S.H. 1990. Female-biased sex ratio at hatching in the Green Wood Hoopoe. Auk 107: 765-771.
Maynard Smith, J. 1980. A new theory of sexual investment. Behavioral Ecology and Sociobiology 7: 247-251.
McGinley, M.A. 1984. The adaptive value of male-biased sex ratios among stressed animals. American Naturalist, 124: 597-599.
Merilä, J., Sheldon, B.C. & Ellegren, H. 1997. Antagonistic natural selection revealed by molecular sex identification of nestling Collared Flycatchers. Molecular Ecology 6: 1167-1175.
Myers, J.H. 1978. Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? American Naturalist 112: 381-388.
Nishiumi, I. 1998. Brood sex ratio is dependent on female mating status in polygynous Great Reed Warblers. Behavioral Ecology and Sociobiology 44: 9-14.
Nishiumi, I., Yamagishi, S., Maekawa, H. & Shimoda, C. 1996. Paternal expenditure is related to brood sex ratio in polygynous Great Reed Warblers. Behavioral Ecology and Sociobiology 39: 211-217.
Olsen, P.D. & Cockburn, A. 1991. Female-biased sex allocation in Peregrine Falcons and other raptors. Behavioral Ecology and Sociobiology 28: 417-424.
Orians G. H. 1969. On the evolution of mating systems in birds and mammals. American Naturalist 103: 589-603.
Patterson, C.B. & Emlen J.M. 1980. Variation in nestling sex ratios in the Yellow-headed Blackbird. American Naturalist 115: 743-747.
Patterson, C.B., Erckman, W.J. & Orians, G.H. 1980. An experimental study of parental investment and polygyny in male blackbirds. American Naturalist 116: 757-769.
Pomiankowski A. N. 1988. The evolution of female mating preferences for male genetic quality. Oxford Surveys in Evolutionary Biology 5: 136-184.
Price T., Kirkpatrick, M. & Arnold, S.J. 1988. Directional selection and the evolution of breeding date in birds. Science 240: 798-799.
Quinn, T.W. 1999. Sex and the single chromosome. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 434-449. Johannesburg: BirdLife South Africa.
Ricklefs R.E. 1968. On the limitation of brood size in passerine birds by the ability of the parents to nourish their young. Proceedings of the National Academy of Sciences of the USA 61: 847-851.
Rosenfield, R.N., Bielefeldt, J. & Vos S.M. 1996. Skewed sex ratios in Cooper's Hawk offspring. Auk 113: 957-960.
Røskaft, E. & Slagsvold, T. 1985. Differential mortality of male and female offspring in experimentally manipulated broods of the rook. Journal of Animal Ecology 54: 261-266.
Sheldon, B.C. & Ellegren, H. 1996. Offspring sex and paternity in the Collared Flycatcher. Proceedings of the Royal Society of London B, Biological Sciences 263: 1017-1021.
Slagsvold, T. & Amundsen, T. 1992. Do Great Tits adjust hatching spread egg size and offspring sex ratio to changes in clutch size? Journal of Animal Ecology 61: 249-258.
Smith H.G. 1995. Experimental demonstration of a trade-off between mate attraction and parental care. Proceedings of the Royal Society of London B, Biological Sciences 260: 45-51.
Svensson, E. & Nilsson, J.-Å. 1996. Mate quality affects offspring sex ratio in Blue Tits. Proceedings of the Royal Society of London B, Biological Sciences 263: 357-361.
Tella, J.L., Donazar, J.A., Negro, J.J. & Hiraldo, F. 1996. Seasonal and interannual variations in the sex-ratio of Lesser Kestrels Falco naumanni broods. Ibis 138: 342-345.
Trivers, R.L. & Willard, D.E. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179: 90-92.
Urano, E. 1990. Factors affecting the cost of polygynous breeding for female Great Reed Warblers Acrocephalus arundinaceus. Ibis, 132: 584-594.
Veiga, J.P. 1990. Infanticide by male and female House Sparrows. Animal Behaviour 39: 496-502.
Weatherhead, P.J. 1983. Secondary sex ratio adjustment in Red-winged Blackbirds (Agelaius phoeniceus). Behavioral Ecology and Sociobiology 12: 57-61.
Weatherhead, P.J. & Robertson, R.J. 1979. Offspring quality and the polygyny threshold: ‘the sexy son hypothesis’. American Naturalist 113: 201-208.
Weatherhead, P.J. & Teather, K.L. 1991. Are skewed fledgling sex ratios in sexually dimorphic birds adaptive? American Naturalist 138: 1159-1172.
Werren, J.H. & Charnov, E.L. 1978. Facultative sex ratios and population dynamics. Nature 272: 349-350.
Westerdahl, H., Bensch, S., Hansson, B., Hasselquist, D. & von Schantz, T. 1997. Sex ratio variation among broods of Great Reed Warblers Acrocephalus arundinaceus. Molecular Ecology 6: 543-548.
Westneat, D.F., Clark, A.B. & Rambo, K.C. 1995. Within-brood patterns of paternity and paternal behavior in Red-winged Blackbirds. Behavioral Ecology and Sociobiology 37: 349-356.
Wiebe, K.L. & Bortolotti, G.R. 1992. Facultative sex ratio manipulation in American kestrels. Behavioral Ecology and Sociobiology 30: 379-386.
Williams, G.C. 1979. The question of adaptive sex ratio in outcrossed vertebrates. Proceedings of the Royal Society of London B, Biological Sciences 205: 567-580.
Yasukawa, K., McClure, J.L., Boley, R.A. & Zanocco, J. 1990. Provisioning of nestlings by male and female Red-winged Blackbirds, Agelaius phoeniceus. Animal Behaviour 40: 153-166.
Zijlstra, M., Daan, S. & Bruinenberg-Rinsma, J. 1992. Seasonal variation in the sex ratio of Marsh Harrier Circus aeruginosus broods. Functional Ecology 6: 553-559.
Appendix 1. Studies examining offspring sex ratios in relation to variables associated with parental quality.

