S08.2: Sex and the single chromosome
Thomas W. Quinn
Department of Biological Sciences, University of Denver, Denver, Colorado, 80208, USA, fax 303 71-3466, e-mail tquinn@du.edu
Quinn, Thomas W. 1999. Sex and the single chromosome. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
434-449. Johannesburg: BirdLife South Africa.Gender identification is difficult in many bird species because of extreme morphological similarity between males and females. In recent years many new methods that detect DNA from the female-specific W chromosome have been developed. These often can be performed with relatively little technical expertise or equipment needs. Some methods target repetitive sequences, others target non-repetitive DNA on that chromosome. Several methods involve amplification of template material using PCR, making them applicable to source material with very small amounts of DNA present. Some of the more promising approaches are compared in this review.
INTRODUCTION
For many years there has been a strong interest in the development of methods that can be used to determine gender in birds because for a large fraction of all avian species, morphological characters are unreliable. This fraction is still higher if only the young are considered. Some zoos and veterinary practices employ surgical endoscopy on a routine basis, but this technique carries various risks related to its invasive nature and requires that birds be mature (Harrison 1978; Jones et al. 1984; Prus and Schmutz 1987). Various biochemical (e.g. Bercobitz et al. 1978; Baverstock et al. 1982; Lacson and Morizot 1988; Tell and Lasley 1991) and immunological (e.g. Wachtel et al. 1983) methods have also shown promise for gender identification, although their ease of application to diverse species has not been tested, and all cannot be used with confidence for all ages or breeding states of the subject (Scherr 1986; Prus and Schmutz 1987; Nakamura et al. 1990). Hildebrandt et al. (1995) have described the use of ultrasonography for sex identification in raptors, but again, described applications have been taxonomically narrow.
In birds, the female is the heterogametic sex. The sex-specific chromosome is termed the W chromosome and the other sex chromosome, the Z chromosome. Female genotypes are ZW and males are ZZ. Because the W chromosome is generally much smaller than the Z chromosome, cytogenetic approaches can provide a general approach for gender identification. However, the time and expertise required preclude their general application for this purpose, despite the vital role that these techniques have played in providing an overall understanding of the avian genome. Furthermore, their accuracy relative to surgical techniques has been questioned in some taxonomic groups (Prus and Schmutz 1987).
Another approach that takes advantage of the size difference between the W and Z chromosomes is flow cytometry. Flow cytometry uses an argon ion laser to measure DNA content of large numbers of cell nuclei that have been stained with propidium iodide. It has been used with success on 29 species from seven orders with an error rate of less than 1% (Nakamura et al. 1990). While Redelman et al., (1997) have argued that flow cytometry ‘ is clearly less laborious and time-consuming than the PCR-based procedures’ , this depends on the number of species, number and variety of tissue samples, and the relative availability of equipment and expertise, which will collectively usually favour PCR-based methods. Interested readers should consider the additional discussion of this issue by Ellegren and Sheldon (1997b).
In recent years, with the development of molecular biological tools, a great amount of information has been gathered about the avian genome at the molecular level. This has led to new approaches to avian gender identification. This review specifically focuses on those molecular methods. To avoid confusion, I will use the term ‘gender identification’ to indicate how one can differentiate the male sex from the female sex, and reserve the term ‘sex determination’ to describe the genetic (or environmental) mechanism(s) that trigger their development.
SEX DETERMINATION IN BIRDS
In mammals, it has been shown that a single sex-determining gene (SRY) encoded on the Y chromosome is responsible for triggering the genital ridge to differentiate into testes rather than ovaries during embryonic development (Gubbay et al. 1990; Sinclair et al. 1990; but see Fredga (1994) for an unusual exception). This step is generally viewed as the critical switch that determines maleness (presence of testes). By analogy, it seems possible that birds might have a gene on the female-specific W chromosome that triggers a cascade that leads to the development of ovaries. Tiersch et al., (1991) and Griffiths (1991) studied sequences homologous to SRY in birds, but they were not female-specific. Whether another gene will be found that fulfils that role remains unknown.
Alternatively, birds might have a sex-determination system that is conceptually more similar to that found in Drosophila where the ratio of the X chromosome to autosomes is thought to be critical (Bridges 1921; Cline 1993). By analogy, if ZZW karyotypes were found to exist in phenotypically male birds and/or ZO karyotypes were found in females, this would provide support for such a system (Mittwoch 1996). While Abdel-Hameed and Shoffner (1971) described triploid Chickens (3A, ZZW; Gallus gallus) with testis-like gonads, suggesting that the W chromosome is not strongly female-determining, Bloom (1974) points out that a better test would be in birds with a normal diploid autosomal complement. A single report of a diploid ZZW rooster exists (Crew 1933; see also Sittmann 1984) seemingly fulfilling that criterion, but the karyotype of this individual was inferred indirectly and the study has not been considered conclusive.
In animals with male heterogamy, females possess two X chromosomes while males have only one. Presumably to keep the gene products of the X chromosome in equal proportion in the two sexes, one of the female X chromosomes is typically inactivated (Lyon, 1989). In birds, the analogous Z chromosomal inactivation does not take place in males (Cock 1964; Baverstock et al. 1982). Chandra (1994) has used this difference in a third hypothesis by proposing that one or more genes involved in sex determination are carried on the Z chromosome (and on the W chromosome where they are presumed to be inactive). When two chromosomal gene copies are expressed (ZZ), testes develop, and when just one is expressed (ZW), ovaries result.
Regardless of which sex determination system(s) is used within Aves, W chromosomes are normally found only in females.
CHARACTERISTICS OF THE AVIAN W CHROMOSOME
While the Z chromosome is typically the fourth or fifth largest chromosome in avian karyotypes and comprises approximately 8-9% of the chromatin, the W chromosome is usually smaller, in the range of the 6th to 10th largest chromosome pair (Krishan et al. 1965; Shields et al. 1982). The DNA content of the Chicken W chromosome has been estimated as 1.4% of the entire genome based on the relative lengths of mitotic chromosomes (Saitoh et al. 1991) and as 2.3% based on synaptonemal complex relative lengths (Solari 1977). Like the mammalian Y chromosome, it is highly heterochromatic and late replicating (Schmid 1962; Galton and Bredbury 1966; Stefos and Arrighi 1971). An exceptional review of early cytogenetic and biochemical studies of the avian sex chromosomes is provided by Bloom (1974) (see also Bloom et al. 1993).
Some of the most detailed molecular studies of the W chromosome were presented in a related series of papers by Tone et al. (1984), Kodama et al. (1987), Saitoh et al. (1991) and Saitoh and Mizuno (1992), again focusing primarily on the domestic Chicken genome. Collectively, these studies showed that approximately 65% of the W chromosome contains two related tandemly repeated sequences, the XhoI and EcoRI families. The two repeat families are located in separate domains on the W chromosome.
