S07.2: The basal metabolic rate and its relationship to social and reproductive status in individual passerine birds
A.B. Kerimov & E.V. Ivankina
Department of Vertebrate Zoology and General Ecology, Moscow State University, 119899, Russia
Kerimov, A.B. & Ivankina, E.V. 1999. The basal metabolic rate and its relationship to social and reproductive status in individual passerine birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
370-389. Johannesburg: BirdLife South Africa.A recently-developed model of avian energetics (Gavrilov, 1997) predicts that the basal metabolic rate (BMR) of an individual bird reflects its long-term working capacity, and consequently its competitive potential. Unfortunately, the relationship of BMR to fitness is not well studied in birds. We observed male wild Great Tits Parus major near Moscow during the non-breeding season and recorded those aspects of their flocking and territorial behaviour, that more or less affect subsequent reproductive success. The birds were captured opportunistically, and their rates of oxygen consumption at night time were measured as estimates of their BMR. The mass specific BMR was higher in young males than in old males and higher in resident flock members than in transient birds. The BMR of territorial males exceeded that of non-territorial males; this trend was more pronounced in young birds from the beginning of the winter season. BMR correlated with features of social behaviour related to flock rank only in new members of basic flock. Prior residency time influenced the BMR of old and young flock members in opposite directions. A high BMR occurred in yearlings during their first month after joining the flock; BMR in these birds dropped to lower levels thereafter. In older flock members BMR tended to increase with the time spent in the flock area. In accordance with the energetic model, the BMR increase associated with an increase in status in the flock appears to reflect the additional energy cost of the better social position. New members of the population (primarily young birds) have to spend to spend more energy during the formation of social bonds to compensate their lack of social experience.
INTRODUCTION
Basal metabolic rate (BMR) variations due to taxonomic status, phase of annual cycle and climate have been interpreted earlier in terms of bioenergetics and ecological adaptations (Lasiewski & Dawson, 1967; Kendeigh et al., 1977; Dolnik, 1995). A recently-developed model of avian energetics (Gavrilov, 1997) predicts that BMR of an individual bird reflects its long-term working capacity, and consequently its competitive potential. Thus, it is particularly interesting to evaluate BMR variation in relation to social and other population factors in free-living birds and to use this index as an indicator of an individual’s functional capacities. However, such attempts have been infrequent (Roskaft et al., 1986; Hogstad,1987; Gavrilov et al., 1993; Reinertsen & Hogstad, 1994) and the relationship of BMR to fitness remains poorly studied in birds.
This study was designed to evaluate variations in the BMR of individuals in relation to male social position in free-living Great Tits Parus major wintering in prime habitat. We attempted to evaluate whether:
(1) BMR was related to male territorial status.
(2) BMR was related to social rank in the flock
(3) Any relationship between BMR of a male and duration of its previous stay in the flock range existed.
MATERIAL & METHODS
Study area and flock structure
The research was carried out in 1992-1997 at Zvenigorod Biological Station (Moscow State University) in the valley of the Moskwa river (55o44’N, 36o51’E), in mixed and coniferous forests. Great Tit populations in the northern and mid-latitude parts of European Russia as well as in some regions of continental Western Europe (Perrins, 1979; Noskov & Smirnov, 1981) consist of sedentary and migratory birds. During autumn and winter, tits form flocks. Flock ranges are amorphous and may overlap. The site where tits occurred in large numbers both in winter and spring was defined as prime habitat ( Kerimov et al., 1994). In prime habitat, the flock range is subsequently used by a majority of regular flock members for breeding. While switching over to their breeding behaviour the males gradually break the bonds of flocking and begin to establish and maintain territories, defending them from intruders of the same species. Besides previous flock members a breeding population consists of spring immigrants. In the study site the flock range was a square of about 7 ha located in prime habitat. It included the small Station settlement, enclosed by a mixed forest. During autumn and winter the tits were provided with a constant food supply of sunflower seeds at feeding tables. Nest boxes, which were used by tits for roosting and nesting, were set out in abundance near the buildings and in the adjacent forest. We distinguished three groups of tits differing in their association with the flock area. The first group formed the flock nucleus (similar to the structure defined as ‘basic flock’ by Saitou, 1978; 1979). Its size was relatively stable in different years, numbering 11-15 males and 2-5 females. These birds settled in the flock range until the current October and were tightly connected with this area during a greater part of non-reproductive season. About 70% of them (so-called residents) subsequently occupied individual territories within the flock range. The second group consisted of the birds, which episodically visited the flock range or joined the flock for a short time. Their number varied markedly between years. Only few of them subsequently hold territories in the flock range. The third group consisted of transient birds, passing through the flock range. The majority of them were observed during autumn or spring migrations. The relations between flock members were based on social hierarchy. Social rank determined the order of access to food at feeding tables and influenced the location of breeding territory of a male in relation to the centre of the flock range (Kerimov et al., 1994).
Methods
From September until May tits were captured in mist nets and in cage traps. Besides standard aluminium rings they were tagged with a combination of colour rings on their legs and paint on their plumage for later visual identification. Age was estimated by coloration of primary coverts (Vinogradova et al., 1976) and verified by ringing data. Body mass and standard lengths were measured before release. Visual observations were done nearly weekly throughout the entire non-reproductive season to determine the presence of the bird in the flock area.
BMR measurements.
