S07.3: Relationships between field metabolic rate, basal metabolic rate and territoriality in passerines
Kenneth A. Nagy1, Valery M. Gavrilov2, Anvar B. Kerimov2, & Elena V. Ivankina2
1Department of Biology, University of California, Los Angeles, California, 90095-1606, USA, fax 1 310 825 9433, e-mail kennagy@biology.ucla.edu; 2Department of Vertebrate Zoology and General Ecology, Moscow State University, Moscow 119889, Russia.Nagy, K.A., Gavrilov, V.M., Kerimov, A.B., & Ivankina, E.V. 1999. Relationships between field metabolic rate, basal metabolic rate and territoriality in passerines. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 390-400. Johannesburg: BirdLife South Africa.
Reproductive effort is the portion of its total food energy intake that an animal allocates to production of offspring, and is probably a key aspect of species fitness. For female birds, most energetic costs of production are evident, but reproductive costs paid by males are harder to define and measure. For example, some male birds initiate breeding behaviour many months before the breeding season by establishing and maintaining territories. We attempted to measure this aspect of male reproductive effort by determining field metabolic rates (FMRs, via doubly labelled water) and basal metabolic rates (BMRs, with open-flow respirometry) in free-living Great Tits Parus major in a forest near Moscow during spring (March), when birds were establishing breeding territories. FMR was positively correlated with territorial status (as determined by daily behaviour observations). The intercepts of the regression line indicate that the cost of territory maintenance increased mass-specific FMR by about 45%. BMR was not correlated with residency times in this sample, but the FMR/BMR ratio was: FMR increased from 2.6 times BMR in non-territorial males to 3.7 in fully territorial males.
INTRODUCTION
Energy, in the form of food, is often presumed to be a major limiting factor for bird populations. It follows that individual birds that are more effective in converting dietary energy into offspring which breed successfully should achieve a selective advantage, and should contribute more genetic information to the next generation. Using energy as a currency, the concept of reproductive effort (Fisher 1928; Stearns 1976) expresses the proportions of total energy flow through a bird that is allocated to reproductive processes. Reproductive energy includes the chemical potential energy accumulated in biomass of new born offspring plus growth of neonates resulting from food the parents provide, plus the extra energy ending up as heat via the metabolic processes associated with reproduction that parents pay. For birds, these metabolic expenditures include costs of courtship, nest-building, incubation, and food-provisioning and defending nestlings through fledging (Drent & Daan 1980). Females pay additional costs to obtain and process the extra food needed to synthesise eggs, and males may incur costs for establishing and maintaining territories before and during the nesting phase.
A variety of studies have been done on breeding birds (Immelman 1971; Carey 1996) and other vertebrates (Congdon, Dunham and Tinkle 1982; Nagy 1983; Loudon and Racey 1987). Most of the measurements were for females, which bear the brunt of reproduction regarding investment of energy into new biomass as eggs. However, the energetic cost of reproduction to males (prior to conception) can be quite large, increasing field metabolic rates (FMR) by 55% in rutting antelope (Nagy and Knight 1994), by about 45% in breeding lizards (Nagy 1983a), and perhaps by similar amounts in birds (Dolnik 1995). This study was done to determine the energetic costs of territoriality in male birds.
The Great Tit Parus major is the common hole-nesting species of moderate and high latitudes of Europe and Asia. Around Moscow and in other regions of Europe, Great Tit populations consist of both sedentary (resident) and migratory individuals. In optimal habitats, resident birds form 'flocks' having a social hierarchy during autumn and winter. The boundaries of adjacent flocks are flexible and they overlap (Perrins 1979; Ekman 1989). Breeding males maintain and defend territories, primarily by song (Dhondt 1971; Krebs 1977), against the intruders. Various aspects of resting and basal metabolic rate (BMR) of Great Tits in Europe have been measured (Hissa and Palokangas 1970; Mertens 1977, 1980; Gavrilov 1994; Gavrilov et al. 1994, 1995; Kerimov et al. 1994). In this study, we measured both BMR and FMR in male tits that were territorial to varying degrees before nest building commenced.
