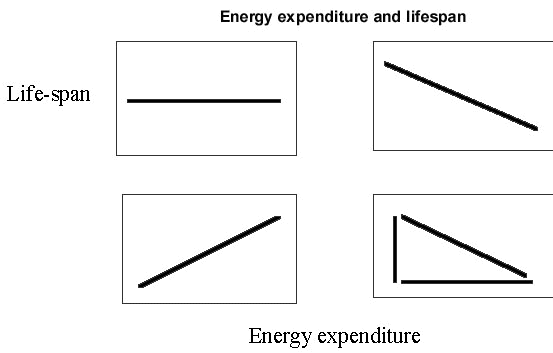
S07.5: Energetics and lifespan in birds
David M. Bryant
Institute of Biological Sciences, University of Stirling, Stirling. FK9 4LA.UK
Bryant, D.M. 1999. Energetics and lifespan in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
412-421. Johannesburg: BirdLife South Africa.The energetics of five components of avian life histories are discussed: body size, reproduction, growth, ageing and survival. Specifically, I consider evidence, mainly from field studies, concerned with intraspecific variation in the energetic costs of the following: (1) Body size of adults. (2) Success in mating, laying, incubation and raising offspring. (3) Chick growth rates. (4) Ageing, from hatching to senescence. (5) Survival and subsequent reproductive success. Components 4 and 5 will be considered in greatest detail. The heritability of energetics traits will also be discussed. Evidence from these sources will be used to evaluate the hypothesis that energy expenditure serves as a reliable token of fitness. Studies using doubly labelled water have revealed a striking variability in energy expended by individuals: In some species, for certain life history components, intra-individual trade-offs between energy costs and fitness have been shown. This demonstrates that energy can be a valuable currency for exploring the evolution of avian life history traits and for revealing underlying mechanisms, although it does not confirm that energy expenditure itself causes fitness costs. Many potential life history trade-offs warrant further study, particularly reaction norms and intergenerational effects involving physiological trade-offs, which have been hardly explored to date.
INTRODUCTION
Life history theory is concerned with relationships between components of fitness, including trade-offs between parental survival and reproductive success (Stearns 1992). Raising additional offspring will normally elevate energetic expenditures, as will other behaviours which contribute to breeding success, but at the same time may reduce the chance of subsequent survival.
There are eight reasons why variation in sustained rates of energy expenditure, such as those occurring during rearing of altricial young, might have consequences for longevity. They are offered not as examples of cause and effect, but rather as observed or suggested relationships between whole-animal or mass-specific metabolism which are consistent with greater expenditures having a depressing effect on survival. 1. Resources are often limited. Therefore, high rates of daily energy expenditure are less likely to be satisfied, other things being equal, and so survival prospects may be reduced. 2. High mass-specific energy expenditures will often increase indirectly the frequency of related events, such as accidents, disease transmission or predation. 3. Allometric analyses suggest that different organisms display some constancy with regard to the lifetime number of ‘cyclic-events’ (i.e. heartbeats) (Lindstedt & Calder 1981). It follows, presuming cause and effect, that higher ‘cyclic-event’ rates would be followed by a corresponding reduction in lifespan. 4. Since body mass and survival are correlated (Saether 1988), as are mass and metabolic rate (Kleiber 1975), metabolic variation is a candidate cause of lifespan variation. 5. Negative correlations link survival and vigorous, energy-expensive activity, such as flight, mainly in insects (Neukirch 1982). 6. Diet-restricted animals have reduced daily energy expenditures and extended lifespans (Hart & Turturro 1998). 7. High rates of mass-specific energy expenditure may entail physiological costs and so carry a mortality risk (Drent & Daan 1980, Daan et al. 1996): possibly because tissue repair is neglected (Kirkwood 1993). 8. Models suggests that high prevailing mortality rates will encourage a greater expenditure of energy on reproduction (Teriokhin 1998). As a result, mortality will increase in excess of that attributable to extrinsic factors, due to strategic resource allocation for current reproduction at the expense of maintenance. These points allow that longevity in wild birds might be jeopardised by an increase in energy expenditure and, taken together, provide a pretext for further investigation.
