S06.2: Demography of forest birds in Panama: How do transients affect estimates of survival rates?
Jeffrey D. Brawn1, James R. Karr2, James N Nichols3, W. Douglas Robinson4
1Illinois Natural History Survey, 607 East Peabody Drive, Champaign, IL 61820 USA, fax 217 333 4949,
e-mail j-brawn@uiuc.edu;2University of Washington, PO Box 357980, 104 Fisheries Center, Seattle, WA, 98195-7980 USA, e-mail jrkarr@u.washington.edu; 3USGS Patuxent Wildlife Research Centre, Merriam Lab, 11510 American Holly Dr., Laurel, MD 20708-4017 USA, e-mail Jim_Nichols@usgs.gov;4Department of Zoology and Wildlife Science, 331 Funchess Hall, Auburn University, Auburn, AL 36849-5414 USA, e-mail songwren@pop.life.uiuc.eduBrawn, J.D., Karr, J.R., Nichols, J.N., & Robinson, W.D. 1999 Demography of forest birds in Panama: how do transients affect estimates of survival rates? In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
297-305. Johannesburg: BirdLife South Africa.Estimates of annual survival rates of neotropical birds have proven controversial. Traditionally, tropical birds were thought to have high survival rates for their size, but analyses of a multispecies assemblage from Panama by Karr et al. (1990) provided a counterexample to that view. One criticism of that study has been that the estimates were biased by transient birds captured only once as they passed through the area being sampled. New models that formally adjust for transient individuals have been developed since 1990. Preliminary analyses indicate that these models are indeed useful in modelling the data from Panama. Nonetheless, there is considerable interspecific variation and overall estimates of annual survival rates for understorey birds in Panama remain lower than those from other studies in the Neotropics and well below the rates long assumed for tropical birds (i.e. > 0.80). Therefore, tropical birds may not have systematically higher survival rates than temperate-zone species. Variation in survival rates among tropical species suggests that theory based on a simple trade-off between clutch size and longevity is inadequate. The demographic traits of birds in the tropics (and elsewhere) vary within and among species according to some combination of historical and ongoing ecological factors. Understanding these processes is the challenge for future work.
INTRODUCTION
Estimate of survival rates of tropical birds have proven controversial. Prior to Karr et al. (1990), it was commonly held that the survival rates of tropical birds are systematically higher than those of their temperate-zone counterparts. This assumption was untenable for two reasons. First, there were few supportive data. Sampling had been conducted on a small set of species and estimates were often based on a restricted sample of individuals such as territorial males. Close inspection of these studies also reveals some estimated annual survival rates much less than those commonly assumed for tropical birds (see Karr et al. 1990 for a complete discussion). Second, virtually all estimates were derived from the technique known as the 'minimum number known alive' or 'return rate' method. This method yields biased parameter estimates and erroneously equates recapture or resighting rates with survival rates (Nichols and Pollock 1983, Lebreton et al. 1992).
The survival rates presented by Karr et al. (1990) were for a multispecies assemblage of forest birds (N = 25) from Panama and contradicted the prevailing view of markedly high avian survival rates in the humid tropics. These estimates were the first for tropical birds to be derived from Cormack-Jolly-Seber type models for mark-recapture data and were well below the oft-assumed annual survival of > 0.80. A key innovation of these and related models is that recapture or resighting probability is incorporated explicitly in the analysis and is not confounded with estimates of survival rates (Lebreton et al. 1992). One criticism of Karr et al. (1990), however, has been a failure to account for effects of transient birds that were captured once as they passed through the area (Johnston et al. 1997). These individuals would, by definition, have zero probability of recapture, and failure to account for transients can lead to underestimates of survival rates (Pradel et al. 1997). In analysing the Panama data, Karr et al. (1990) did formally test for differences in survival rates between newly banded individuals and previously banded individuals (i.e. the two-age class model of Brownie and Robson [1983]). Tests for all 25 species detected significant differences in only one species. In addition, the ad hoc approach presented by Pradel et al. (1997), in which first captures of each individual are ignored, was carried out for several of the 25 species and overall results were not changed significantly (Karr unpublished analyses).
