S05.5: Environmental endocrinology and the timing of reproduction: Interaction of photoperiod and temperature
Donna L. Maney1 Stephan J. Schoech2 & John C. Wingfield3
1Rockefeller University Field Research Center, Tyrrel Road, Millbrook, NY 12545, USA, fax 914 677 6491, e-mail maneyd@rockvax.rockefeller.edu; 2Department of Biology, Indiana University, Bloomington, IN 47405, USA, e-mail sschoech@indiana.edu; 3Department of Zoology, University of Washington, Seattle, WA 98195, USA, e-mail jwingfie@u.washington.edu
Maney, D.L., Schoech, S.J. & Wingfield, J.C. 1999 Environmental endocrinology and the timing of reproduction: Interaction of photoperiod and temperature. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 279-294. Johannesburg: BirdLife South Africa.
The predictability of the environment largely determines which cues are valuable for the timing of reproduction. In arctic-breeding species, the breeding season is highly predictable and short, making proximate cues such as temperature less important than in temperate-breeding species with more flexible breeding seasons. Because White-crowned Sparrow species Zonotrichia leucophrys is divided into a number of subspecies, each of which breeds in different environments, these birds provide opportunities to compare sensitivities to various proximate cues. In the subspecies Z. l. gambelii, which breeds in arctic regions, gonadal maturation is less affected by temperature cues than in the subspecies Z. l. pugetensis, which breeds at temperate latitudes. Because of such differences among subspecies, White-crowned Sparrows also provide opportunities to study possible mechanisms underlying the responses to proximate cues. Preliminary data suggest that, unlike increases in photoperiod, variations in temperature do not result in robust effects on gonadotropin secretion. Recent evidence shows that another pituitary hormone, prolactin, increases in plasma during exposure to warmer temperatures. Perhaps prolactin mediates temperature effects on gonadal maturation by altering gonadal responsiveness to gonadotropins.
ENVIRONMENTAL PREDICTABILITY
The onset of breeding in many temperate zone species is timed to maximise reproductive success in the face of variable environmental conditions. The environmental cues that regulate gonadal development and reproductive function can be organised into several types based on their major biological effects (Wingfield 1980; 1983; Wingfield and Kenagy 1991). One type, called 'initial predictive information', provides a reliable long-term forecast about the onset of ideal breeding conditions, enabling an individual to begin preparing for reproduction several weeks or even months in advance. For example, lengthening days in late winter and early spring reliably predict the onset of the breeding season and can act as a signal to promote gonadal development. This is essential to ensure that the reproductive system is functional at the time conditions become conducive to breeding.
Species living in highly predictable environments with a precisely timed breeding season may rely primarily on initial predictive information to time many aspects of reproductive effort. In most habitats, however, the breeding season fluctuates temporally from year to year because of variations in factors affecting food availability, such as temperature and rainfall. Such factors can serve as a second type of environmental cue, called 'supplementary information'. These cues provide predictive information in the short-term, and can accelerate or slow down the gonadal development induced by initial predictive information such as photoperiod (see also Marshall 1959; Wingfield and Kenagy 1991; Wingfield et al. 1992). The reproductive maturation process involves two stages that are most distinct in females. First, the gonads grow to a 'prebreeding stage', which depends primarily on initial predictive information. This is followed by synthesis and deposition of yolk leading to ovulation, triggered when local conditions are favourable (Marshall 1959; 1970; King et al. 1966; Wingfield and Farner 1980; Wingfield and Kenagy 1991). Gonadal growth and yolk deposition provide an easily quantifiable indication of both stages. Supplementary information may affect both processes, although the mechanisms remain essentially unknown.
The degree to which individuals integrate these two types of environmental signals that induce gonadal development depends upon the predictability of the breeding seasons (see Wingfield et al. 1992). Cohen (1967) suggested that if a future event such as onset of breeding is highly predictable, then only one or a few reliable environmental cues should be required to time breeding, and many other potential environmental signals should be ignored. On the other hand, if the breeding season is less predictable, for example, because of cold or warm spring seasons, then an individual should integrate many environmental cues to ensure that reproduction is timed optimally. Colwell (1974) pointed out that predictability has two components: constancy (i.e. the environment is predictable because conditions do not vary) and contingency (i.e. major fluctuations occur). Wingfield et al. (1992) applied Colwell's ideas to matrices of egg-laying dates of several avian species, and suggested that the ratio of contingency to constancy indicates the level of environmental predictability for each population. Using this figure, one can estimate the degree to which an individual within a population should integrate several environmental cues--both long-term (initial predictive) and short-term (supplementary).