What constitutes a ‘high’ sustained energy expenditure for any given bird species? It could derive from: a. Extended aerobic activity (i.e. foraging), b. Persistent thermostatic demands, c. Continuous expenditure of relatively high cost (i.e. load-carrying, disease, injury, reproduction), d. Frequent and costly ephemeral activities, probably involving at least some anaerobic metabolism (i.e. escape behaviour), and e. Rapid growth. These would be likely to have an effect on mass-specific energy expenditure of a sufficient magnitude to be detected using current techniques and would be likely to result in a daily energy expenditure (DEE) which approached the upper bounds of observed ranges (Drent & Daan 1980, Bryant & Tatner 1991, Bryant 1997, Hammond & Diamond 1997). An assumption is made that the most appropriate way to investigate the consequences of high sustained energy expenditures amongst wild birds is to integrate activities over periods of a day or more, and therefore DEE (or field metabolic rate, FMR) is adopted as the principal measure of interest. To discard other energetics data at this stage, however, risks overlooking some useful comparative evidence. Hence, I consider basal metabolic rates (BMR or RMR, resting metabolic rate), albeit briefly, as well as DEE (or AMR, active metabolic rate).
Evidence in support of an interaction between energy expenditure and longevity falls into two familiar categories: correlative evidence on one hand and experimental evidence on the other. Cursory examination of data on DEEs gives an impression that birds with high expenditures tend to be short-lived, whereas others with low expenditures are long-lived. This impression is consistent with the extremes of the expenditure continuum (Weathers & Sullivan 1989, Bryant & Tatner 1991): for example, the short-lived Tree swallow Tachycineta bicolor, and the long-lived Kakapo Strigops habroptilis, have average DEEs estimated at >5xBMR (Williams 1988) and <1.5xBMR (Bryant & Elliott in prep) respectively. Furthermore, the active flight-foraging lifestyle of the migrant swallow might be expected to increase mortality risks, whereas the flightless habit of the Kakapo (in the absence of introduced mammalian predators) would reduce such risks. Is this impression supported by a rigorous analysis, because it risks being confounded by body size, habits and phylogeny? Trevelyan et al. (1990) examined variation in RMR and AMR in relation to lifespan and other life-history variables, using phylogenetically independent contrasts (Harvey & Pagel 1991). A significant negative relationship for RMR and lifespan was found, after controlling for body mass, but it was critically dependent on one outlier (i.e. a petrel Pteroderma) amongst 103 comparisons, and so the relationship was discounted. Similarly no correlation between AMR and lifespan was detected. The authors concluded that metabolic rate and lifespan, after controlling for phylogeny and body size, were unrelated. Three problems affected this analysis. Firstly, RMR includes both BMR and SMR (standard metabolic rate, for which conditions were not constant), which introduces heterogeneity and thereby reduces the chance of detecting significant relationships. An analogous criticism may be levelled at the AMR data, which were drawn from a mix of sources. Lifespan was derived from the age of the longest-lived individual on record; an imprecise measure of survival rate, which is likely to show marked biases. Finally, the analysis did not distinguish between mortality (or lifespan) variation which might have been due to facultative variation in metabolism, and that which was due to extrinsic mortality. This also being generally so, correlations between energy expenditure and lifespan will always be hard, and may be impossible to demonstrate, because precise segregation of extrinsic and facultative mortality is unlikely to be achieved on a substantial scale. Nevertheless, when more consistent energetics data are analysed for birds from various taxa, across a range of precisely-known survival rates, we could be more confident we are not witness to a Type II error.
Life history theory has attempted to link changes in reproductive effort with age (Stearns 1992). If these were found to occur, involving an increase in energy expenditure (here equated with effort) with age for example, it would be consistent with a trade-off between reproductive effort and survival. Comparisons between juveniles and adults are unsuitable as test data, however, because the former’s costs derive from other factors, including inexperience in foraging (Weathers & Sullivan 1989). Studies of known-age populations, which allow comparisons amongst adults alone, however, remain few. Amongst these, only one of three has shown a significant correlation of energy expenditure with adult age (Bryant & Tatner 1991) and this did not prevail within a subsequent, larger and more homogeneous data set on the same species (Dipper Cinclus cinclus, Bryant & Newton in prep.).