Detailed cytogenetic studies by Rahn and Solari (1986) and by Solari (1992) suggest that true homologous pairing between the W and Z chromosomes is restricted to a small telomeric region. The consistent observation of a recombination nodule in this region makes it likely that crossing over is frequent or obligatory and hence that this represents a ‘pseudoautosomal region’ (Solari et al. 1988). Genes located in such regions are difficult to distinguish by their patterns of mendelian inheritance from typical autosomal genes. Rather, it is those areas that do not recombine with any regularity between the W and Z chromosomes, called the ‘differential region’, that have diverged significantly between the sex chromosomes and can therefore provide material that is potentially useful for gender identification.
THE USE OF MOLECULAR GENETIC METHODS FOR GENDER IDENTIFICATION
All molecular techniques for sexing birds require a sample of DNA. In principle, this could be extracted from any tissue, but the fact that birds (unlike mammals) have nucleated red blood cells means that large amounts of high molecular weight DNA can be extracted from relatively small blood samples (e.g. 40 mg DNA from 50 ml blood; Quinn and White 1987). This has been invaluable because the earliest molecular sexing techniques required considerable amounts of DNA.
More recent techniques have been based on the polymerase chain reaction (PCR, Saiki et al. 1985). Because PCR is an efficient way to make many copies from a small amount of the original organismal DNA (template), the need for large amounts of extracted DNA has been eliminated for many types of DNA-based study, including gender identification. This means that sufficient DNA can now be obtained from feathers (Smith et al. 1991; Ellegren 1992), and even from museum specimens (Ellegren 1991; Cooper et al. 1992). PCR relies on a pair of single-stranded short oligonuclotide DNA ‘primers’ that can be synthesised such that they bind by base complementation to the template at specific locations that flank a region of interest. The reaction itself consists of three steps that are repeated many times. First, double-stranded template DNA (previously extracted from the specimen of interest) is heated to approximately 94o C to ‘unzip’ the complementary strands into a denatured single-stranded state. As the reaction mixture is cooled (typically to 50 to 65o C) the synthetic primers anneal to the template DNA by base complementation. In the last part of the cycle, the temperature is raised to a temperature that is optimal for DNA polymerase to synthesise copies of the original template DNA, starting at the bound primers. By repeating this cycle 30 or more times, the area flanked by the primers (theoretically) doubles in number each cycle, to yield an enormous number of target copies. The simplicity, efficiency and specificity of the technique has led to its widespread use by both ‘specialists’ and ‘nonspecialists’.
Membrane-based techniques
Many molecular sexing techniques involve binding the DNA to a nitrocellulose or nylon membrane. Usually the sample DNA is ‘digested’ with a restriction endonuclease, which cuts the DNA where specific base pair sequences occur, and the fragments of DNA are then separated according to size using electrophoresis. The resultant pattern of DNA fragments is transferred from the gel onto a more convenient and durable membrane by ‘Southern blotting’. The presence of DNA fragments containing particular DNA sequences can then be detected by probing the membrane with purified single-stranded DNA, which will ‘hybridise’ to the membrane-bound DNA wherever the base sequences are complementary. The position of DNA fragments where such hybridisation has occurred can be determined because the probe DNA is labelled. For instance, if the probe is radioactively labelled, an X-ray film will show the position of DNA fragments where hybridisation has occurred as a series of dark bands. If the DNA contained in the band originated on the W chromosome, the band will occur in DNA samples from females only, and hence provide a means of gender identification. ‘Dot blots’ can also be used to immobilise sample DNA on membranes. With this method high numbers of purified DNA samples can be transferred directly onto the membrane (typically in a grid pattern) without any intervening electrophoretic size fractionation. When hybridised with a W-specific probe, only female ‘dots’ will result in dark spots on the final X-ray.
In practice, many probes from the avian W chromosome hybridise to other areas in the genome, especially the Z chromosome, making interpretation of the resultant patterns more complex. The Z chromosomal homology could reflect areas that were common to both sex chromosomes prior to their evolutionary differentiation, or there may be occasional translocations between the sex chromosomes.
Some of the earliest discoveries of avian female-specific DNA fragments were fortuitous, coming about as researchers noticed female-specific bands on X-rays that had been prepared for ‘other purposes’ (such as DNA fingerprinting or restriction fragment length polymorphism analysis). Other discoveries were made more intentionally, as researchers applied methods designed to increase the prevalence of W-specific material.
Repetitive probes
The presence of repetitive DNA sequences on the avian W chromosome was originally suspected because the chromosome was known to be highly heterochromatic in somatic cells, and highly repetitive sequences had been shown to be associated with heterochromatin (John and Miklos 1979). Singh et al. (1976) used a type of centrifugation that separates DNA on the basis of density to detect the presence of a ‘satellite’ band in female Chicken DNA that flanked the major genomic DNA band and was absent (or less apparent) in male-derived DNA. This too is indicative of repetitive DNA sequences which are often of a different average density than other genomic DNA. None of the methods that use repetitive DNA to detect the W chromosome have proven to be effective across taxonomically distant species. More recent molecular biological studies have allowed more direct characterisation of the W-chromosomal repetitive sequences, especially in Chickens.
Tone et al. (1982) discovered that when the restriction enzyme XhoI was used to cut Chicken genomic DNA samples, electrophoretic separation by size on agarose gels revealed two prominent bands superimposed upon the background genomic smear in female but not in male samples. This results from repetitive sequences that are in high copy number in the genome. When a restriction enzyme is used that has a recognition sequence within a repeat sequence, a large number of fragments of equal size are generated. Similar findings have been reported in the Lesser Black-backed Gull (Larus fuscus) and the Herring Gull (Larus argentatus; Griffiths and Holland 1990).
Uryu et al. (1988), and Griffiths and Holland (1990) were able to use repeat sequences to devise a highly efficient assay for avian gender identification. Both groups used a standard ‘dot blot’ apparatus to transfer genomic DNA from male and female samples to discrete spots on a nylon membrane and later hybridised cloned and labelled repeat DNA to the membrane. Because of the high copy number of each repeat on the W chromosome relative to the rest of the genome, intense hybridization to female samples and weak or negligible hybridization to male samples was observed. While the taxonomic range of sex-specific detection was narrow in both studies, the detection format itself is of general interest, as it would be a very efficient assay to use on large numbers of samples because electrophoretic fractionation is not needed. Uryu et al. (1988) also showed that blood cells could be lysed and the lysate transferred to a membrane directly, without the need for DNA purification.