METHODS
Study Site
Field work was done at the Zvenigorod Biological Station (Moscow State University), located about 70 km west of Moscow (55o44’N, 36o51’E). Great Tit (Parus major) males living in flocks in mixed and coniferous forest near the Moskva River were studied (Kerimov et al. 1994; Gavrilov et al. 1996). There were 500 nest boxes set out on tree trunks in the forest around the station’s buildings, the nest boxes were present in excess, and were used by several bird species, including Great Tits. The FMR measurements were done in late March (25 to 28 March 1995) when spring migration was peaking. The ground was covered by some snow, and it snowed during FMR measurements. A minimum-maximum thermometer indicated that daily air temperatures ranged between -3 and +4 oC, and the mean daily temperature during FMR measurements was +1.0 oC.
Territoriality
The Great Tits using this site have been studied for many years. The 'flock range' in prime habitat is where Great Tits occur in large numbers throughout the year. During autumn and winter, they organise flocks in such habitats, and during the reproductive period in spring and summer, they form dense breeding settlements consisting of small, adjacent territories. There was intense competition for centrally-located territories within the flock range (Kerimov et al. 1994; Gavrilov et al. 1996). Because we wished to measure the cost of establishing and maintaining new breeding territories by males, we did not study males that returned to territories they held the previous year.
Beginning in January, birds were captured in mist nets and in cage traps at feeding stations using lure birds of the same or a related species (Parus ater). Captured birds were ringed with aluminium leg bands and were given coloured leg bands for later visual identification. Birds were weighed and standard lengths were measured before release. Visual observations were done nearly daily between late February and late April to determine establishment and maintenance of territories by banded males. Territory status was given a value of two if the male showed strong territorial behaviour (singing plus displaying and fighting) within the flock range. A value of one described strong territorial behaviour at the periphery of the flock range, and a value of zero was applied to birds not showing territorial behaviour at all in the study area.
Metabolic rate measurements
Field metabolic rates (FMR) were measured using the doubly labelled water method. Captured males were immediately injected in the pectoral muscle with 50.6 microlitres of distilled water containing 96 atom percent oxygen-18 and 17 microcuries (630 becquerels) of tritium. We held the birds in cloth bags or small cages for 60 minutes to allow the isotopes to equilibrate completely in body water, then took a small (50 microlitre) blood sample from a wing vein. Blood samples were flame-sealed in heparinised microhematocrit tubes and refrigerated until analysis at the University of California, Los Angeles. Of the 25 males injected, 12 were recaptured once and one was recaptured three times within the two-day duration of the isotope dose. Blood samples from three uninjected males were taken for measurement of isotope natural abundances. In addition, samples from four recaptures of six injected females were analysed to increase the sample size and reliability of the photoperiod correction of FMR results (see below).
Pure water was distilled from blood samples under vacuum (Nagy 1983). Aliquants were analysed in duplicate for tritium activity by liquid scintillation spectrometry, and in triplicate for oxygen-18 concentration by proton activation analysis (Wood et al. 1975). Body water volumes, necessary for the FMR calculations, were estimated for the times of injection from the dilution volumes of injected oxygen-18 (Nagy 1983). Body water volumes at recapture were estimated from body mass, assuming individuals maintained the same fractional body water content during the study. Field metabolic rates were calculated using equation (2) in Nagy (1980), as modified from Lifson and McClintock (1966). Rates of CO2 production were converted to units of energy (J or joules) using the relationship 25.7 J/ml CO2 produced for an insectivorous diet (Nagy 1983).