The BMR was determined as the rate of oxygen consumption by a resting bird at midnight using a modified Kalabukhov closed system (Gavrilov, 1979; Gavrilov et al, 1996). The birds captured in evening were studied that night, then released the next morning. A bird was placed in small cage, kept in the dark without food at 20-23°C for at least 4 hours. Then, the cage with a bird was placed in a glass 3-litre metabolism chamber and kept in the dark in an incubator at 26°C . One hour was allowed for adaptation and thermal stabilisation. The chamber was then sealed and O2 consumed was replaced by metering in pure O2. CO2 in the chamber was removed using KOH. Oxygen consumption was measured over 10-minute intervals for one hour. Temperature in the chamber was measured by mercurial thermometer. A bird was weighed after removal from chamber. Oxygen consumption values were corrected to standard temperature and pressure (Depocas and Hart, 1957). 601 measurements of BMR were taken from 363 males. Many birds were measured several times during non-reproductive season, but not oftener than once in two weeks. Only birds which finished autumn moult were measured. We calculated mass specific rates of oxygen consumption per 1 hour (ml O2 consumed * h-1*g-1 ) as estimates of bird’s BMR. These values may be converted to energy units using the relation 19.7 kJ = 1 litre O2 consumed (for fat metabolism).
Age, season and behavioural variables
Age factor
Two age classes were distinguished in this study: ‘young’ (yearlings, subadult) and ‘old’ (adult).
Seasonal factor
Five periods of the annual cycle were selected to test for seasonal effects on BMR. The exact dates of these periods shifted slightly from year to year in relation to variation in weather conditions between years:
(1) Period of autumn migration and flock formation (from the end of the tit moult in mid September - October until formation of a stable snow cover in November - early December).
(2) Period of long-term residency and sustainable flocking bonds in wintering great tits (from December to February, before some wintering residents begin to sing).
(3) Period of early territoriality (February - March, before spring migration).
(4) Period of spring migration (March - early April).
(5) Beginning of the nesting period (late April - May).
The 1st through the 4th periods are the non-reproductive season. Additionally, we assigned the value of 1 to periods 1 and 2, and the value of 2 to periods 3 and 4.
Territorial status
Various aspects of male spatial behaviour were taken into account to determine individual territorial status. The point of reference was the time just after ending of moult in the population in the current non-reproductive season. The degree of membership in the wintering group was assigned two values:
0 - transient tits, which spent less than a week in the flock area in the current non-reproductive season;
1 - wintering tits or ‘flock members’ (2 weeks and more in the flock area in the current non-reproductive season).
The factor ‘ territorial status-1’ reflected the total duration of male connections with the flock area during the current season. The numerical values assigned were:
1 - weak bonds with flock area (for birds which were observed at least once during one calendar month);
2 - moderate bonds (birds were observed during two calendar months);
3 - strong bonds (presented three and more calendar months).
The factor ‘territorial status -2’ reflected the date when a male settled in the flock area in the current non-reproductive season. The following values of this factor were used for wintering young males:
1 - late date (after the end of autumn migration);
2 - intermediate date (during the period of autumn migration );
3 - early date (before the beginning of autumn migration).
For old males, which as a rule joined the flock later than did young birds, the dates were delayed one month.
The factor ‘ territorial status -3’ reflected whether a male had participated in the division of the flock area into territories in the subsequent or (and) preceding reproductive season:
1 - territorial male (who manifested active forms of territorial behaviour in the flock area);
0 - non-territorial male.
The factor ‘territorial status -4’ represented the prior residency time spent by a wintering male in the flock area in the current non-reproductive season before the day it was captured for BMR measurement. Values assigned were:
1 - less than one month in the flock area before BMR measurement;
2 - from one to two months;
3 - more than two months.
In the simplified classification of this factor, the first two categories were combined and given a value of one, and the third category was graded as two.
Special consideration was given to long-term participation of old males in the flock area (factor ‘territorial status - 5’):
0 - an old male was not observed in the flock area in preceding seasons;
1 - it was observed in the flock area before the current season.
The result of each BMR measurement represented a sample unit in analysis of the relationships between BMR and mentioned above variables.
Indexes of male flocking behaviour
The social relationships of males were evaluated from observations of interactions of wintering great tits on the feeding tables from October to early February. In this part of the study, each male was characterised by its mean BMR value determined from separate measurements at a given phase of the annual cycle. Estimation of social rank was based on outcomes of fights and ‘pecking order’ for feeding. From the results of 65 observation sessions, each of which lasted for 0.5 to 1.5 h, the following indexes were calculated:
(1) the feeding index, defined as the average number of successful visits to a feeding table over 0.5 h;
(2) the attack index, defined as the average number of attacks towards other great tits of both sexes over 0.5 h;
(3) the defeat index, defined as the average number of successful aggressions by conspecific birds towards a given male over 0.5 h;
(4) the victory index, defined over the entire period of observation as a percentage of aggressive interactions with other great tits in which a given male won;
(5) the participation index, calculated over the entire winter period as a ratio of observation sessions during which a given male was noticed in the flock near the feeding table to those during which this male could be expected to attend the feeding table (because it was observed in the study site), but had not done it.
Statistical analysis
In many sets of analyses, the variation due to year or season was accounted for by standardisation of the individual measurements according to the relation (xi - x )/sx. Here, xi is the individual value of the parameter to be analysed, x is its mean value for a given year or season, and sx denotes the standard deviation of the sample mean. Analysis of variance (one-way and two-way ANOVA), t-tests and Spearman rank correlation were used to process the data statistically.