Another type of repeat sequence that has led to the detection of W-specific bands are those visualised in DNA fingerprinting studies. DNA fingerprinting began when Jeffreys et al. (1985) discovered that a related set of repeat sequences are found scattered throughout the genome. Typically, they occur in disjunct locations as tandem repeats. The fact that each ‘island’ of tandem repeats contains related sequences provides a way that all can be detected simultaneously, and hence a way that several locations within the genome can be studied simultaneously. This is done by digesting genomic DNA samples with restriction enzymes that lack recognition sequences within the repeat islands (so that most islands remain ‘intact’) then electrophoresing the cut DNA samples on agarose gels. Southern blots of these gels can then be hybridised with a labelled probe that includes the core sequences, to reveal complex bands distributed along each lane. In birds, DNA fingerprinting is often used to study mating behavior. Because the location of the repeat islands is somewhat random, on occasion they are found on the W chromosome and are detected as bands that are present in females but absent in males. Rabenold et al. (1991a) discovered such a pattern in a DNA fingerprinting study of Stripe-backed Wrens (Campylorhynchus nuchalis). They subsequently made use of these markers to better understand maternal patterns of dispersal (Rabenold et al. 1991b). Longmire et al. (1991) made a similar discovery of a W-linked marker in Peregrine Falcons (Falco peregrinus), as did Graves et al. (1993) in the Shag (Phalacrocorax aristotelis) and Millar et al. (1997) in the South Polar Skua (Stercorarius maccormicki). Again, the taxonomic range of detection of W-specific islands was narrow or untested for each of these studies. Longmire et al. (1993) has also reported detection of microsatellite repeats (tandem repeats composed of units just a few nucleotides long) on the W chromosome.
Rather than using membrane-bound DNA samples as described above, methods that use PCR primers located within a repeat have also been developed. Such assays allow increased sensitivity of detection, as illustrated by Petitte and Kegelmeyer (1995) who used PCR primers internal to the Chicken XhoI repeats to determine the sex of Chicken embryos at a very early stage of development. They were able to use as few as two cells in their determinations. Simkiss et al. (1996) developed similar primers within the EcoRI repeat of Chicken so that they could track the fate of female cells in male germline chimaeras.
Overall, repetitive sequences have led to some highly efficient methods for gender identification of specific species and their close relatives. However, they typically need to be ‘rediscovered’ for each new taxonomic group, due to the rapid rate of evolutionarily change that is typical of such regions (but see Jones and Singh (1985) for an exception in snakes).
Non-repetitive probes
While repetitive sequences seem to predominate on the W chromosome in those few species characterised thus far, W-chromosomal sequences that are not repeated more than a few times within the genome have also being discovered and used for sex identification. Of these, most detect one or more additional related sequences that are also located on another chromosome within the avian genome.
Quinn et al. (1990) cloned a piece of the Z chromosome that contains sequences related to those also found in a small section of the W chromosome in Snow Geese (Anser caerulescens). Hybridization of this clone to a Southern blot that included both male and female genomic DNA samples digested with the restriction enzyme TaqI, revealed one band that was only present in females (a W chromosomal piece) and several other bands that were present in both males and females (Z chromosomal pieces). There was a 98% concordance between the presence of the one band in adult females and its absence in adult males (n=83), using cloacal eversion to determine gender. Because the error rate of cloacal sexing of Snow Geese in the field is approximately 2%, it is probable that most or all of the nonconcordance was due to those errors rather than a molecular ‘mismatch’. The other detected bands were shown to be Z-chromosomal based on their intensity and patterns of polymorphism.
Because Quinn et al. (1990) isolated their clone from a collection of clones prepared randomly from the entire genome, it is probably noncoding, as most of the avian genome does not code for protein or RNA products. In contrast, Dvorak et al. (1992) began with a set of clones that were specifically isolated from coding regions, using RNA extracted from turkey embryos in their third to fifth day of development. From 92 clones screened, three detected Z-linked fragments and one detected both Z and W-linked fragments (and perhaps autosomal fragments as well). This clone could also be used to determine gender. While the taxonomic range of the probe isolated by Quinn et al. (1990) was not tested, that isolated by Dvorak et al. (1992) could be used in other Galliformes, but was not tested outside of that order.
In an elegant series of experiments, Ogawa et al. (1997) used a laser microbeam to obliterate all but a single W chromosome on a Chicken metaphase preparation, and used PCR to amplify and clone the resultant DNA. From this material, they have isolated several clones that hybridise only to female-specific DNA. They further isolated and characterised one large (25,000 base pair) non-repetitive piece of DNA from that chromosome, and found within it, a segment that is highly conserved across Carinatae birds. They tested this by probing a Southern blot containing male and female samples from 18 species representing 8 different orders. Of these, the gender of all but one could be determined by a female-specific band. In some species there was also hybridization to weaker bands common to both sexes that are probably Z chromosomal, as they are approximately twice the intensity in males versus females.
A fourth non-repetitive region that is present on both the W and Z chromosomes is called the chromodomain-helicase-DNA-binding (CHD) gene. This gene is remarkable because it is evolutionarily highly conserved at the DNA sequence level among distantly related vertebrates (and invertebrates). Because of its association with the sex chromosomes in birds, it has proven especially important in the development of near-universal methods for avian gender identification, as discussed in more detail below.
As for repetitive DNA sequences, some of the non-repetitive DNA sequences mentioned in this section have been used to develop PCR-based assays that no longer require the binding of sample DNA to membranes. For example, Ogawa et al. (1997) developed a primer pair that can be used for gender identification in various ways. In some species, a single female-specific PCR product is seen. In others, there were intensity differences between single male and female products. Samples of a third set of species generated a single female-specific band plus one or more additional bands shared between the sexes. Primers have also been developed for CHD, and these are described below.
Unlike repetitive sequences, there appear to be high levels of sequence conservation across large taxonomic distances with some of the clones that have been isolated from non-repetitive sequences on the W chromosome. Such conservation is typically associated with coding regions, and is very important for the development of universally applicable methods for gender identification, as the requirement for rediscovering W-specific material for each major taxonomic group is removed.
Techniques based on RAPD primers
Several W chromosomal segments of DNA have been isolated using a recently described PCR-based method (Williams et al. 1990) that requires relatively little technical expertise and/or specialised equipment. This technique has been given the acronym ‘random amplified polymorphic DNA’ (RAPD). It uses randomly chosen short (usually 10 bases long) primers rather than primers designed to amplify specific DNA sequences. Because they are short, specificity is reduced, and the primers anneal at a large number of locations on a given chromosome simultaneously. These primers often amplify sections of DNA that are polymorphic in terms of both size and their presence or absence in different individuals. Using a variety of primers with different combinations of bases, an enormous number of genomic regions can be sampled with little or no prior characterisation of the genome being studied.