Nonetheless, new analyses by Johnston et al. (1997), for a multispecies assemblage from second growth forests in Trinidad, clearly identify the importance of accounting for effects of transients or other possible sources of heterogeneity between newly and previously captured birds. Several species were common to the Trinidad and Panama samples and, on average, survival rates were significantly greater in Trinidad. These results, combined with those from another mark-recapture study in subtropical forests in Puerto Rico (Faaborg and Arendt 1995) and a different approach by Ricklefs (1997), have revived the notion that survival rates of tropical birds may indeed be greater than those of birds at temperate latitudes.
Life history theory cannot advance without a clear understanding of the patterns and sources of variation in vital demographic parameters such as survival rates (Lebreton et al. 1992, Brawn et al. 1995). Many models in avian life history theory virtually 'require' high survival rates of tropical birds. For example, tropical birds have comparatively small clutches and a persistent explanation has been that this illustrates a classic life history tradeoff between fecundity and lifespan (Ricklefs and Bloom 1977, Murray 1985, Martin 1996, Johnston et al. 1997). Understanding the demography of tropical birds is also vital from a conservation perspective (Karr 1990, Dhondt and Matthyssen 1993, Brawn et al. 1998). Clearly, estimating survival rates in tropical birds remains an important issue.
We analysed a subset of the data from the Panama study and present new estimates of survival rates of forest birds. Our data and analyses differ from those of Karr et al. (1990) in two ways. First, we formally account for possible effects of transients using the set of models and techniques presented by Pradel et al. (1997). Second, sample size has increased and the time span of sampling are longer. We present these new analyses and discuss results with respect to geographic variation in tropical survival rates and small clutch size in the tropics.
METHODS
Sampling was conducted from 1977 to 1997 in central Panama in moist lowland forest that varies in age from 60 to about 400 years old and lies within a relatively flat 600 ha basin. The study site is located within Parque Nacional Soberania, a park created in 1979 that comprises about 16,000 ha. Rainfall in the region is highly seasonal and about 90% of the annual rainfall (mean = 2600 mm) occurs in the wet season from late April to early January. Detailed descriptions of the site’s floristic composition and forest structure are presented in Karr and Freemark (1983), Karr et al. (1990), and Robinson et al. (In Press).
Mark-recapture sampling was carried out with mist nets. Net lines consisted of 12 to 22, 36-mm mesh nets. Nets were open from dawn to dusk, usually for about 3 days / line. Sampling generally occurred in March (dry season) and July (wet season). A total of 12 different lines were used over the course of the study, but the same two to four 'core' net lines were operated within all sampling periods. We sampled every year except 1989 and 1991 and in the wet season only in 1992 and 1993.
With few exceptions (i.e., hummingbirds until 1995), first-capture birds were given numbered aluminum leg bands and released. Unique combinations of colour bands were applied to nearly all captured individuals beginning in 1992.
The most general model we used is parameterised with time-specific survival (f i) and capture (r i) probabilities for resident birds, as well as with time-specific probabilities (g i) that an unmarked bird is a resident. We parameterized the model using the program TMSURVIV (Hines 1998) which is a modification of the program SURVIV (White 1983) with adjustments for transients. Karr et al. (1990) used the contingency table test of Robson (1969) and Brownie and Robson (1983) to test between the Cormack-Jolly-Seber model ([f i, r i]; Cormack 1964, Jolly 1965, Seber 1965) and the Brownie-Robson (1983) model (g i,f i,r i). Here we use the asymptotically equivalent approach of a likelihood ratio test between the two models. In cases where full time-specificity is not necessary, we also used likelihood ratio tests between reduced parameter models that do (g ,f ,r ) and do not (e.g., [f ,r ]) account for transients among unmarked birds. Tests using these reduced-parameter models are frequently more powerful than tests between fully time-specific models (Pradel et al. 1997) thus providing a motivation for the present analysis. As noted in Loery et al. (1997), 'transient effects' on estimates of survival rates can be obtained when true transients are sampled, when there is heterogeneity in survival rates (e.g., age effects) between the first year after initial marking and subsequent years, or when some birds develop net shyness subsequent to their initial capture.