RESULTS
Age-dependent BMR variation
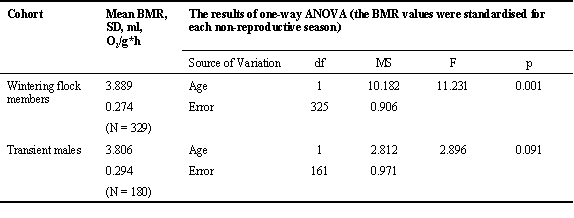
Overall, BMR was significantly affected by age during non-reproductive season. Its level was higher in young males than in old ones (N = 530; F = 5.059; p = 0.025; ANOVA; the sample consisted of wintering males, transients and spring immigrants, which occupied breeding territories in the flock range). However, the effect of age was pronounced only in wintering flock members. Similar relation in transient males was not significant (Table 1).
Annual and seasonal variations of BMR
The effect of year on BMR in the non-reproductive season was clearly expressed in the overall sample (p = 0.000; F = 9.53; n = 550 for 5 years; ANOVA), as well as in different age and territorial groups (Table 2). BMR change due to year was mainly resulted from its marked variation in two of five years (Table 3).
BMR was not related to the period of the non-reproductive season (1-4 periods) in the overall sample (p = 0.06) and in subsamples of transients and flock members (Table 2; Fig.1). However, BMR decreased in spring at the beginning of reproductive season (5-th period). This effect was pronounced in both age groups (N = 450; F = 7.29; p = 0.000 by two-way ANOVA with age taken into account as the second factor).
Mean, minimal and maximal ambient temperatures during a day and during the 5 days period preceding BMR measurement did not correlated with BMR during the non-reproductive season or within its individual periods (p = 0.5 - 0.9; n = 313-350).
Influence of male territorial status on BMR during the non-reproductive season
Mean BMR in flock members was higher than in transients (df = 505; T-value = 3.499; p = 0.0005 for data standardised by year). A similar trend was observed in both age groups, but among young males this difference was larger (Table 4; Fig.2). The total duration of association with the flock area (factor ‘territorial status 1’) significantly influenced BMR (Table 4). BMR was maximum in males which spent the most time in the flock area. However, a linear increase of BMR occurred only in old males. Among young males, all birds observed in the flock area during more than one calendar month had similar high mean BMR.
At the end of winter and in early spring (periods 3-4) the BMR of flock members was related to the terms of their settling in the flock area (factor ‘territorial status 2 ‘; Table 4; Fig. 3). It was highest in the birds which joined the flock in early autumn.
Territorial males had higher BMR than non-territorial males during overall non-reproductive season (factor ‘territorial status 3’; Table 4; Fig.4). Among young males, the increase in BMR due to subsequent territoriality was more pronounced and took place at different phases of non-reproductive season (Table 4). It was most clearly exhibited in the young members of basic flock (N = 120; F = 9.308; p = 0.003 by two-way ANOVA, with season period as the second factor).
The energetic correlate of territoriality was similar both in residents and spring migrants, which tried to establish breeding territories in the flock area. However, residents were establishing their territories earlier than spring migrants. When the latter achieved their BMR peak the residents were in the stage of seasonal BMR decline to the summer level. Thus, BMR of territorial migrants tended to be higher than in residents in April (N = 35; F = 5.26; p = 0.028; ANOVA).
The effects of the above-mentioned territorial factors appeared to be partly interconnected, because local territory holders were mainly recruited from the birds which were tightly associated with the flock area.
The relation of BMR to prior residency time prior residency time spent in the flock area (factor ‘territorial status 4)’influenced BMR of old and young males in opposite directions. This is indicated in the interaction between age and ‘status’ factors in analysis of the overall sample of wintering males in 1-3 season periods (Table 5; Fig.5). Among old males, the BMR increased in relation to prior residency time. This process was most noticeably exhibited during the first part of the non-reproductive season, reflecting a BMR increase in old males which settled in the flock area from early autumn (N = 88; F = 6.024; p = 0.016 for periods 1-2; ANOVA). On the contrary, the BMR was high during the first month after joining the flock and low afterwards in young males (N = 165; F = .364; p = 0.038 for periods 1-3; ANOVA).
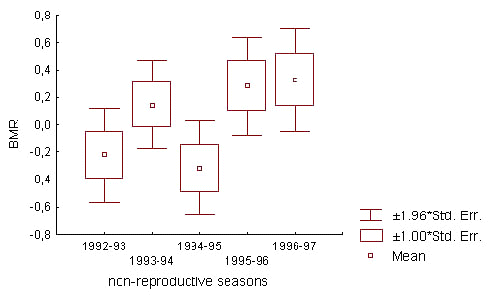
The alternative tendencies in BMR trends, displayed by old and young males, were differently expressed in particular seasons. Annual variations in the effect of prior residency appeared to be related to differences in the flock social structure. Two groups of winter seasons were distinguished according to social features of the flock: ‘stable’ (1992-93 and 1994-95) and ‘unstable’(1993-94, 1995-96 and 1996-97). These years differed in the pattern of seasonal BMR change in wintering males, that was expressed in year to year variation of the mean annually standardised BMR values at the end of winter (Fig.6). In ‘stable’ seasons the flock hierarchical structure, which was established in autumn, did not change until the end of winter. The tendency of the BMR drop due to prior residency time was exhibited very sharply in young males. The BMR value of old males almost did not vary, remaining at low level (Table 5). In spite of the BMR decline with the course of the winter season, the effect of prior residency time was significant in young males, when the period of season was taken into account (N = 77; F = 5.647; p = 0.020 by two-way ANOVA, with period of season as the second variable). In ‘unstable’ seasons the composition of dominant males in the flock range changed from first to second halves of winter, or a large number of competitors for high social positions persisted for a long time. In such seasons the BMRs of young males were high during the fall-winter season and were not affected by prior residency time. Old birds demonstrated increasing BMR in accordance with increase of their prior residency time. Their maximum BMR reached the minimal BMR in young birds (Table 5). The influence of residency duration on BMR in old males remained very pronounced, with season as a second factor (N = 79; F = 9.263; p = 0.003; two-way ANOVA).