By comparing RAPDs from a male bird with those from a female bird it is often possible to identify W-chromosomal amplification products by their presence in females and absence in males. A potential difficulty with this approach is that many of the differences between any two individuals could be due to autosomal (or Z chromosomal) polymorphisms rather than differences originating from the presence or absence of the W chromosome. To circumvent this potential problem, DNA from several individuals of the same sex can be pooled and used as a template so that most of the common polymorphisms present in a population will be amplified within each pool, an approach called ‘bulked segregant analysis’ (Michelmore et al. 1991). Thus, when applied to gender identification in birds, several male samples could be combined to form one pool and several female samples combined to form another. Both pools should contain most polymorphic variants, thereby sharing many identically sized products when compared by electrophoresis. Those bands that are found only in the female pools are now more likely to originate from W chromosomal products rather than autosomal or Z chromosomal polymorphisms. However, as Lessells and Mateman (1998) point out, other factors should be taken into account. Since females are expected to have sex-specific bands, using just a single female minimises the number of polymorphic bands generated while the number of bands related to the W chromosome remain the same. False positives (female-specific bands that are due to polymorphisms rather than due to W-specific amplification) can still be identified by pooling several male samples. However, this format is also problematic if there is any doubt concerning the original identification of sex in the samples being used, or if samples are mixed up during experimental manipulations. Particularly difficult would be the unknown presence of female DNA within the male DNA pool, as this would prevent the isolation of W-specific material regardless of the number of experiments performed. These factors should all be considered by anyone embarking on such an approach to isolation of W-specific material.
Griffiths and Tiwari (1993) used bulked segregant analysis to generate a female-specific fragment in each of three species of passerine after screening twenty 10-mers and sixteen 23-mers that had been designed for other purposes. When the female-specific bands were isolated, radioactively labelled, and used as probes to hybridise with Southern blots containing restriction digested genomic DNA, all detected a female-specific band in addition to other bands shared between the sexes. Sabo et al. (1994) used the same approach to amplify a female-specific fragment in Roseate Terns (Sterna dougallii). In total, 180 10-mer primers were screened against male and female pools, each including DNA from 12 individuals. A total of 1,400 ‘readable’ bands were recorded, and six primers amplified an extra band in the female pool. Of these, W- linkage of four was contradicted when screening 12 individual males and 12 individual females, but two remained consistent with W-linkage. One of these was cloned and sequenced. By sequencing the W-specific band, Sabo et al. (1994) were able to design primers that would amplify material from female samples but not from male samples (termed a sequence-characterised amplified region or ‘SCAR’ (Paran and Michelmore 1992)). A benefit of this additional step is that the primers become more specific with their increased length. A potential drawback is that templates producing no amplification product could either have come from a male individual or represent a failed PCR reaction. To control for this, autosomal primers could be included in a ‘multiplex’ PCR reaction, or, as Sabo et al. (1994) suggest, the annealing temperature could be lowered enough to produce nonspecific autosomal (or Z chromosomal) bands, although the practicality of this for a wide variety of primers has yet to be tested. It may be preferable to simply use the original 10-mer primer for gender identification provided one is confident in the reproducibility of the reaction conditions. Nonetheless, conversion to SCARs does create additional capabilities related to genomic characterisation .
The most intensive use of RAPD primers for the production of avian female-specific markers was done by Lessells and Mateman (1998) who successfully identified female-specific material in seven of the ten species that were screened with a maximum of 69 different 10-mer primers (see also Lessells et al. 1996). They noted that primers that detect female-specific bands have a tendency to have a higher than average C + G content (approaching significance; p=0.06).
They also identified a potential problem with the use of such an approach for gender identification. If a primer amplifies a W-specific segment in some, but not all W chromosomes in a population due to W chromosomal polymorphism, this could lead to misidentification of sex. Such nonamplifying regions are called ‘null alleles’, and could be caused by chromosomal sequence differences at primer binding sites, deletion of binding sites or large insertions between primer pairs. Analysis of birds also sexed by morphological means, excluding identifiable recording errors in either technique, suggested a maximum 0.6% error in the molecular gender identification among over 290 female Oystercatchers (Haematopus ostralegus) due to null alleles and none among 241 female Great Tits (Parus major). It is important to note that as theirs was a maximum estimate; null alleles may have been entirely absent in both surveys.
While other more universal assays for gender identification are now available for most species (next section), this set of techniques represents one of few simple ways to deliberately isolate W-chromosomal material. The fact that Lessells and Mateman (1998) were successful with 7/10 avian species studied shows its efficiency to be high. To date, the majority of RAPD primers that amplify W-specific targets have shown a restricted taxonomic range of effectiveness, particularly beyond the intrageneric level. For example, among the 7 species for which a W-chromosomal product was discovered by Lessells and Mateman (1998), just one primer amplified a female-specific product from a congeneric species. Nonetheless, while primer sites themselves seem poorly conserved among species, the W-chromosomal DNA amplified between them may or may not follow a similar pattern, depending on whether they contain conserved sequences. It was a female-specific amplification product obtained using bulked segregant analysis that led to the isolation of a fragment of the very highly conserved CHD gene (see next section) that hybridises throughout (and beyond) Aves (Griffiths and Tiwari 1995). More recently, E.R.A. Flood and T.W. Quinn (unpublished data) used Southern blots to show that a female-specific amplification product isolated using bulked segregant analysis in Snow Geese hybridised to females throughout the order Anseriformes.
Techniques based on the CHD gene
One of the most exciting discoveries leading to further development of methods for avian gender identification was the finding that two related copies of the chromodomain-helicase-DNA-binding (CHD) gene previously described in mammal and Drosophila (Delmas et al. 1993; Stokes et al. 1996) are located on the avian W and Z chromosomes respectively (Griffiths et al. 1996; Griffiths and Korn 1997). Griffiths and Tiwari (1993) originally isolated a portion of the avian CHD gene using bulked segregant analysis to identify a W-specific band in the Great tit, using a set of 21 to 29-mer primers that had originally been designed for ‘other purposes’. One of these (primer ss2) produced a 724 bp band in female great tits that was later shown to be homologous to the mammalian CHD gene. Because CHD is so highly conserved, with 86% nucleotide sequence identity between bird and mouse coding regions, Griffiths and Tiwari (1995) were able to develop a set of primers that amplify the same region in widely divergent avian species. The procedure that they developed (Griffiths and Tiwari 1995; Griffiths et al. 1996) requires two main steps. First a PCR reaction is performed using their P2 and P3 primers. These primers flank a short coding region within both the W (CHD-W) and the Z (CHD-Z) homologue of CHD, and in each case the amplified fragment is identical in length (110 bp including the primers), but somewhat different in sequence. The amplification product is then digested with the restriction endonuclease HaeIII. This enzyme cuts the CHD-Z but not the CHD-W fragment at a single site in those species included in their study. As a result, in males, the two CHD-Z-derived (allelic) products are cut into two pieces, 45 bp and 65 bp. These are small enough that, relative to the 110 bp undigested product, they are poorly resolved on agarose gels. In females, the Z-derived product is similarly cut in two, but the W-derived product is not. The net result of this is that in females there are three post-digestion products, including a 110bp fragment that is easily visualised and two smaller fragments that are more difficult to visualise. In contrast, male lanes do not contain the 110bp fragment.