Following recent work on model selection and parameter estimation, we based model selection on the QAICc statistic, a small-sample modification (Hurvish and Tsai 1989) of Akaike’s Information Criterion (Akaike 1973) adjusted for overdispersion using a quasi-likelihood approach (Lebreton et al. 1992, Burnham et al. 1995). Under this approach, model selection is viewed as an optimisation problem with the objective function depending on both fit and number of parameters. The goal is to select the most parsimonious model that adequately describes the variation in the data with a small number of parameters.
RESULTS
Here we report on new estimates of survival rates for 11 of the species originally considered by Karr et al (1990). We are in the process of re-analysing data for all 25 species from the original paper, additional tropical species that can now be analysed owing to larger sample sizes, as well as the temperate-zone species considered by Karr et al. (1990).
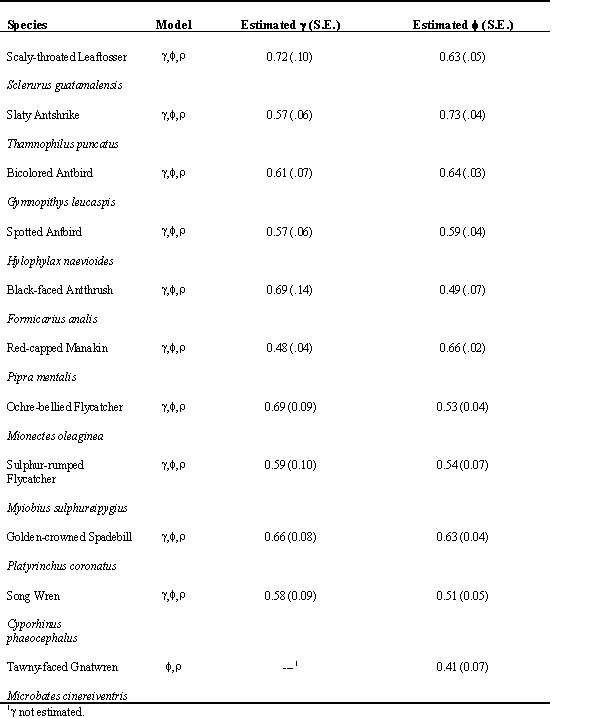
The new analyses clearly identified the importance of accounting for transients in the mist net samples. Likelihood ratio tests and QAICC indicated that a model with parameterisation for transients (g ,f ,r ) was the most appropriate for 10 of 11 species (Table 1). We found no evidence of time specific variation in probability of capture or annual variation in survival rate; results that, qualitatively, are consistent with the original analyses of Karr et al. (1990).
Estimates of g , the probability that an unmarked bird is a resident, averaged 0.61 (S.E. = 0.02) and ranged from 0.48 for the Red-capped Manakin (see Table 1 for scientific names of all species considered) to 0.72 for the Scaly-throated Leaftosser (Table 1). Thus, on average, assuming the 'transient effects' were due to true transients, about 40% of the unmarked birds present in the area being sampled were transient birds. Interspecific variation in g likely reflects variation in spacing systems, territoriality, and frequency of annual or seasonal movements. For example, the Red-capped Manakin is a highly vagile, lek-breeding species with no fixed territories whereas the leaftosser is comparatively sedentary on fixed territories. Note again, however, that g can reflect different demographic and sampling processes.
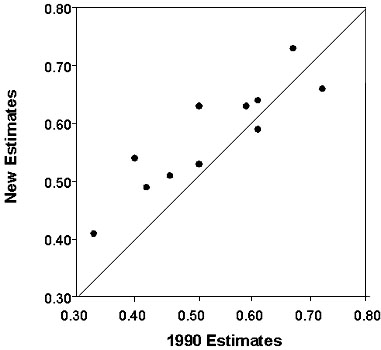
Estimated survival rates for the 11 species ranged from 0.41 for the Tawny-faced Gnatwren to 0.73 for the Slaty Antshrike and averaged 0.58 (S.E. = 0.03, Table 1). The average survival rate for our 11 species sample using models without transient parameterisations and data from 1977 to 1986 (Karr et al. 1990) was 0.53 (S.E. = 0.04). Overall, estimated survival rates increased with the new analyses in nine of the 11 species (Fig. 1). The largest positive differences in estimated survival rates from the old to the new analyses using the g ,f ,r model were for the Sulphur-rumped Flycatcher which increased by from 0.40 to 0.54, the Golden-crowned Spadebill (0.51 to 0.62) and the Tawny-faced Gnatwren (0.33 to 0.41). Note that the change in the gnatwren was due to the addition of new data (an increase from 40 to 81 individuals in the sample) since the f ,r model was appropriate for this species. Elsewhere, we analyse the larger dataset in more detail, but failure to detect temporal variation in f suggests that survival rates did not change substantially since 1986. The largest decrease in estimated survival rate was with the Red-capped Manakin (6%). This change may stem from additional data since the model for this species presented in Karr et al. (1990) is equivalent to the most general model of Pradel et al. (1997). Differences between estimated survival rates for the two sets of analyses were significant (paired t-test with arcsine transform; t-ratio = -2.7, df = 10, P = 0.022).