Preceding long-term connections with the flock area (factor ‘territorial status 5’) influenced the BMR of old males at initial phase of flock formation (N = 26; F = 8.017; p = 0.009; one-way ANOVA). In autumn (period 1) the newcomers had relatively high BMRs. This factor did not influence the BMR later in the season.
Social rank and BMR
The social rank in the flock hierarchy affected all behavioural indexes (except for the defeat index), which were estimated from interactions between free-living great tits at feeding tables (Kerimov & Ivankina, 1997). The effect of the male territorial status was similar. Territorial males (which subsequently or previously established territories in the flock area) were more successful than non-territorial males. In young birds, this superiority was most pronounced in the feeding and attack indexes. With the exception of the victory index, the social success indexes decreased with age. The influence of the social rank on the defeat index was expressed only among old males (N = 33; F = 5.916; p = 0.021; one-way ANOVA). Low ranked old males were most frequently exposed to successful attacks from the flock members.
A simple correlation between the BMR and social rank was not found. In the total sample, BMR correlated with only the index of participation in flock feeding (N = 61; F = 3.25; p = 0.019; two-way ANOVA). However, in the subsample of young and old birds that newly joined the flock nucleus (basic flock) in the current season, BMR positively correlated at strong and moderate level with all rank-related characteristics (Table 6).
Influence of age and social factors on male body mass
The slope of BMR(kJ/day) on body mass ranged from 0.66 to 0.75 in accordance to the allometric equations derived from wide size scale of passerine species (Lasiewski & Dawson, 1967; Dolnik, 1995; Gavrilov, 1997). We calculated the regression of lg BMR (kJ/day) on lg of body mass. This regression reflected the allometric relationship in spite of the narrow size range in the total sample of great tit males (N = 550; p = 0.0000 for intercept and slope):
BMR(kJ/day) = 3.02 *MASS 0.83
The slope lowered in the subsample of winter flock members and became similar to the value 0.75 from theoretical equation (N = 350; p = 0.0000 for intercept and slope):
BMR(kJ/day) = 3.72 * MASS 0.76
Hence, the mass specific BMR index (per 1 gram of body mass), analysed in the present work, can be proportional to mass to a power close to -0.25. It means that the body mass decrease should increase the mass specific BMR due to the allometric relationship. Some of considered trends of BMR variation, at least partly, can be stipulated by body mass change as if the analysed factors affected body mass and the BMR changes in opposite direction. To test this point, we examined the effect of population factors on body mass (Table 7). Such factors as age, date of establishing in the flock area and territoriality did not affect body mass. Prior residency time in old males and male membership in wintering group correlated with body mass in the same way as did mass specific BMR. Rank-related indexes of behaviour at feeding tables did not correlate with body mass in the total sample and among the new members of basic flock (rs = -0.08 - 0.16 at p = 0.52-0.83). In all these cases the correlation between energetics and social variables was exhibited independently of the allometric relationship. Prior residency time in young males appeared to be the only factor whose influence on mass specific BMR may be explained from allometric positions. The mass of wintering young birds increased in accordance with prior residency time, while their mass specific BMR decreased. Nevertheless, in groups that had a stable social structure for a long time, the energetic effect of this factor was so strong in young males, that it can be hardly explained by single allometric relation. In ‘stable’ seasons prior residency time was correlated with the BMR recalculated to the 0.75 power of body mass (N = 79; F = 8.971; p = 0.004 by one-way ANOVA.
The energy cost of differences in BMR influenced by social factors Maximal energy measure of BMR change due to social variables may be calculated using mean annual values and standard deviation of BMR (Table 3) and formula of standardisation of data by year applied in this study: xsti = (xi - x)/ SD, where x and SD are the mean value and standard deviation of the sample mean in a given non-reproductive season, respectively. Thus, the values to be compared may be presented as:x1 = x + xst1* SD and x2 = x + xst2 * SD. The per cent difference between them may be computed as
100%* (x1- x1)/ x1 = 100% *(xst2- xst1)* SD/ (x + xst1 *SD).
The calculation showed the following differences in BMR within age classes in certain non-reproductive seasons:
(1) flock membership corresponded to 3.6% increase in BMR;
(2) territoriality resulted in 2.6% increase of BMR;
(3) prior residency time corresponded to 6.9% BMR decrease in young members of the flock in seasons with stable social structure of the flock; this factor was related to 8.5% BMR increase in old males in ‘unstable’ seasons;
(4) early settling in the flock area was related to 6.9% increase of BMR in young males and 3.6% in old ones during the end of winter.
(5) The combination of social factors may result in more pronounced BMR change. For example, in the beginning period of joining the flock, the mean BMR of young members of basic flock which subsequently hold territories in the flock area exceeded that of young transients by 8.7%.