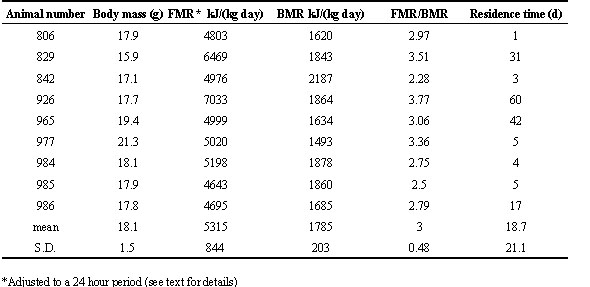
Because recapture of injected birds was opportunistic, FMR measurement intervals were sometimes less than or greater than 24 hours, so some FMR values included disproportionate amounts of daylight or darkness hours. We corrected FMR values to the natural 24 h photoperiod of 12.7 h light: 11.3 h dark during the study, as follows. The proportion of each FMR measurement interval that was light was calculated using times of sunset (plus 30 min. for twilight) and sunrise (minus 30 min.), and expressed as light time divided by total time. A least squares linear regression of FMR, in units of kJ (kg day)-1, on proportion of light time per total time for all males was highly significant (F = 6.86, df = 18, P = 0.0074), and the positive slope indicated that a bird’s FMR was higher when its FMR measurement interval included more daylight time (Fig. 1). We used the resulting regression equation [kJ (kg day)-1 = 2673 + 4632 ´ (time light/total time), r = 0.59] along with each bird’s FMR and time light/total time ratio to predict the intercept for that bird (assuming the slope of 4632 is applicable to all study birds). Then, we used the same equation, given that intercept and the common slope, to calculate FMR when the time light/total time ratio = 0.528, which was the natural photoperiod during our field measurements. This correction changed FMR values only a little (mean change = 3%, SD = 8%) because most FMR measurement intervals were near 24 or 48 hours.
Basal metabolic rate (BMR) measurements were done at night in an open-flow system using glass 3 litre metabolism chambers kept in the dark in incubators at 26 oC, which is well within the thermal neutral zone of Parus major (Gavrilov 1997). BMR measurements were done on the same birds used for FMR measurements, but BMRs were usually measured within a few days of FMR measurements, and not during FMR measurements. Birds captured in the evening were placed in small cages without food at 20 to 23 oC for at least seven hours before measurements began. Cages containing the birds were placed inside the metabolism chambers singly for at least one hour for thermal stabilisation and for the bird to settle down. CO2 produced by the bird was absorbed by KOH placed in the chamber (Dolnik and Gavrilov 1979; Gavrilov 1997). Chamber pressure was measured with a water-filled manometer. Pure O2 was metered into the chamber from an attached 2-liter glass container of O2, fitted with a water-filled burette, in order to exactly replace O2 consumed, and return the animal chamber to its original pressure. Oxygen consumption was measured in 10-minute intervals for one hour, and measurement sets with high variation between the six 10-minute intervals were discarded. Measurements were made only between four hours after dusk and two hours before dawn. Metabolism chambers were confirmed to be air-tight at the beginning and end of each experiment. Birds were weighed after removal from metabolism chambers. Oxygen consumption values were corrected to standard temperature and pressure according to Depocas and Hart (1957), and oxygen measurements were converted to units of energy using the factor 19.7 kJ (litre O2 consumed)-1 for fat metabolism.
Results are reported as mean and standard deviation (S.D.). Correlations were tested for statistical significance (P < 0.05) using least squares regression analysis of log10 transformed or untransformed data.
RESULTSOf the 13 male Great Tits recaptured for FMR measurements, we could obtain reliable BMR along with territory status measurements for only nine, and these data are reported herein. Average body mass was 18.1 (+/- 1.5) g (Table 1), and body water contents (from 18O dilution spaces) averaged 68.7 (+/-3.6)% of body mass. Water influx rates, which the doubly labelled water method also estimates, averaged 590 (+/- 207) ml H2O (kg day)-1. The mean rate of body mass change was -0.47 (+/- 4.43)% (day)-1, which was not significantly different from zero mass change (= weight maintenance).