A few studies have considered correlations between energy expenditure and survival more directly. Such correlational evidence is likely to be most useful when the extrinsic mortality risk is constant or more likely to be so. This will be commoner within the bounds of single species studies. For example, in Red Knot Calidris canuta which winter off north-west Europe and tropical West Africa, a higher annual expenditure amongst temperate-wintering birds, principally for thermostatic reasons, is matched by a poorer survival (Piersma 1994). This is consistent with a model which assumes that lifespan and energy expenditure are inversely related (Fig. 1B) rather than being unrelated (Fig. 1A). Alternatives are possible, however: if a higher expenditure brings greater net energy returns, then lifespan and expenditure might be positively related (Fig.1C). A further option is that energy expenditure may be unrelated to lifespan at low levels of expenditure but inhibits survival at high expenditures (Bryant 1988). If so, high expenditures would not co-occur with long lifespans, but low expenditures and short lifespans would occur together (Fig. 1D). Lifespan of individually marked Dippers was examined in relation to single measurements of energy expenditure (Bryant et al. in prep.). DEE was measured during one stage of the annual cycle (rearing young, n=84), which assumes a high level of repeatability (Schoeller & Richert 1993), and compared with their lifespans, which were spread over the years before and after the measurements were made. All the birds involved are now known to be dead. Body mass was entered into the analysis as a covariate, along with other factors (see Logie 1998) which may have influenced longevity. Lifespan and energy expenditure were related in a way consistent with models 1B and 1D: either they were inversely related, or more likely, high expenditures in particular induced a survival penalty. Hence, few birds showed both high FMRs and long lifespans (Fig. 2).
It is clear, however, that conclusions which extend our understanding of energy-lifespan interactions are unlikely to follow via this route; for every plausible explanation there are alternatives, and what we seek are exclusive explanations. For example, the negative association between lifespan and DEE in Dippers may have been a product of the habitat characteristics where each individual spent its life and had its metabolism measured, or to other factors. An alternative, therefore, is to adopt an experimental approach, to identify causal factors with greater confidence. While repeated manipulations throughout the lifespan of an individual is one option (i.e. manipulations of brood size to affect energy expenditure during breeding, in the same direction over a lifetime), which could increase lifespan energy expenditures, it is at the extreme of logistic and research risk-return options. Accordingly, shorter-term experiments are generally to be preferred.
Manipulation of energy budgets ideally takes birds away from their chosen expenditures and elicits higher or lower alternatives. Effects on survival, and other components of fitness, can then be assessed. Not uncommonly, brood size has been the life-history component manipulated, but it has often failed to affect energy expenditures reliably because no behavioural response was induced in birds being measured using the doubly labelled water (DLW) method. In our work on Atlantic puffins Fratercula arctica, food supplementation to chicks drastically reduced adult provisioning-rates and induced a suite of life-history consequences, including effects manifest in the year following, so implying a strong interaction between parental effort and life history, including lifespan (Wernham & Bryant 1998). Nevertheless, while we inferred that the benefits to parents of ‘load-lightening’ operated via an energetic route, we did not demonstrate in a parallel study that feeding visit rates by puffins were related to energy expenditure, possibly because behaviour was disturbed. Manipulations, therefore, should change costs in isolation and they must have an effect which can be demonstrated or assumed with confidence. In the European Kestrel Falco tinnunclus, Daan et al. (1996) showed a behavioural response to experimental treatments, and also that the resulting energy expenditures were indeed associated with changed rates of survival. While confounding factors cannot be wholly eliminated, a higher energy expenditure was itself identified as a risk factor. Further work of this type is needed if its generality is to be accepted. Even so, it is consistent with a large body of research on life-histories, where broods have been experimentally adjusted; an increased reproductive effort later exacting a ‘cost of reproduction’ (Partridge 1989). The problem remains, however, when proposing further work of this type, of treated birds failing to respond consistently to experimental manipulations, particularly when positive brood size manipulations are combined with use of the DLW method. This argues for consideration of alternative approaches.
An ideal experimental treatment is one that cannot be side-stepped or immediately compensated for, and is precisely controlled. One approach is to manipulate energy budgets, positively and negatively, by a physiological rather than behavioural route, and observe subsequent behaviour and energy expenditure which may affect survival. Manipulation of body mass, which can be taken to affect energy expenditure, has been used to show that take-off trajectories of heavier birds change in a way which makes them more liable to predation (Witter & Cuthill 1993, Metcalfe & Ure 1995). Hence energy expenditure and survival will be related inversely although, in this case, not causally. In a field study of the same phenomenon, Great Tits Parus major (Gosler et al. 1995) became lighter when Sparrowhawks Accipiter nisus re-colonised after a period of absence. Again, while the heavier, less-mobile individuals were at a disadvantage; results were apparently consistent with selection against individuals with a higher energy expenditure, which might, as a result, have been less well nourished and more vulnerable. Under either view, however, the causal factor is unclear: indeed, in both cases energy expenditure is most likely a correlate rather than a cause of changes in behaviour and mortality risk.