DISCUSSION
Initial reanalyses of mark-recapture data for forest birds in Panama indicated two major points. First, our results demonstrate that the models of Pradel et al. (1997) can be useful in analysing data from mist nest samples of tropical birds just as they are for similar data for temperate-zone species (DeSante et al. 1995). Whereas the extent of bias experienced by ignoring transients likely varies among species, the estimated proportion of transients in samples of new captures of tropical birds was generally more than 40%, and in nearly all cases survival rates were estimated best by transient models with parameters constant over time. Second, there is evidence of geographic variation in the survival rates of tropical birds. Average estimated survival rates for the 17 species sample from Trinidad was 0.65, about 12% greater than that reported here. This estimated difference between Panama and Trinidad is greater than that reported for Panama and North America reported by Karr et al. (1990). Further, the Ochre-bellied Flycatcher is common to the Panama and Trinidad samples and has been analysed with transient models; the estimated survival rate in Panama is about 8% lower than that in Trinidad. In assessing why results from Panama demonstrate lower survival rates than elsewhere in the tropics, Johnston et al. (1997) emphasised differences in how the data were modelled and offered that a definitive conclusion awaits further analyses of the Panama data. More complete analyses with adjustments for phylogeny and body size are underway, but differences between the Panama and Trinidad estimates do not appear to be methodologically based.
We conclude that there may be true differences in the demography of birds among sites within the tropics. The ecological or historical sources of this variation offer a promising avenue of research for understanding the demography of tropical birds. Comparisons of Trinidad and Panama, for example, suggest hypotheses about island effects and habitat effects. Several avian life history traits are known to vary with island-mainland and with habitat structure (Cody 1966, Brawn 1991, Blondel et al. 1992) but variation in survival rates has not been explored extensively (but see Faaborg and Arendt 1995). Note , however, that estimates of survival rates in forest birds from a mainland site in Peru indicate higher survival rates that than those in Panama (Francis et al., this volume).
One ecological difference between the Panama and Trinidad study sites is the presence of Micraster spp. forest falcons which are known predators on forest birds. No forest falcons are found on Trinidad (ffrench 1991), but three species are common at the Panama site (Karr et al. 1990, Robinson et al. In Press). Further, based on the habitat descriptions and the species list reported in Johnston et al. 1997, the Trinidad sites are located in comparatively immature forest. The habitats sampled by Faaborg and Arendt (1995) in Puerto Rico were, in part, also regenerating forest. How successional stage and the various ecological factors that co-vary with succession might affect survival rate requires investigation.
We also believe that the more general comparison of survival rates in tropical and temperate latitudes requires much additional study. Couching the comparison in a 'yes-no' dichotomy reduces the vitality of the question. Notwithstanding the present study, several analyses have reported a tendency for tropical birds to have greater survival; comparatively great survival rates (i.e., > 0.70) are nearly always reported with the tropical samples (but see Murphy 1996 for an example from North America). Careful inspection, however, reveals considerable overlap between nearly all reported temperate and tropical samples. The unconditional statement that 'tropical birds have greater survival rates than temperate birds' is not supported by the evidence. Understanding sources of variation within and between regions is key to understanding avian life histories.
Methodological issues may still obscure latitudinal comparisons. Estimates of survival rates for temperate-zone species are also sensitive to the effects of transients (i.e., DeSante et al. 1995, Chase et al. 1997, J. Nichols personal observation). Several of the estimates cited by Johnston et al. (1997) from north-temperate zones were not generated by modern methods and await re-analyses and possible adjustments for transients.