DISCUSSION
Two main points may be derived from the results. First, the BMR was found to relate to various indexes reflecting the position of a bird in social and demographic structure of population. Increased BMR was peculiar to the males that raised their social status. They managed to settle early in the flock area, they became the permanent flock members, established breeding territories in the preferable habitat and were successful in winter aggressive interactions and feeding. These social features are related to fitness since they more or less affect subsequent reproductive success in great tits and in other species with similar pattern of territoriality (Perrins, 1979; Ekman, 1989; Matthysen, 1990; Kerimov et al., 1994; Ilyina, 1996).
Second, the relationship between energetics and social variables was more pronounced in new recruits of local population (in general, in young males and in those old males who newly joined the flock in the current season).Our approach did not permit to decide definitely whether the higher BMR was a reason of social success or successful birds developed higher BMR. The first version is more likely in the case of territoriality. Among young males, the future territory holders had higher BMR well before the beginning of spring territorial behaviour. On the contrary, prior residency time appears to be a predictor of BMR. BMR of young males was high during the first phase of formation of their flocking bonds which included intense aggressive interactions. It dropped to lower levels thereafter when the males acquired stable position in the flock.
Does the increase in BMR reflect the energetic cost that a bird has to pay for improvement of its social position? Physiologically BMR and the resting metabolic rate may be influenced by a change in the levels of steroid hormones related to social factors (Roscaft et al., 1986). However, gonadotropin and testosterone plasma levels of free-living Swedish great tits, which are very similar to Moscow tits in terms of non-breeding ecology and reproduction, have pronounced seasonal variation (Rohss, Silverin, 1983; Silverin, 1984). Both young and adult birds had sharp peak plasma levels in March, well before the phase of accelerated testicular growth. These results do not correspond to the seasonal pattern in BMR changes observed in this study and permit rejection of the ‘hormonal’ hypothesis.
According to a recent energetic model, the BMR is unshared component of energy expenditure. It determines maximal long-term level of work output (Gavrilov, 1995; 1997). Increase of the BMR causes increase both the levels of maximal potential and potential productive energy that may be transformed into additional work. The model predicts the quantitative relation between daily energy expenditure (DEE) and the BMR at different ambient temperatures and at a given level of activity. The tendency to maintain a constant level of DEE at different temporal composition of behaviour was proposed to be related to the existence of an optimal DEE/BMR ratio in birds. This ratio varies in different avian taxonomic groups (Dolnik, 1982; 1996). In this respect, such processes as joining the basic flock, acquisition of dominant rank and establishing a territory appeared to have high energy cost. They relate to BMR increase that, in turn, indicates the raise of total energy expenditure of a bird, or provides its ability to use expensive strategies of behaviour. The model predicts the existence of long-term 4-fold ratio between MPE and BMR. It means that 3-8% of BMR increase due to social variables is related to additional energy of 0.09- 0, 24 BMR, which may be loaded for different functions besides the maintaining of basal metabolism. This amount is comparable with daily energy cost of moult (0.36 BMR), daily cost of feeding (0.42 BM) and territorial defence (0.04-0.5BM) in different species (see review by Dolnik, 1995).
Among sedentary Great Tits, the improved social position in the winter flock gives both short-term and long-term benefits related to future access to reproductive resources. The members of the basic flock have an advantage in competition for preferable breeding territories in the flock area and dominants have a priority in the choice of central territories (Kerimov et al., 1994). Thus, in terms of the energetic model the increase of BMR due to social status in wintering great tits appears to reflect the indirect energy cost of subsequent reproductive success. Further field observations based on the double-labelled water method are required to test the assumption about the increase of total energy expense in relation to increase of both BMR and social status in individual birds. The first steps in this direction were taken by Nagy et al. (in press) who showed increased DEE and DEE/BMR ratio in territorial males of great tits during the middle phase of competition for territories. At the same time, the transients that were not trying to establish territories had lower DEE levels, which were similar to those of existence metabolism predicted by the model for this species (Gavrilov et al., 1996).
Why do novices demonstrate more pronounced pattern of the BMR variation due to social factor? If the BMR, as predicted by the model, reflects the maximal level of work output, then the above mentioned results may support rather common idea that additional energy expense by an animal is required to compensate the lack of its social experience or the absence of a stable social position. Switching over to a high power regime facilitates the prolonged use of expensive activities, for example, such as fights and pursuit flights (Dolnik, 1995). These activities are usually allocated to the beginning phase of formation of new social relations. On the other hand, the maintaining of established social position in stabilised population system is based mainly on social learning and ritualised forms of behaviour with low energy cost.
Alternative effects of prior residency time on the BMR in young and old wintering males appear to be related to peculiarities of seasonal patterns of their flocking behaviour. Young males enter intense flock interactions from early autumn. The decline of their BMR with increasing residency time takes place during the greater part of the non-reproductive season. Old males show on average a lesser intensity of flock interactions (Kerimov & Ivankina, 1997). The terms of their entering tight relations with other flock members shift to midwinter, when noticeable food shortage occurs. This time coincides with the period of their BMR increase, which represents a main component of seasonal variation in their BMR in some years. Thus, for a large portion of the sample of old males, the increasing of residency time corresponds to the intensification of their flocking behaviour. From this point of view, the BMR increase in old males reflects the energy cost of establishing of tight social bonds.