Warming and cooling treatments while birds are at rest are other options, but ones with more specific outcomes for energy expenditure. Cooling treatments impose a cost, either because they move the subject away from their ‘chosen’ optimum or because they result in reduced reserves (Fig. 3). The effect of warming is less clear, however. It may be negative because again it involves a shift from the chosen optimum. More likely, especially in an energy-limited environment (i.e. during breeding or the ‘lean’ season), it will confer a benefit because survival prospects seem likely to be enhanced. Whatever the outcome, with a suitable experimental design, birds cannot avoid the impact of treatments, which have quantifiable and repeatable effects; shifting energy stores to relatively high or relatively low levels. It follows that if subsequent energy expenditure changes substantially as a result of body-state manipulations, then survival prospects and expenditure are related causally, although it does not specify the direction of such effects. We have conducted three experiments involving temperature manipulations in association with doubly labelled water (DLW) measures of energy expenditure by free-living birds. Here I report on one study (Spencer & Bryant in prep).
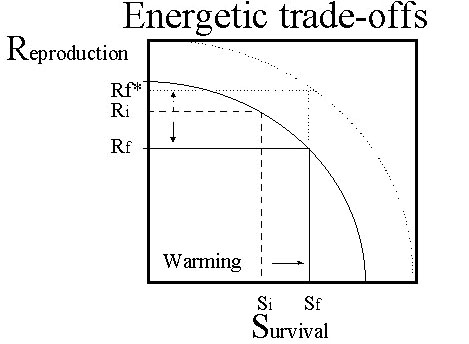
Life-history theory posits a trade-off between survival and reproduction (Fig. 4). Increased survival, implies a reduction in allocation to reproduction and vice versa (Sibly & Calow 1986). Were a notional trade-off function to be shifted as a result of experimental treatments, however, different outcomes would be predicted (Fig. 4). Under this scenario, manipulations of energy budgets which alter survival (or here, maintenance requirements), due to warming or cooling, are predicted to increase or decrease respectively the energy-allocations to reproduction. Does this occur?
Breeding Barn Swallows Hirundo rustica were exposed over-night to heating and cooling treatments. Treatments are assumed to enhance or depress survival prospects via effects on energetic state. We ask whether this elicits a positive or negative effect on subsequent energy expenditure and behaviour. Warming treatments on females reduced overnight metabolism and had a significant positive effect on subsequent daytime metabolic rates measured using DLW, most likely due to the concurrent increase in nest provisioning rate (Spencer & Bryant in prep). Since treatments did not affect overnight mass changes, an effect via body mass (i.e. due to load carrying) is unlikely. While the underlying model linking energy expenditure with survival and reproduction remains unclear, the results show that body state (itself related to survival) and energy expenditure are inter-dependent. This does not demonstrate that variation in energy expenditure has a direct effect on survival, rather the reverse, but it does show that such a link is possible. The principal merit of this approach, however, is that changes of energetic-states themselves influence energy expenditure and behaviour in a way which affects components of fitness, while also involving a common energetic currency. The size of the observed effect is indicative of state-dependent, and therefore presumably mostly facultative energy expenditure, being a substantial but previously unrecognised cause of variation in free-living energy expenditure.
In similar work, we have also examined the following: effects of heating and cooling on territorial behaviour of Robins Erithacus rubecula (Godfrey & Bryant in prep) and incubation schedules in Great Tits (Bryan & Bryant in prep). We have also manipulated energetic expenditure by adjusting plumage, specifically the tail streamers of swallows, which again affects costs in a manner which cannot be avoided (Hall & Bryant in prep). In all cases, an effect on free-living energy expenditure was detected.
It is concluded that the case for investigating the relationship between energy expenditure and survival remains strong, but convincing evidence for such a link is still rare. Future work should build on the experimental approach, whereby short-term manipulations of energy budgets are examined in relation to components of fitness, including survival prospects. Also, further experiments involving brood manipulations, and concurrent direct observation of relevant parameters, is likely to be both challenging and productive.