Finally, latitudinal variation in survival rates and its association with clutch size was first questioned by Karr et al (1990). We further believe that evidence for this life-history trade-off is doubtful based on tropical samples alone. All analyses of tropical species presented to date, regardless of method, have found considerable interspecific variation in estimated survival rates. Yet, clutch size is nearly invariant within these samples. For example, 23 of the 25 species considered by Karr et al. (1990) have a clutch size of two. Variation in clutch size among tropical forest birds is so low that there is virtually no correlation between clutch size and survival rate. If avian clutch size is comparatively small in the tropics owing to some property of longevity, then the theory to account for this relationship has yet to be presented. We reiterate Karr et al. (1990) and concur with a recent review by Martin (1996) that small clutch size in the tropics is a complex question that requires more information on many aspects of the reproductive ecology of tropical birds. Examples are age-specific survival rates, the extent and function of post-fledging parental care, multi-brooding, length of the breeding season, rates of nest loss, and feeding conditions for nestlings.
In summary, re-analyses to date indicate that the results presented by Karr et al. (1990) are robust qualitatively. Survival rates of forest birds appear to be lower in Panama than Trinidad (Johnston et al. 1997) or Puerto Rico (Faaborg and Arendt 1995). If ecological conditions in the forests of Panama are somehow different than elsewhere, the challenge for empirical work is to identify why, and for theoretical models to account for this variation.
ACKNOWLEDGEMENTS
We thank the Republic of Panama’s Instituto Nacional de Recursos Naturales Renovables (IN.RE.NA.RE.) for permission to work in Parque Nacional Soberania and continue our studies. The Smithsonian Tropical Research Institute - especially Joe Wright - provided logistical support. Major funding sources since 1977 for the continuing demographic studies of birds in Panama include: the Smithsonian Tropical Research Institute’s Environmental Studies Program (J.D.B.), the U.S. Department of Defense’s Legacy Program (J.D.B.), the National Science Foundation (DEB 82-06672 to J.R.K.), The Center for Field Research/Earthwatch (J.R.K.) and the University of Illinois (W.D.R.). We thank the many field assistants and colleagues who provided support on the project over the years; especially Tara Robinson, George Angehr, Belkys Jimenez, and Jacobo Ortega. Jim Hines kindly provided computational and analytic support.
REFERENCES
Akaike, H. 1973. Information theory and an extension of the maximum likelihood principle. In Petrov, B.N. and Csaki, F (eds) Second International Symposium on Information Theory; Budapest, Akademiai Kaido: 267-281.
Blondel, J., Pradel, R., Lebreton, J.-D. 1992. Low fecundity insular blue tits do not survive better as adults than high fecundity mainland ones. Journal of Animal Ecology 61: 205-213.
Brawn, J. D. 1991. Environmental effects on variation and covariation in reproductive traits of Western Bluebirds. Oecologia 86:193-201
.Brawn, J.D., Karr, J.R. & Nichols, J.D. 1995. Demography of birds in a neotropical forest: effects of allometry, taxonomy, and ecology. Ecology 76:41-51.
Brawn, J.D., Robinson, S.K., Stotz, D.F. & Robinson W.D. 1998. Research needs for the conservation of Neotropical birds. In Marzluff, J. & Sallabanks, R. (eds) Avian Conservation: Research and Management; Washington, D. C., Island Press: 323-335.
Brownie, C., & Robson, D.S. 1983. Estimation of time specific survival rates from tag-resighting samples: A generalization of the Jolly-Seber model. Biometrics 39: 437-453.
Burnham, K.P., White G.C., & Anderson, D.R. 1995. Model selection in the analysis of capture-recapture data. Biometrics 51: 888-898.
Chase, M.K., Nur, N., Geupel, G.R. 1997. Survival, productivity, and abundance in a Wilson’s Warbler population. Auk 114: 354-366.
Cody, M.L. 1966. A general theory of clutch size. Evolution 20: 174-184.
Cormack, R.M. 1964. Estimates of survival from resighting of marked animals. Biometrika 51: 429-438.