Griffiths et al. (1996) tested this assay in 13 avian species including representatives of both major branches of the avian phylogeny as reported by Sibley et al. (1988), namely, Eoaves (represented by Chicken) and Neoaves (all other samples) and pointed out that the implication of this is that CHD-W was linked to the W chromosome before the diversification of the class Aves from a single avian common ancestor. However, at least one other species (Ostrich, Struthio camelus) that is considered a member of Neoaves by Sibley et al. (1988), does not show obvious W-linkage for the CHD gene (Ellegren 1996). It is possible that the ratite lineage is the most primitive among extant birds and that the CHD gene is absent on the W, or that, in light of the lack of heteromorphism and considerable banding homology between the sex chromosomes in ratites (Takagi et al. 1972; Ansari et al. 1988) they ‘behave’ like a pair of autosomal alleles. With the exception of at least some ratites, the technique of Griffiths and Tiwari (1995) and Griffiths et al. (1996) represents the first near-universal method for molecular sex identification in birds.
Ellegren (1996) used a cDNA library prepared from Chicken embryonic spinal tissue to ‘walk’ out from the P2-P3 amplified region described by Griffiths and Tiwari (1995) along CHD-W by using PCR and information from mouse (Delmas et al. 1993) and the Chicken CHD-Z (Funahashi et al. 1993). In this way he sequenced 2,686 bp of the Chicken CHD-W coding region. He also developed several additional approaches to using the CHD region to determine gender. First, P2 and P3 amplification products, rather than being digested with a restriction enzyme as was done by Griffiths et al. (1996) could be resolved in Chickens as one (male) or two (female) bands by using single strand conformational polymorphism (SSCP) analysis and acrylamide gels. Attempts with other species were not reported, but would probably be successful in most instances because this technique is sensitive enough to detect single base pair differences in 100-300 bp fragments 99% of the time (Lessa and Appelbaum 1993). A second approach (also used by Griffiths et al. 1996) was to use portions of the CHD-W gene directly as a probe and hybridise it to Southern blots of restriction-digested genomic DNA samples. This was done with nine species and discerned one or more W-specific bands in all but one (Ostrich). A third approach was developed for a single species (Collared Flycatcher, Ficedula albicollis) and used a set of three primers that generated different numbers and sizes of products in male versus female. One primer (cFR) matched an intronic region on CHD-W and did not bind to CHD-Z. The other two primers (2945F and 3224R) could be used together to amplify an identically sized 630 bp fragment from CHD-W and CHD-Z. By using all three primers at the same time, multiplex PCR would produce two bands in females, a 210bp fragment produced by cFR paired with 2945F, and a 630 bp fragment produced by the 2945F and 3224R pair. In males the cFR primer would be inactive and a 630 bp fragment would be the only product produced. By using this multiplex format, the 630 bp fragment provided a control for the success of the PCR reaction itself, so that failed reactions would not be mistaken for male samples as would be the case in amplifications using just cFR and 2945F. This particular method is unlikely to be ‘universal’ as introns typically diverge more rapidly than exons in conserved genes. The fourth approach used by Ellegren (1996) took advantage of size differences in Collared Flycatcher introns located within CHD-W and CHD-Z. He designed primers flanking those introns and showed that consistent size differences were apparent between the two chromosomal locations. On denaturing acrylamide gels, male PCR products were resolved as a single band, while female products resolved into two bands. These primers were presumably not effective among divergent avian taxa (Ellegren and Sheldon 1997a), but highly conserved and near universal primers using a similar approach have now been developed (below).
Griffiths et al. (1998) and Kahn et al. (1998) independently developed primer pairs flanking an intron that varies in size between CHD-W and CHD-Z in almost all species tested. Griffiths et al. (1998) used sequences they gathered from the Chicken CHD-Z aligned with those from the mouse CHD1 gene (Delmas et al. 1993) to design a new primer (P8) that, could be paired with their previously described P2 primer (Griffiths and Tiwari 1995). The priming sites of P8 and P2 were sequenced in Chicken, Zebra Finch Taenopygia guttata, and Mouse, verifying that the 3’ end of P2 and P8 was conserved in each. In total, 28 species from 11 avian orders were tested in PCR reactions. Of these, the gender of all but one could be identified by the occurrence of two sizes of amplification product in females but only one in males. The exception was a ratite (Ostrich). While most size differences in females could be discerned using agarose gel electrophoresis, the introns in some species were close enough in size that an 8% denaturing acrylamide gel was necessary to resolve the size difference.
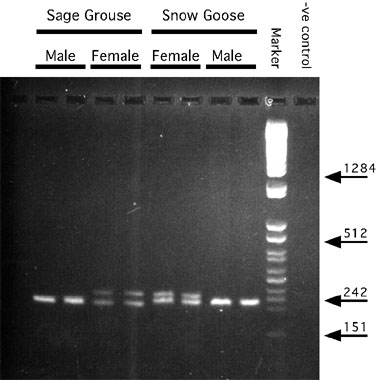
Kahn et al. (1998) discovered the same intron by cloning and sequencing this region in Sage Grouse Centrocercus urophasianus and Snow Geese Anser caerulescens using primers they designed by aligning published cDNA sequence of Chicken (Funahashi et al. 1993) and mouse (Delmas et al. 1993), and the P2 primer of Griffiths and Tiwari (1995). By comparing this sequence information with that from Chicken (Funahashi et al. 1993) and mouse (Delmas et al. 1993), a second set of primers were designed to match flanking conserved coding regions. Using these primers, they amplified the intervening region in 18 avian species belonging to 9 different orders. In 11 species the two chromosomal products in females were of sufficiently different size that they could be resolved by conventional agarose gel electrophoresis (Fig. 1). In another seven species (including two ratites; Ostrich and Emu Dromaius novaehollandiae) only one band was resolved on agarose gels for both male and female targets. Two of these (Great Horned Owl, Bubo virginianus and Red-tailed Hawk, Buteo jamaicensis) could be resolved as two bands in females and one band in males using 5% non-denaturing acrylamide gels, the ratites (Ostrich and Emu) remained unresolved, and the other three were not analysed further. One observation that remains puzzling is that the female-specific intron was always heavier than the male-specific intron for all species in which resolution was possible by Kahn et al. (1998) and in all but two cases in the study of Griffiths et al. (1998).