SUMMARY
Life history theory is concerned with relationships between components of fitness, including trade-offs between number of offspring and parental survival. Raising additional offspring will normally elevate energetic expenditures, as will other behaviours which contribute to reproductive success. These may have consequences for subsequent survival. Interactions of this type, between energy expenditure and lifespan amongst free-living birds, are considered. Eight reasons are offered which suggest that higher energy expenditures, including both whole-animal and mass-specific expenditures, might reduce lifespans. Correlational evidence from birds is conflicting, but with the strongest supporting evidence coming from single-species studies of energy expenditure in the field. Experimental studies using brood manipulations to adjust rates of energy expenditure have provided the firmest evidence for energy expenditure-lifespan trade-offs. Short-term experiments which manipulate expenditures directly, using physiological means rather than a behavioural route, provide an alternative approach which shows that energy expenditure has a state-dependent component. This demonstrates a direct link between energy storage (which affects survival chances) and energy expenditure.
REFERENCES
Bryant, D.M. 1988. Energy expenditure and body mass changes as measures of reproductive costs in birds. Funct. Ecol. 2: 23-34.
Bryant, D. M.(1997. Energy expenditure in wild birds. Proc. Nut. Soc. 56: 1025-1039.
Bryant, D. M. & Tatner, P. 1991. Intraspecies variation in avian energy expenditure:correlates and constraints. Ibis 133: 236-245.
Daan, S., & Deerenberg, C. 1996. Increased daily work precipitates natural death in the kestrel. J. Anim. Ecol. 65(5): 539-544.
Drent, R. & Daan, S. 1980. The prudent parent. Energetic adjustments in avian breeding. Ardea 68: 225-252.
Gosler, A.G. & Greenwood, J.J.D. 1995. Predation risk and the cost of being fat. Nature 377: 621-623.
Hammond, K.A. & Diamond, J. 1997. Maximal sustained energy budgets in humans and animals. Nature 386: 457-462.
Hart, R.W. & Turturro, A. 1998. Evolution and dietary restriction." Exptl. Gerontol. 33: 53-60.
Harvey, P.H. & Pagel, M.D. 1991. The comparative method in evolutionary biology. Oxford, Oxford University Press.
Kirkwood, T.B.L. 1993. The disposable soma theory: evidence and implications. Neth. J. Zool. 43(3-4): 359-363.
Kleiber, M. 1975. The fire of life: an introduction to animal energetics. New York, R.E.Krieger.
Lindstedt, S.L. & Calder, W.A. 1981. Body size, physiological time, and longevity in homeothermic animals. Q. Rev. Biol. 56: 1-16.
Logie, J.W. 1998. Population ecology and lifetime reproductive success of dippers Cinclus cinclus. PhD thesis, University of Stirling.
Metcalfe, N.B. & Ure, S.E. 1995. Diurnal variation in flight performance and hence potential predation risk in small birds. Proc. Roy. Soc. Lond., B 261: 395-400.
Neukirch, A. 1982. Dependence of the lifespan of the honeybee (Apis melifera) upon flight performance and energy consumption. J. Comp. Physiol. 146: 35-40.
Partridge, L. 1989. Lifetime reproductive success and life-history evolution. Lifetime reproduction in birds. I. Newton. London, Academic Press: 422-440.
Piersma, T. 1994. Close to the edge: energetic bottlenecks and the evolution of migratory pathways in knots, University of Groningen.
Saether, B.E. 1988. Patterns of covariation between life history traits of European birds. Nature 331: 616-617.
Schoeller, D.A. & Richert, J.H. 1993. Repeatability of energy expenditure in women measured by doubly-labeled water. Faseb J., 7: 839.
Sibly, R. M. & Calow, P. 1986. Physiological ecology of animals. Oxford, Blackwell.
Stearns, S.C. 1992. The evolution of life histories. Oxford, Oxford University Press.
Teriokhin, A.T. 1998. Evolutionarily optimal age schedules of repair: computer models of energy partition between current and future survival and reproduction. Evol. Ecol. 12: 291-307.
Weathers, W.W. & Sullivan, K.A. 1989. Juvenile foraging proficiency, parental effort, and avian reproductive success. Ecol. Monogr. 59(3): 223-246.
Wernham, C. & Bryant, D.M. 1998. An experimental study of reduced parental effort and future reproductive success in the puffin Fratercula arctica. J. Anim. Ecol. 67(1): 25-40.
Williams, J.B. 1988. Field metabolism of tree swallows during the breeding season. Auk 105: 706-714.
Witter, M.S. 1993. The ecological costs of avian fat storage. Phil. Trans. Roy. Soc. Lond. B. 340(1291): 73-92.