Neither BMR nor FMR were significantly correlated with body mass in allometric analyses (regressions of log10 whole animal BMR or FMR vs. log10 body mass), which is not surprising given the sample size of nine and the narrow range of body masses (15.9 to 21.3 g). Nevertheless, because larger animals usually use more energy than do smaller ones, we expressed these rates in mass specific units of kg-1 for subsequent analyses. The mean BMR of the nine males was 1785 (+/- 203) kJ (kg day)-1 and FMR averaged 5315 (+/- 844) kJ (kg day)-1 (Table 1). FMR was 3.0 ´ BMR on average. Only two of the nine males we studied were strongly territorial in the centre of a flock range, and one showed intermediate territoriality. Individual males arrived at the study site at various times during this two-month period, so we obtained FMR values for birds that may have been just beginning territorial behaviour, or may have been holding territories for some time at the study site. BMR was not significantly correlated with territory status (F = 0.05, P = 0.83) in this study. However, FMR was significantly related to territory status (F = 23.9, P = 0.002), such that the birds displaying strong territoriality had relatively high energy expenditures in the field (Fig. 2). Similarly, the ratio of FMR to BMR (Fig. 3) increased significantly with increasing territory status (F = 8.00, P = 0.026). There was no relationship between body mass and territory status (F = 1.17, P = 0.32).
DISCUSSION
The significant positive correlation between FMR and territory status in male Great Tits (Fig. 2) indicates that there is a measurable energetic cost of territorial behaviour . The r2 value for this regression is 0.77, suggesting that about three quarters of the variation in FMR in these males was associated with territoriality. The two intercepts of the regression line, one at 0 territory status and one at status two (maximum territoriality), can be used to estimate the energetic cost of territorial behaviour. The difference between the two intercepts [4571 and 6607 kJ (kg day)-1] is 2036 kJ (kg day)-1 which is an increase of 44% in FMR. This is equivalent to an increase in the FMR to BMR ratio from 2.63 to 3.63 (Fig. 3). Our measurements were made during the middle of the 2-month territory establishment period.
The FMRs of the Great Tits we studied were relatively high. The mean of 5315 kJ (kg day)-1 for these 18.1g birds is significantly greater [judging by its 95% confidence intervals of 4666 and 5964 kJ (kg day)-1] than the expected mean FMR of 3888 kJ (kg day)-1 for a typical bird of its size (Williams et al. 1993), or the 4293 kJ (kg day)-1 expected for a typical passerine bird (Nagy 1987). Nagy’s (1987) equations (kJ d-1 = 9.57 g0.689 for all birds, and kJ d-1 = 8.88 g0.749 for passerines) are based on FMRs measured in birds living under a variety of conditions and in different seasons, including the breeding season. A more recent equation by Masman et al. (1989) includes 13 species of passerine birds whose FMRs were measured only during parental care (FMRpar: kJ d-1 = 17.07 g 0.57). This relationship yields a predicted FMRpar of 4914 kJ (kg day)-1 for an 18.1 g bird. Similarly, the equation of Daan et al. (1991) for 28 species of altricial birds during parental care (kJ d-1 = 13.93 g0.657) yields an expected value for an 18.1 g bird of 5158 kJ (kg day)-1. Both of these predictions are within 8% of the mean FMR of our male Great Tits, and are within the 95% confidence interval of the mean.
The FMRpar of female Great Tits has been measured while tending their 11-day old nestlings (Tinbergen and Dietz 1994). These 17.7 g birds were living in woods in the Netherlands (Ta 9-22oC) and had an average FMRpar of 5373 kJ (kg day)-1, which is the same as we measured in territorial males. Male Great Tits maintaining territories prior to breeding apparently were working as hard as were females caring for nestlings; however this comparison is complicated by the substantial differences in thermal environment between the two study sites.
Great Tits and other small passerines are quite successful in northern forest habitats. It has been suggested that their capabilities for high rates of energy metabolism (Gavrilov 1997) and nest building abilities (Collias 1997) are important reasons for this success.
ACKNOWLEDGMENTS
This study was funded by the Russian Fund of Basic Research, and by the Department of Biology, University of California, Los Angeles. We thank Dr. T.A. Ilyina, student V.G. Grinkov, and Dr. V.V. Gavrilov for assistance in the field.