The strength of conserved primers that amplify an intron with different sizes on the two sex chromosomes is that they are almost universal, they require relatively simple laboratory procedures, and they (like most RAPD primer-based fragments) amplify not just a female-specific fragment, but also one found in both sexes that serves as a valuable control for the PCR reaction.
CONCLUSION
With the recent development of simple and near-universal methods for avian gender determination, researchers will be freed from the relatively tedious task of isolating W-specific fragments for their taxonomic group of interest (see Table 1 of Ellegren and Sheldon, 1997). The fact that a single PCR reaction and electrophoresis is now all that is required for most taxonomic groups (Griffiths et al., 1998; Kahn et al., 1998) makes the expertise required minimal (although an awareness of proper controls remains important) and equipment for such reactions is widely available. Those methods that take advantage of intron size differences should be the most straightforward for most species. However, it remains important that caution be used when these ‘universal’ methods are applied to new species, because the last 20 years of research have brought the realisation that vertebrate genomes are much more fluid than previously thought, and the possibility of additional or missing gene copies in the genomes of some avian species should at least be considered.
While amplification across CHD introns will likely become the favoured approach in many laboratories, the dot blot format such as that used by Griffiths and Holland (1990) and Uryu et al. (1988) may become more efficient for processing very large numbers of samples provided the more demanding technical expertise and equipment needs can be met. If a conserved gene or gene family that is found exclusively on the W chromosome is available, or if an equivalent fragment can be generated by another technique such as with RAPD-based primers, it should become possible to place hundreds of unknown samples onto a single membrane and process them simultaneously. The ‘EE0.6’ clone isolated by Ogawa et al. (1997) appears to have the required characteristics for many (not all) species, although this has not yet been tested directly. With assays such as this, that use W-specific probes, it will remain important to verify that lack of signal accurately identifies male samples (as opposed to poor/absent transfer of DNA, membrane inconsistencies, etc.) but this should be relatively straightforward by hybridisation with autosomal probes. Nonetheless, for most labs with average needs, PCR-based methods will likely be more efficient.
Until recently, very little was known about DNA sequences on the W chromosome. Now, with the application of PCR-based approaches and with the recent revitalisation of cytogenetic approaches that has been driven by their synergistic relationship with molecular techniques, it is likely that a great deal more will be learned in the immediate future. Of particular importance for this larger goal has been the development of techniques that allow the isolation of specific chromosomal DNA directly from metaphase chromosomal spreads such as those used by Ogawa et al. (1997) in isolating W-chromosomal material and by Ambady et al. (1997) and Zimmer et al. (1997) in isolating Z-chromosomal material.
ACKNOWLEDGEMENTS
I thank Nate Kahn and Judy St. John for supplying the photo used in Fig. 1 and Kate Lessells and Jim Quinn for many thoughtful comments that improved this paper. National Sciences Foundation provided support via a grant to TWQ.
REFERENCES
Abdel-Hameed, F. & Shoffner, R.N. 1971. Intersexes and sex determination in chickens. Science 172: 962-964.
Ambady, S., Ciufo, S., Robl, J.M., Smyth, J.R. & Ponce de León, F.A. 1997. Development of a chicken Z-chromosome-specific DNA library. Journal of Heredity 88: 247-249.
Ansari, H.A., Takagi, N. & Sasaki, M. 1988. Morphological differentiation of sex chromosomes in three species of ratite birds. Cytogenetics and Cell Genetics 47: 185-188.
Baverstock, P.R., Adams, M., Polkinghorne, R.W. & Gelder, M. 1982. A sex-linked enzyme in birds-Z chromosome conservation but no dosage compensation. Nature 296: 763-766.
Bercobitz, A.B., Czekala, N.M. & Lasley, B.L. 1978. A new method of sex determination in monomorphic birds. Journal of Zoo Animal Medicine 9: 114-124.
Bloom, S.E. 1974. Current knowledge about the avian W chromosome. BioScience 24: 340-344.
Bloom, S.E., Delany, M.E. & Muscarella, D.E. 1993. Constant and variable features of avian chromosomes. In Manipulations of the Avian Genome (R.J. Etches and A.M.V. Gibbins, Eds.). CRC Press, Ann Arbor. pp. 39-59.
Bridges, C.B. 1921. Triploid intersexes in Drosophila melanogaster. Science 54: 252-254.
Chandra, H.S. 1994. Proposed role of W chromosome inactivation and the absence of dosage compensation in avian sex determination. Proceedings of the Royal Society of London. Series B: Biological Sciences 258: 79-82.
Cline, T.W. 1993. The Drosophila sex determination signal: how do flies count to two? Trends in Genetics 9: 385-390.
Cock, A.G. 1964. Dosage compensation and sex-chromatin in non-mammals. Genetical Research 5: 354-365.
Crew, F.A.E. 1933. A case of non-disjunction in the fowl. Proceedings - Royal Society of Edinburgh. Section B: Biology 53: 89-104.
Delmas, V., Stokes, D.G. & Perry, R.P. 1993. A mammalian DNA-binding protein that contains a chromodomain and an SNF2/SWI2-like helicase domain. Proceedings of the National Academy of Sciences of the United States of America 90: 2414-2418.
Dvorák, J., Halverson, J.L., Gulick, P., Rauen, K.A., Abbott, U.D., Kelly, B.J. & Shultz, J.T. 1992. cDNA cloning of a Z- and W-linked gene in gallinaceous birds. Journal of Heredity 83: 22-25.
Ellegren, H. 1991. DNA typing of museum birds. Nature 354: 113.
Ellegren, H. 1992. Polymerase-chain-reaction (PCR) analysis of microsatellites-A new approach to studies of genetic relationships in birds. The Auk 109: 886-895.
Ellegren, H. 1996. First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proceedings of the Royal Society of London. Series B: Biological Sciences 263: 1635 -1641.
Ellegren, H. & Sheldon, B.C. 1997a. New tools for sex identification and the study of sex allocation in birds. Trends in Ecology and Evolution 12: 255-259.
Ellegren, H. & Sheldon, B.C. 1997b. Reply from H. Ellegren & B.C. Sheldon. Trends in Ecology and Evolution 12: 489-490.
Fredga, K. 1994. Bizarre mammalian sex-determining mechanisms. In: The Difference Between the Sexes (Short, R.V. & Balaban, E., eds), pp 419-431, Cambridge University Press.
Funahashi, J-I., Sekido, R., Murai, K., Kamachi, Y. and Kondoh, H. 1993. d -crystallin enhancer binding protein d EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development 119: 433-446.