Similar trends in the BMR variation in accordance to age (old - young) and the novelty level of social relations (previous social bonds - new bonds) may be regarded from the point of long-term and short-term aspects of social adaptation. The increased energetic potential of young birds gives strategic benefit, allowing a bird to acquire better social position at an early stage of life in spite of little experience. The acquired social status may influence access to the vital resources in the future due to inertia of social processes. The short-term energetic adaptations are related to transformation of social bonds that can take place at any phase of the life cycle. In this case, the increased working capacity of a bird provides an additional advantage in social competition.
ACKNOWLEDGEMENTS
We especially thank Prof. K. Nagy, Prof. I. A. Shilov, Dr. T. A. Ilyina and V. G. Grinkov who provided useful comments and helped us to improve style and the content of the manuscript. The assistance of administration of Zvenigorod Biological Station (Moscow State University) made this study possible. The study was funded by Russian Fund of Basic Researches and by Higher School Program ‘Universities of Russia’.
REFERENCES
Depocas, F. & Hart, J.S. 1957. Use of the Pauling oxygen analyzer for measurement of oxygen consumption of animals in open-circuit systems and in a shortlag, closed-circuit apparatus. J. Applied Physiology 10: 388-392.
Dolnik,V.R. 1982. Methods of time and energy budgets study. In : Dolnik, V.R. (ed.) Time and energy budgets in free-living birds. Leningrad: Nauka: 3-37 (in Russian, English summary).
Dolnik, V.R. 1995. Energy and time resources of free-living birds. St. Petersburg, ‘Nauka’ Publ. House (in Russian, English summary).
Dolnik, V.R. 1996. Passerine birds as energetic phenomenon. J. General Biology (JOB) 57(5): 533-566 (in Russian, English summary).
Ekman, J. 1989. Ecology of non-breeding social systems of Parus. Willson Bull. 101: 263-288.
Gavrilov, V.M. 1979. Bioenergetics in large passerine species. I. Resting and existence metabolism. Zool. Journ. 58 (4): 530-541 (in Russian, English summary).
Gavrilov, V.M., Kerimov, A.B. & Ivankina, E.V. 1993. Population and geographic variation of plumage color and metabolism in males of different color type in The Pied Flycatcher Ficedula hypoleuca. Dokladi AN 333 (6): 608-610 (in Russian).
Gavrilov, V.M. 1995. Maximal, potential productive and normal levels of existence metabolism in passerine and non-passerine birds: II. The relation with external work, energetic and ecological consequences. Zool. Journal 74 (4): 108-123 (in Russian, English summary).
Gavrilov, V.M., Kerimov, A.B., Golubeva, T.B. 1996. Energetics, morphological and physiological heterogeneity and population structure in birds: I. Energetics, morfological and physiological heterogeneity and population structure in great tit in Moscow region. Ornithologia. Moscow Univ. Press. 27: 34-73 (in Russian, English summary).
Gavrilov, V.M. 1997. Energetics and avian behavior. Physiology and General Biology Reviews. Harwood Acad. Publ. CmbH. 11 (1): 1-225.
Hogstad, O. 1987. It is expensive to be dominant. Auk. 104 (2): 332-336.
Ilyina, T.A. 1996. The role of energetics in social relations in birds. Ornitologia. Moscow Univ. Press. 27: 98-126 (in Russian, English summary).
Kendeigh, S.C., Dolnik, V.R. & Gavrilov, V.M. 1977. Avian energetics. In: Pinowsky, G. & Kendeigh, S.C.(eds) Granivorous birds in ecosystems. Int. Biol. Programs. Cambridge: Cambridge Univ. Press.12: 127-204, 363-378.
Kerimov, A.B., Ivankina, E.V., Ilyina, T.A. & Gavrilov, V.M. 1994. Dynamics of morphophysiological correlatives of social status during the annual cycle of the Great Tit (Parus major, Passeriformes, Aves). Doklady Biological Sciences 334: 57-60.
Kerimov, A.B. & Ivankina, E.V. 1997. The energetics and social status of a specimen: relationship between the basal metabolism rate and the social position of a specimen in wintering groups of Great Tits (Parus major, Passeriformes, Aves). Doklady Biological Sciences 357: 565-568.
Lasiewski, R.C. & Dawson, W.R. 1967. A re-examination of the relation between standart metabolic rate and body weight in birds. Condor 69(1): 13-23.
Matthysen, E. 1990. Non-breeding social organization in Parus. Current Ornithology 7: 209-249.
Nagy, K.A., Gavrilov, V.M., Kerimov, A.B., Ivankina, E.V. Relationships between field metabolic rate, basal metabolic rate and territoriality in passerines. Proc. 22 Int. Ornithol. Congr.,Durban, University of Natal., in press.
Noskov, G.A. & Smirnov, O.P. 1981. Territorial behaviour and migrations in the Great Tit. In: Ecology of birds in Ladoga region. Leningrad. 100-130 (in Russian).
Perrins, C.M. 1979. British tits. Collins, London.
Reinertsen, R.E. & Hogstad, O. 1994. Influence of social status on the nocturnal energy expenditure of the Willow Tit Parus montanus. Fauna norv. Ser. C. Cinclus 17: 43-48.
Rohss, M. & Silverin, B. 1983.. Seasonal variation in the ultrastructure of the Leyding cells and plasma levels of luteinizing hormone and steroid hormone in juvenile and adult male Great Tits Parus major. Ornis Scandinavica 14: 202-212.