DeSante, D.F., Burton, K.F., Saracco, J. F., & Walker B. L. 1995. Productivity indices and survival rate estimates from MAPS, a continent-wide programme of constant-effort mist-netting in North America. Journal of Applied Statistics 22:935-947.
Dhondt, A., & Matthysen, E. 1993. Conservation biology of birds: can we bridge the gap between head and heart? Trends in Ecology and Evolution. 8:160-161.
Faaborg, J. & Arendt, W. 1995. Survival rates of Puerto Rican birds: are islands really different? Auk 112:503-507.
ffrench, R. 1991. A guide to the birds of Trinidad and Tobago. Ithaca, Cornell University Press: 426pp.
Hines, J.E. 1998. TMSURVIV Users Manual. Biological Resources Division, United States Geological Survey, Patuxent Wildlife Research Center, Laurel, Maryland, USA.
Hurvich, C.M., & Tsai, C. 1989. Regression and time series model seletion in small samples. Biometrika 76:297-307.
Johnston, J.P., Peach, W.J., Gregory, R.D., & White, S.A. 1997. Survival rates of tropical and temperate passerines: a Trinidadian perspective. American Naturalist 150: 771-789.
Jolly, G.M. 1965. Explicit estimates from capture-recapture data with both death and immigration-stochastic models. Biometrika 38:301-321.
Karr, J.R. 1990. Avian survival rates and the extinction process on Barro Colorado Island, Panama. Conservation Biology 4:391-396.
Karr, J.R., Nichols, J.N., Klimkiewicz, M.K., & Brawn, J.D. 1990. Survival rates of birds of tropical and temperate forests: will the dogma survive? American Naturalist 136: 277-291.
Karr, J.R., & Freemark, K.E. 1983. Habitat selection and environmental gradients: dynamics in the 'stable' tropics. Ecology 64:1481-1494.
Lebreton, J.-D., Burnham, K.P., Clobert, J., & Anderson, D.R. 1992. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecological Monographs 62:67-118.
Loery , G., Nichols, J.D., & Hines, J.E. 1997. Capture-recapture analysis of a wintering Black-capped Chickadee population in Connecticut, 1958-1993. Auk 114:431-442.
Martin, T.E. 1996. Life history evolution in tropical and south temperate birds: what do we really know? Journal of Avian Biology 27: 1-10.
Murray, B.G., Jr. 1985. Evolution of clutch size in tropical species of birds. Ornithological Monographs 36: 505-519.
Murphy, M.T. 1996. Survivorship, breeding dispersal, and mate fidelity in Eastern Kingbirds. Condor 98: 82-92.
Nichols, J.N. & Pollock, K.H. 1983. Estimation methodology in contemporary small mammal capture-recapture studies. Journal of Mammalogy 64:253-260.
Pradel, R., Hines, J.E., Lebreton, J.-D., & Nichols, J.D. 1997. Capture-recapture survival models taking account of transients. Biometrics 53: 60-72.
Ricklefs, R.E. 1997. Comparative demography of New World populations of thrushes (Turdus spp.). Ecological Monographs 67:23-43.
Ricklefs, R.E. & Bloom, G. 1977. Components of avian breeding productivity. Auk 94:86-96.
Robinson, W.D., Brawn, J.D., & Robinson, S.K. In Press. Structure of a tropical forest bird community: influence of scale and biogeographic history. Ecological Monographs.
Seber, G.A.F. 1965. A note on the multiple recapture census. Biometrika 52:249-259.
White, G.C. 1983. Numerical estimation of survival rates from band-recovery and biotelemetry data. The Journal of Wildlife Management 47:716-728.
Table 1. Estimated parameters for 11 species of forest birds in central Panama for data from 1977-1997. 'Model' refers to the model determined to be most appropriate based on likelihood ratio tests and QAICc (see Methods); 'g ,f ,r ' refers to model with parameterisation for transients, 'f ,r ' refers to traditional Cormack–Jolly–Seber model.

Fig. 1. Scatterplot comparing estimated survival rates for 11 species from Panama with (new) and without (1990) adjustments for transient birds. Data for 1990 estimates are from 1977 to 1986 and are presented in Karr (1990). New estimates are for data from 1977 to 1997.