REFERENCES
Carey, C. 1996. Female reproductive energetics. In: Carey, C. (ed.) Avian Energetics and Nutritional Ecology. New York: Chapman & Hall: 324-374.
Collias, N.E. 1997. On the origin and evolution of nest building by passerine birds. Condor 99:253-270.
Congdon, J.D., Dunham, A.E. & Tinkle, D.W. 1982. Energy budgets and life histories of reptiles. In:Gans C. and Pough, F.H. (eds.). 1982 Biology of the reptilia, volume 13. London: Academic Press:233-271.
Daan, S., Masman, D., Strijkstra, A.M. and Kenagy, G.J. 1991. Daily energy turnover during reproduction in birds and mammals: its relationship to basal metabolic rate. Acta XX Congressus Internationalis Ornithologici:1976-1987
Depocas, F. & Hart, J.S. 1957 Use of the Pauling oxygen analyzer for measurement of oxygen consumption of animals in open-circuit systems and in a short-lag, closed-circuit apparatus. J. Applied Physiology 10:388-392.
Dhondt, A.A. 1971. Some factors influencing territory in the Great Tit, Parus major L. Le Gerfault 61:125-135.
Dolnik, V.R. 1995. Energy and time resources in free-living birds. St. Petersburgh: Nauka (in Russian, English summary).
Dolnik, V.R., & Gavrilov, V.M. 1979. Bioenergetics of molt in the chaffinch (Fringilla coelebs). Auk 2:253-264.
Drent, R.H. and Daan, S. 1980. The prudent parent: energetic adjustments in avian breeding. Ardea 68: 225-252.
Ekman, J. 1989. Ecology of non-breeding social systems of Parus major. Wilson Bulletin 101:263-288.
Fisher, R.A. 1928. Genetical theory of natural selection. London; Oxford University Press
Gavrilov, V.M. 1994. General principles of temperature effects on energetics of homeothermic animals (with reference to Parus major, Passeriformes, Aves). Dolk. Akad. Nauk. 334:121-126 (in Russian).
Gavrilov, V.M. 1997. Energetics and avian behaviour . Physiology and General Biology Reviews 11:1-225 Amsterdam: Harwood Academic Publishers.
Gavrilov, V,M., Kerimov, A.B., Golubeva, T.B., Ivankina, E.V., Ilyina, T.A., Karelin, D.V., & Kolyaskin, V.V. 1994. Ecological energetics and population ecology of the Great Tit (Parus major). Pages 111-158 in Rings in the Study of Bird Migration in Eastern Europe and Northern Asia, Moscow: Nauka (in Russian, English summary).
Gavrilov, V.M., Kerimov, A.B., Golubeva, T.B., Ivankina, E.V., Ilyina, T.A., Karelin, D.V., and Kolyaskin, V.V. 1996. Energetics, morphological and physiological heterogenity and the population structure in birds. I. Energetics, morphological and physiological heterogenity and the population structure in Great Tits in the Moscow region. Ornithologia 27:34-73 (in Russian, English summary).
Hissa, R., & Palokangas, R. 1970. Thermoregulation in the Titmouse (Parus major L.). Comp. Biochem. Physiol. 33:941-953.
Immelman, K. 1971 Ecological aspects of periodic reproduction. In: Farner, D.S., King, J.R. and Parkes, K.C. (eds.) Avian Biology, Vol. 1. New York: Academic Press: 341-389.
Kerimov, A.B., Ivankina, E.V., Iliyna, T.A., and Gavrilov, V.M. 1994. Dynamics of morphophysiological correlates of social status during the annual cycle of the Great Tit (Parus major, Passeriformes, Aves). Dolklady Biological Sciences 334:57-60.
Krebs, J.R. 1977. Song and territory in the Great Tit Parus major. In: Stonebhouse, B. and Perrins, C. -(eds) Evolutionary ecology. Baltimore: University Park Press: 47-62.