Roskaft E., Jarvi T., Bakken M. Bech, C. & Reinertsen, R.E. 1986. The relationship between social status and resting metabolic rate in Great Tits Parus major) and pied flycatcher (Ficedula hypoleuca). Anim. Behav. 34 (3): 838-842.
Saitou, T. 1978. Ecological study of social organization in the Great Tit, Parus major L. I. Basic structure of winter flocks. Japan. J. Ecol. 28: 199-214.
Saitou, T. 1979. Ecological study of social organization in the Great Tit, Parus major L. II. Formation of the basic flocks. Misc. Rep. Yamash. Inst. Ornithol. Zool., 11: 137- 148.
Silverin, B. 1984. Annual Gonadotropin and Testosterone Cycles in Free-living Male Birds. J. Experim. Zool. 232: 581-587.
Vinogradova, N.V., Dolnik V.R., Efremov, V.D. & Pajevski, V.A. 1976. Estimation of sex and age in passerine birds from USSR fauna. Reference book. Moscow: Nauka (in Russian).
Table 1. The influence of age on the BMR in Great Tit males during non-reproductive season

Table 2. Annual and seasonal variation of the BMR in Great Tit males
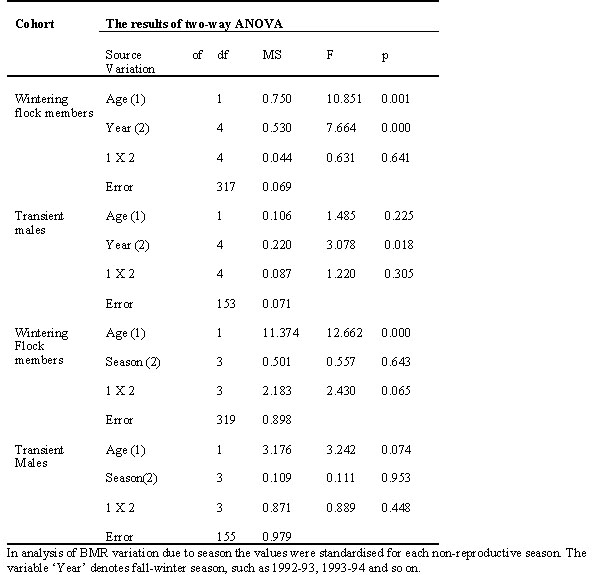
Table 3. Mean basal metabolic rate (BMR) of Great Tit males in different non-reproductive seasons
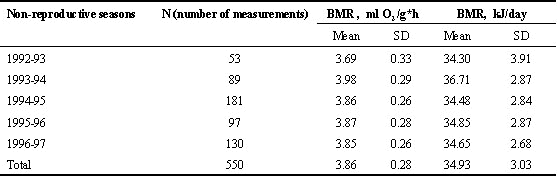
Table 4. The influence of male territorial status on the BMR. The results of two-way ANOVA (the BMR values were standardised for each non-reproductive season)
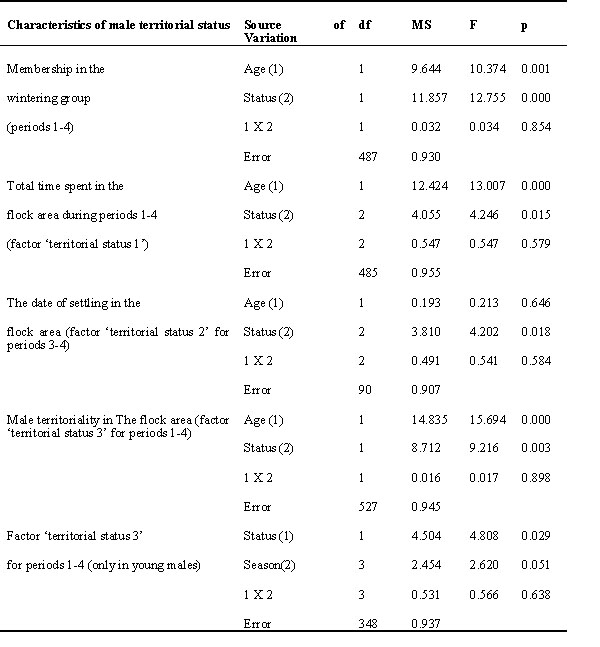
Table 5. The influence of prior residency time on the BMR in male flock members.
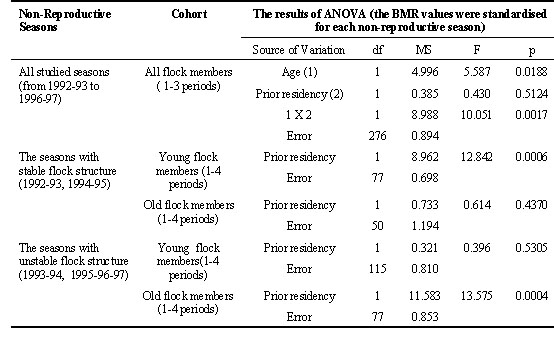
Table 6. The relation between individual BMR and characteristics of social behaviour in wintering Great Tit males. The BMR and behavioural indexes were standardised both for season phase and year.