Galton, M. & Bredbury, P.R. 1966. DNA replication patterns of the sex chromosomes of the pigeon (Columba livia domestica). Cytogenetics 5: 295-306.
Graves, J., Ruano, J.O., Slater, P.J.B. 1993. Sex ratio of chicks in the Shag Phalacrocorax aristotelis determined by a female-specific band in DNA fingerprinting. Ibis 135: 470-472.
Griffiths, R. 1991. The isolation of conserved DNA sequences related to the human sex-determining region Y gene from the lesser black-backed gull (Larus fuscus). Proceedings of the Royal Society of London. Series B: Biological Sciences 244: 123-128.
Griffiths, R. & Holland, P.W.H. 1990. A novel avian W chromosome DNA repeat sequence in the lesser black-backed gull (Larus fuscus). Chromosoma 99: 243-250.
Griffiths, R. & Tiwari, B. 1993. The isolation of molecular genetic markers for the identification of sex. Proceedings of the National Academy of Sciences of the United States of America 90: 8324-8326.
Griffiths, R. & Tiwari, B. 1995. Sex of the last wild Spix’s macaw. Nature 375: 454.
Griffiths, R., Daan, S. & Dijkstra, C. 1996. Sex identification in birds using two CHD genes. Proceedings of the Royal Society of London. Series B: Biological Sciences. 263: 1251-1256.
Griffiths, R. & Korn, R.M. 1997. A CHD1 gene is Z chromosome linked in the chicken Gallus domesticus. Gene 197: 225-229.
Griffiths, R., Double, M.C., Orr, K. & Dawson, R.J.G. 1998. A DNA test to sex most birds. Molecular Ecology 7: 1071-1075.
Gubbay, J., Collignon, J., Koopmen, P., Capel, B., Economou, A., Munsterbert, A., Vivian, N., Goodfellow, P. & Lovell-Badge, R. 1990. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346: 245-250.
Harrison, G.J. 1978. Endoscopic examination of avian gonadal tissues. Veterinary Medicine, Small Animal Clinician 73: 479-484.
Hildebrandt, T., Pitra, C., Sömmer, P. & Pinkowski, M. 1995. Sex identification in birds of prey by ultrasonography. Journal of Zoo and Wildlife Medicine 26: 367-376.
Jeffreys, A.J., Wilson, V. & Thein, S.L. 1985. Hypervariable ‘minisatellite’ regions in human DNA. Nature 314: 67-73.
John, B. & Miklos, G.L.G. 1979. Functional aspects of satellite DNA and heterochromatin. International Review of Cytology 58: 1-23.
Jones, D.M., Samour, J.H., Knight, J.A. & Finch, J.M. 1984. Sex determination of monomorphic birds by fiberoptic endoscopy. Veterinary Record 115: 596-598.
Jones, K.W. & Singh, L. 1985. Snakes and the evolution of sex chromosomes. Trends in Genetics 1: 55-61.
Kahn, N.W., St. John, J. & Quinn, T.W. 1998. Chromosome-specific intron size differences in the avian CHD gene provide a simple and efficient method for sexing birds. The Auk 115: 1074-1078.
Kodama, H., Saitoh, H., Tome, M., Kuhara, S., Sakaki, Y. & Mizuno, S. 1987. Nucleotide sequences and unusual electrophoretic behavior of the W chromosome-specific repeating DNA units of the domestic fowl, Gallus gallus domesticus. Chromosoma 96: 18-25.
Krishan, A., Haiden, G.J. & Shoffner, R.N. 1965. Mitotic chromosomes and the W sex chromosome of the great horned owl (Bubo v. virginianus). Chromosoma (Berl.) 17: 258-263.
Lacson, J.M & Morizot, D.C. 1988. Confirmation of avian sex-chromosome linkage of liver cytosolic aconitase (ACO1). Cytogenetics and Cell Genetics 48: 244-245.
Lessa, E.P. & Applebaum, G. 1993. Screening techniques for detecting allelic variation in DNA sequences. Molecular Ecology 2: 119-129.
Lessells, C.M., Mateman, A.C. & Visser, J. 1996. Great Tit hatchling sex ratios. Journal of Avian Biology 27: 135-142.
Lessells, C.M. & Mateman, A.C. 1998. Sexing birds using random amplified polymorphic DNA (RAPD) markers. Molecular Ecology 7: 187-195.
Longmire, J.L., Abrose, R.E., Brown, N.C., Cade, T.J., Maechtle, T.L., Seegar,, W.S., Ward, F.P. & White, C.M. 1991. Use of sex-linked minisatellite fragments to investigate genetic differentiation and migration of North American populations of the peregrine falcon (Falco peregrinus). In: DNA Fingerprinting: Approaches and Applications (eds T. Burke, G. Dolf, A.J. Jeffreys, R. Wolff), Basle: Bukhauser Verlag, pp 217-229.
Longmire, J.L., Maltbie, M., Pavelka, R.W., Smith, L.M., Witte, S.M., Ryder, O.A., Ellsworth, D.L. & Baker, R.J. 1993. Gender identification in birds using microsatellite DNA fingerprint analysis. The Auk 110: 378-381.
Lyon, M.F. 1989. X-chromosome inactivation as a system of gene dosage compensation to regulate gene expression. Progress in Nucleic Acid Research and Molecular Biology 36: 19-130.
Michelmore, R.W., Paran, I. & Kesseli, R.V. 1991. Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proceedings of the National Academy of Sciences of the USA 88: 9828-9832.
Millar, C.D., Lambert, D. M. & Young, E. C. 1997. Minisatellite DNA detects sex, parentage, and adoption in the south polar skua. Journal of Heredity 88: 235-238.
Nakamura, D., Tiersch, T.R., Douglass, M. & Chandler, R.W. 1990. Rapid identification of sex in birds by flow cytometry. Cytogenetics and Cell Genetics 53: 201-205.
Ogawa, A., Solovei, I., Hutchison, N., Saitoh, Y., Ikeda, J-E, Macgregor, H. & Mizuno, S. 1997. Molecular characterisation and cytological mapping of a non-repetitive DNA sequence region from the W chromosome of a chicken and its use as a universal probe for sexing Carinatae birds. Chromosome Research 5: 93-101.
Paran, I. & Michelmore, R. 1992. Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theoretical and Applied Genetics 85: 985-993.
Petitte, J.N. & Kegelmeyer, A.E. 1995. Rapid sex determination of chick embryos using the polymerase chain reaction. Animal Biotechnology 6: 119-130.
Prus, S.E. & Schmutz, S.C. 1987. Comparative efficiently and accuracy of surgical and cytogenetic sexing in Psittacines. Avian Diseases 31: 420-424.
Quinn, T.W. & White, B.N. 1987. Identification of restriction-fragment-length polymorphisms in genomic DNA of the Lesser Snow Goose (Anser caerulescens caerulescens). Molecular Biology and Evolution 4: 126-143.