Lifson, N. & McClintock, R. 1966. Theory of use of the turnover rates of body water for measuring energy material balance. J. Theoretical Biology 12:46-74.
Loudon, A.S.I. & Racey, P.A. (eds.). 1987. Reproductive energetics in mammals. Oxford:Oxford University Press:371 pp.
Masman, D., Dijkstra, C., Daan, C., Bult, A. 1989. Energetics limitations of avian parental effort: field experiments in the kestrel (Falco tinnunculus). J. Evolutionary Biology 2;435-455.
Mertens, J.A.L. 1980. The energy requirement on incubation in Great Tits and other bird species. Ardea 68:185-192.
Nagy, K.A. 1980. CO2 production in animals: analyses of potential errors in the doubly labelled water method. American J. Physiology 238: R466-R473.
Nagy, K.A. 1983a. Ecological Energetics. In: Huey, R.B., Pianka, E.R., Schoener, T.W. (eds.). Lizard ecology: studies of a model organism. Cambridge, MA: Harvard University Press: 24-54.
Nagy, K.A. 1983b. The doubly labelled water (3HH18O) method: a guide to its use. University of California, Los Angeles, Publication No. 12-1417.
Nagy, K.A. 1987. Field metabolic rate and food requirement scaling in mammals and birds. Ecological Monographs 57: 111-128
Nagy, K.A. & Knight, M.H. 1994. Energy, water, and food use by Springbok antelope (Antidorcas marsupialis) in the Kalahari Desert. Journal of Mammology 75: 860-872
Perrins, C.M. 1979. British tits. Collins, London, 304 pp.
Stearns, S.C. 1976. Life-history tactics: a review of the ideas. Quarterly Review of Biology 51:3-47.
Tinbergen, J.M., Dietz, M.W. 1994. Parental energy expenditure during brood rearing in the Great Tit (Parus major) in relation to body mass, temperature, food availability and clutch size. Functional Ecology 8: 563-572.
Williams, J.B., Siegfried, W.R. Milton, S.J., Adams, N.J., Dean, W.R.J., de Plessis, M., Jackson, S., & Nagy, K.A. 1993 Field metabolism, water requirements, and foraging behaviour of wild Ostriches in the Namib. Ecology 74: 390-404.
Wood, R. A., Nagy, K.A., MacDonald, N.S., Wakakuwa, S.T., Beckman, R.J., & Kaaz, H. 1975. Determination of oxygen-18 in water contained in biological samples by charged particle activation. Analytical Chemistry 47:646-650.
Table 1. Field and basal metabolic rates (FMR and BMR), body masses and territory residence times of nine male Great Tits Parus major in early spring near Moscow.

Fig. 1. Method for correcting FMR values to representative 24 hour day with a natural photperiod of 0.528 days of light (dawn to dusk). Solid line is least squares regression line; dashed lines represent 95% confidence intervals of the regression.
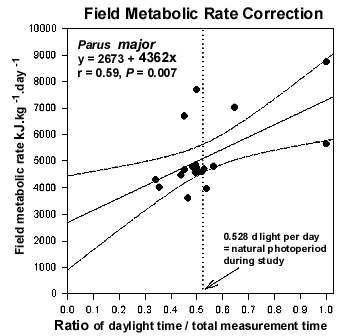
Fig. 2. Relationship between field metabolic rate of male Great Tits and the amount of time they maintained a territory.
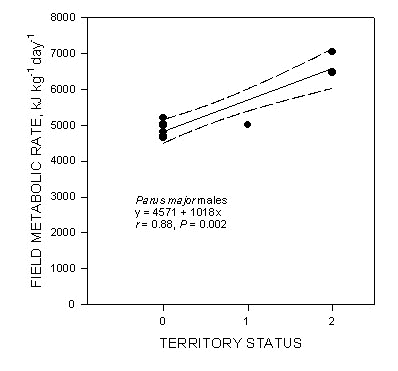
Fig. 3. Relationship between the ratio of field to basal metabolic rates measured in individual male Great Tits and their territorial residency time.