Fig. 1. Theoretical relationships between lifespan and energy expenditure. A (upper left); implies no association. B (upper right); shows a negative relationship consistent with high energy expenditures leading to reduced lifespans and vice versa. C (lower left); higher energy expenditures could generate higher net energy returns, which would enhance survival chances and so provide for longer lifespans. D (lower right); illustrates the distribution of data expected (i.e. within the triangle) when low levels of energy expenditure carry no cost, but modest extrinsic mortality persists. High levels of energy expenditure, on the other hand, make long lifespans unlikely. For simplicity, only linear relationships are shown, although non-linearity is likely (Bryant 1988).
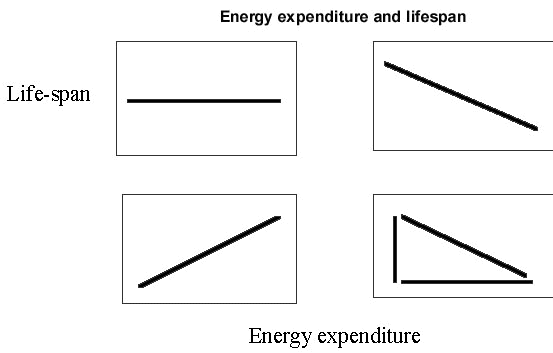
Fig. 2. Lifespans of Dippers Cinclus cinclus in relation to free-living energy expenditure. Lifespan was assessed directly by following marked individuals from hatch-year to death. Energy expenditure data are from single measurements made during the chick rearing period using the doubly labelled water method. Data include both males and females and were collected over 3 breeding seasons: neither sex nor season, nor body mass entered as a covariate, had a significant effect on lifespan. The OLS regression for the fitted line is: Lifespan (y) = 4.33 - 0.147 ADMR (cm3 CO2 g-1 h-1), n=84, P<0.05.
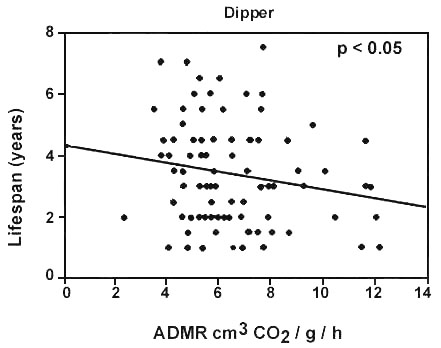
Fig. 3. Predicted effects of treatments on survival prospects. Model 1 (upper figure) implies a reduction in fitness when body reserves are increased or decreased, as a result of warming and cooling treatments, for example. This occurs because body reserves (M) are likely to be optimal under these conditions (Mopt). Model 2 (lower figure) relates to a resource-limited environment. Under these conditions, such as when provisioning chicks, depleted reserves are likely to be commoner (<Mopt). Hence, survival prospects of parents are generally likely to be enhanced by warming and reduced by cooling treatments.
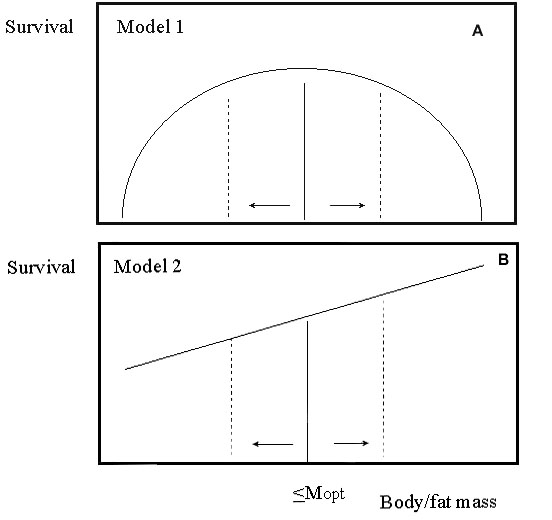
Fig. 4. Model of trade-offs between survival and reproduction. The trade-off function relating energetic investments for survival (i.e. S, energy contained in body reserves) and reproduction (i.e. R, reproductive effort, or energy expenditure when rearing offspring) is shown. The solid-line function denotes normal circumstances. The more favourable circumstances provided by the warming treatments, shifts the function (dotted line) away from the origin. Predicted reproductive effort (Rf*) therefore increases as a result of this treatment, related to the imposed shift in survival from Si to Sf. Alternative models, such as when cooling treatments are applied, suggest different outcomes.