Quinn, T.W., Cooke, F. & White, B.N. 1990. Molecular sexing of geese using a cloned Z chromosomal sequence with homology to the W chromosome. The Auk 107: 199-202.
Rabenold, P.P., Piper, W.H., Decker, M.D. & Minchella, D.J. 1991a. Polymorphic minisatellite amplified on the avian W chromosome. Genome 34: 489-492.
Rabenold, P.P., Rabenold, K.N., Piper, W.H. & Minchella, D.J. 1991b. Density-dependent dispersal in social wrens: genetic analysis using novel matriline markers. Animal Behavior 42: 144-146.
Rahn, M.I. & A.J. Solari. 1986. Recombination nodules in the oocytes of the chicken, Gallus domesticus. Cytogenetics and Cell Genetics 43: 187-193.
Redelman, D., Fleury, S.A. & Garner, D.L. 1997. Flow cytometry for sexing birds. Trends in Ecology and Evolution 12: 489.
Sabo, T.J., Kessell, R., Halverson, J.L., Nisbet, I.C.T. & Hatch, J.J. 1994. PCR-based method for sexing roseate terns (Sterna dougallii). The Auk 111: 1023-1027.
Saiki, R.K., Scharf, S., Faloona, F., Mullis, K.B., Horn, G.T., Erlich, H.A. & Arnheim, N. 1985. Enzymatic amplification of b -globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230: 1350-1354.
Saitoh, Y., Saitoh, H., Ohtomo, K. & Mizuno, S. 1991. Occupancy of the majority of DNA in the chicken W chromosome by bent-repetitive sequences. Chromosoma 101: 32-40.
Saitoh, Y. & Mizuno, S. 1992. Distribution of XhoI and EcoRI family repetitive DNA sequences into separate domains in the chicken W chromosome. Chromosoma 101: 474-477.
Scherr, L.J. 1986. Invalid results from blood sexing. Association of Avian Veterinarians Newsletter 6: 110.
Schmid, W. 1962. DNA replication patterns of the heterochromosomes in Gallus domesticus. Cytogenetics 1: 344-352.
Shields, G.F., Jarrell, G.H. & Redruff, E. 1982. Enlarged sex chromosomes of woodpeckers (Piciformes). The Auk 99: 767-771.
Sibley, C.G., Ahlquist, J.E. & Monroe, B.L. 1988. A classification of the living birds of the world based on DNA-DNA hybridization studies. The Auk 105: 409-423.
Simkiss, K., Luke, G. & Behnam, J. 1996. Female chromosomes in cockerel ejaculates. Proceedings of the Royal Society of London. Series B: Biological Sciences 263: 1245-1249.
Sinclair, A.H. Berta, P., Palmer, M.S., Hawkins, J.R., Griffiths, B.L., Smith, M.J., Foster, J.W., Frischauf, A., Lovell-Badge, R. & Goodfellow, P.N. 1990. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA binding motif. Nature 346: 240-244.
Singh, L., Purdom, I.F. & Jones, K.W. 1976. Satellite DNA and evolution of sex chromosomes. Chromosoma (Berl.) 59: 43-62.
Sittmann, K. 1984. Sex determination in birds: progeny of nondisjunction canaries of Durham (1926). Genetical Research 43: 173-180.
Smith, E.F.G., Arctander, P., Fjeldsa, J. & Amir, O.G. 1991. A new species of shrike (Laniidae: Laniarius) from Somalia, verified by DNA sequence data from the only known individual. Ibis 133: 227-235.
Solari, A.J. 1977. Ultrastructure of the synaptic autosomes and the ZW bivalent in chicken oocytes. Chromosoma 64: 155-165.
Solari, A.J. 1992. Equalization of Z and W axes in chicken and quail oocytes. Cytogenetics and Cell Genetics 59: 52-56.
Solari, A.J., Fechheimer, N.S. & Bitgood, J.J. 1988. Pairing of ZW gonosomes and the localized recombination nodule in two Z-autosome translocations in Gallus domesticus. Cytogenetics and Cell Genetics 48: 130-136.
Stefos, K. & Arrighi, F. 1971. Heterochromatic nature of W chromosome in birds. Experimental Cell Research 68: 228-231.
Stokes, D.G., Tartof, K.D. & Perry, R.P. 1996. CHD1 is concentrated in interbands and puffed regions of polytene chromosomes. Proceedings of the National Academy of Sciences of the United States of America 93: 7137-7142.
Takagi, N., Itoh, M. & Sasaki, M. 1972. Chromosome studies in four species of Ratitae (Aves). Chromosoma (Berl.) 36: 281-291.
Tell, L.A. & Lasley, B.L. 1991. An automated assay for fecal estrogen conjugates in the determination of sex in avian species. Zoo Biology 10: 361-367.
Tiersch, T.R., Mitchell, M.J. & Wachtel, S.S. 1991 Studies on the phylogenetic conservation of the SRY gene. Human Genetics 87: 571-573.
Tone, M., Nakano, N., Takao, E., Narisaws, S. & Mizuno, S. 1982. Demonstration of W chromosome-specific repetitive DNA sequences in the domestic fowl, Gallus g. domesticus. Chromosoma 86: 551-569.
Tone, M., Sakaki, Y., Hashiguchi, T. & Mizuno, S. 1984. Genus specificity and extensive methylation of the W chromosome-specific repetitive DNA sequences from the domestic fowl Gallus gallus domesticus. Chromosoma 89: 228-237.
Uryu, N., Nagata, Y., Ito, K., Saitoh, H. & Mizuno, S. 1988. Research Note: Determination of sex of chickens by a biotin-labelled deoxyribonucleic acid probe. Poultry Science 68: 850-853.
Wachtel, S.S., Wachtel, G.W., Nadamura, D. & Gilmour, D. 1983. H-Y antigen in the chicken. Differentiation 23 (Suppl): s107-s115.
Williams, J.G.K., Kubelik, A.R., Livak, K.J., Rafalski, J.A. & Tingey, S.V. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research 18: 6531-6535.
Zimmer, R., King, W.A. & Gibbins, A.M.V. 1997. Generation of chicken Z-chromosome painting probes by microdissection for screening large-insert genomic libraries. Cytogenetics and Cell Genetics 78: 124-130.
Fig. 1. PCR products generated using 1237L and 1272H primers (Kahn et al., 1998). Approximately 30 ng of template DNA was amplified for 30 cycles and electrophoresed though a 2% NuSieve gel at 5 V/cm. A Z-specific intron product is present in all lanes. A W-specific product is present in female samples only. Selected sizes of marker bands are shown in nucleotide base pairs. F, female samples; M, male samples.