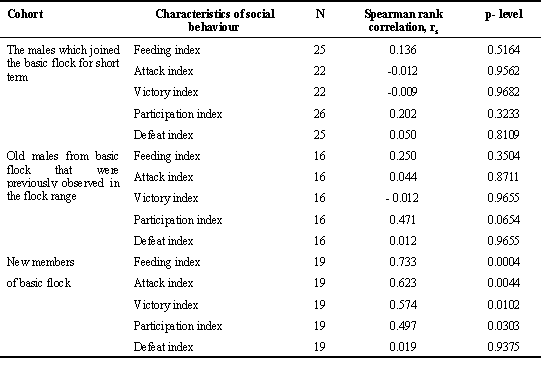
Table 7. The influence of age and social factors on male body mass in Great Tit
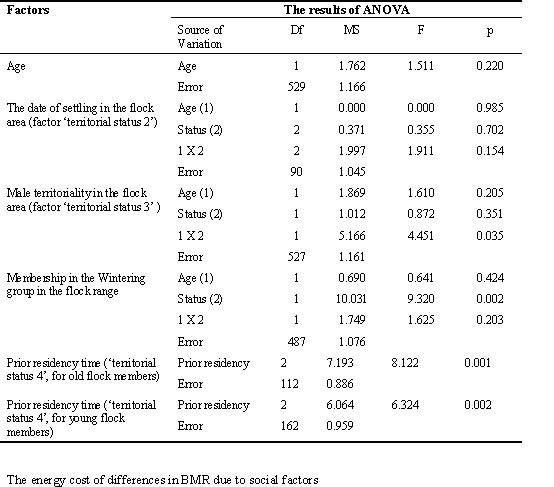
Fig.1. Seasonal variation of the basal metabolic rate (BMR) of flock members in wintering Great Tit males. The BMR values were standardised for each non-reproductive season. See ‘Materials and methods’ for description of seasonal periods
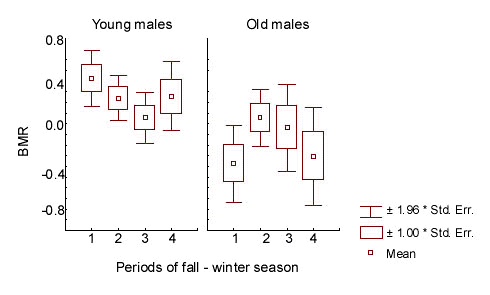
Fig.2. Mean basal metabolic rate (BMR) of flock members and transient birds in Great Tit males. The BMR values standardised for each non-reproductive season are presented for the entire non-reproductive season (n = 491). The sample of transient males includes both seasonal migrants and the birds which crossed the flock area in midwinter.
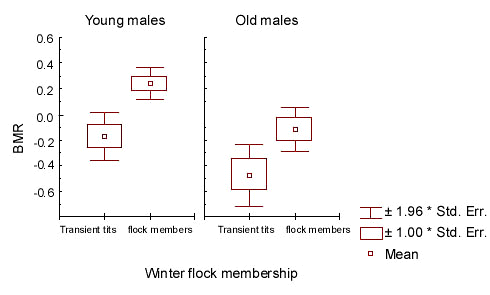
Fig.3. The relation between basal metabolic rate (BMR) and the date of male settling in the flock range. Standardised BMR values of flock members estimated in the end of winter and early spring are shown (n = 96). Terms of settling correspond only to the current non-reproductive season: 1 - late -; 2- intermediate-; 3 - early settling. See ‘Materials and methods’ for more detailed description of gradations.
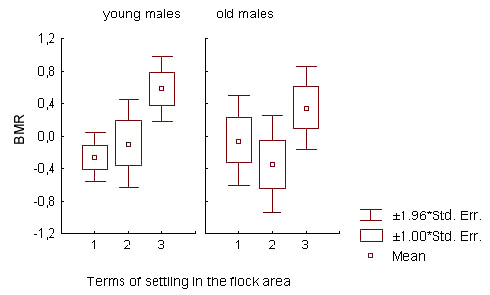
Fig.4. The relation between basal metabolic rate and male territoriality in the flock area. Standardised BMR values correspond to the entire non-reproductive season. The male was defined as territorial if it established or actively tried to establish a territory in the flock area in the subsequent or (and) the preceding breeding season(s). Non-territorial males were those who did not show any defined forms of breeding territoriality in the flock area.
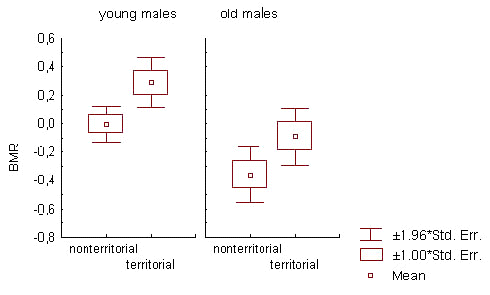
Fig.5. The influence of prior residency time on the basal metabolic rate (BMR) of winter flock members in great tit males. Standardised BMR values correspond to 1-3 phases of non-reproductive season. Prior residency time denotes the time spent by the male in the flock area before the day it was captured for BMR measurement.
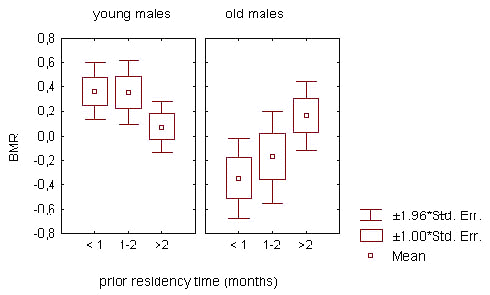
Fig.6. The basal metabolic rate (BMR) of wintering flock members at the end of non-reproductive seasons in different years. The BMR values were standardised in the total annual sample for a given non-reproductive season. Mean values refer to periods 3 and 4.