TEMPERATURE CUES IN ZONOTRICHIA LEUCOPHRYS
For Z. l. gambelii, a subspecies which breeds at high latitudes in Arctic regions of North America, the breeding season is highly predictable; the short breeding season occurs at almost precisely the same time every year. Thus, initial predictive information such as photoperiod is an accurate predictor of optimal conditions. In this situation, an individual should show a strong reliance on photoperiod as a cue for gonadal growth and disregard other potential cues. For Z. l. pugetensis, however, a subspecies that breeds at lower latitudes in the Pacific Northwest region of North America, the breeding season is still predictable, but onset and termination of breeding can vary by three to five weeks from year to year. These birds should use other cues in addition to day length to time reproductive effort. Z. l. oriantha breeds in montane regions of western North America, where the window of optimal breeding conditions also may vary temporally from year to year depending on the depth of the snowpack (Morton and Allan 1990). Thus, the calculations of Wingfield et al. (1992) predict that Z. l. gambelii should be relatively unresponsive to environmental cues other than day length, whereas Z. l. pugetensis and Z. l. oriantha, although still sensitive to day length cues, should also respond to other cues such as temperature.
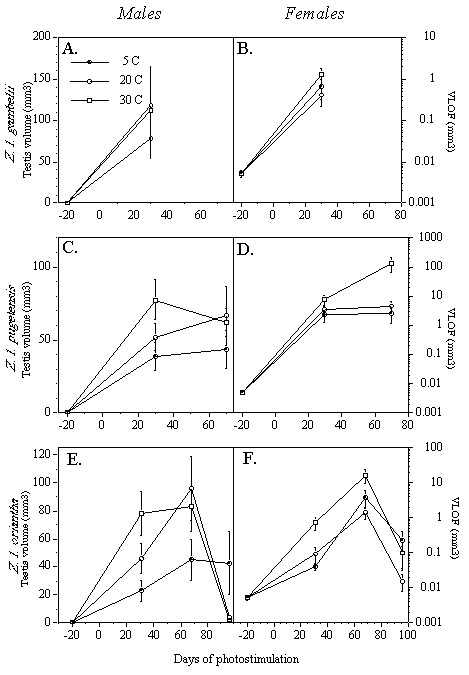
Wingfield et al. (1996; 1997; unpublished manuscript) tested these predictions by photostimulating captive White-crowned Sparrows of these three subspecies held at different temperatures (5°, 20°, and 30°C). As expected, gonadal development was stimulated by increased photoperiod but unaffected by temperature treatment in Z. l. gambelii (Fig. 1, A-B). Note, however, that data are only available for day 30 of photostimulation. In female Z. l. pugetensis, temperature affected gonadal growth later during development; birds held at 30°C showed significantly enhanced gonadal development compared with females held at the other temperatures on day 70 (Fig. 1D). In male Z. l. pugetensis, temperature appears to have affected testis volume at day 30, but this trend was not significant (Fig. 1C). In Z. l. oriantha, temperature affected gonadal maturation in both sexes, significantly so at days 31 and 96 for males, and at all three time points for females (Fig. 1, E-F). These studies also showed that termination of reproduction is accelerated in birds held at warmer temperatures. Gonadal regression and moult are initiated sooner at warm temperatures in all three subspecies (Fig. 1. & Fig. 2).
ENDOCRINE MECHANISMS OF TRANSDUCTION
Endocrine responses to temperature cues
Contrary to what would be expected given the effects of ambient temperature on photo-induced gonadal growth in Z. l. pugetensis and Z. l. oriantha, temperature does not affect plasma luteinizing hormone (LH) concentrations for either sex in any of the Z. leucophrys subspecies tested. Circulating LH increased following photostimulation and then decreased as the birds became photorefractory, but temperature treatment did not affect these LH profiles (Fig. 3). Plasma levels of follicle stimulating hormone (FSH) are similarly unaffected by temperature treatment. It is possible that pulses of LH were missed by the sampling process; however, the homogeneity of most data, together with the fact that blood samples were taken at the same time of day for each experiment, makes this unlikely. We must, therefore, look elsewhere for possible hormonal mechanisms for the transduction of temperature cues.
Glucocorticoids
The adrenal stress hormone corticosterone has been implicated in the suppression of reproductive function in birds (Cain and Lien 1985). Wingfield et al. (1996) hypothesized that although temperatures in the range used in the Z. leucophrys studies would not induce a stress response, temperature might affect corticosterone secretion and thus gonadal development. However, like LH and FSH, corticosterone is relatively unaffected by temperature treatment in each subspecies. In Z. l. gambelii, both males and females housed at low temperatures had higher plasma levels of corticosterone on the first day of photostimulation, but this difference disappeared by day 10 (Wingfield et al. 1996). Likewise, male Z. l. pugetensis held at low temperatures had slightly elevated plasma corticosterone on day 20 of photostimulation but at not at other time points (Wingfield et al. 1997). In both studies, the maximum plasma levels were well below those seen during a stress response (Wingfield et al. 1982). Corticosterone was not measured in Z. l. oriantha.
Thyroxine
Thyroid hormones have long been suspected as possible mediators of temperature cues because of their robust response to changes in ambient temperature (see Wingfield and Farner 1993 for review). Low ambient temperature during the breeding season increases thyroid weight in Z. l. gambelii (Lewis and Farner 1973) and increases thyroid hormone secretion in Japanese quail Coturnix coturnix (Oishi and Konishi 1978). In addition, in some species cold temperatures induce thyroid hormone secretion only during winter, not during the reproductive season (see Wingfield and Farner 1993 for review). In Z. leucophrys, low ambient temperature does not stimulate thyroid hormone secretion during the breeding season (Smith 1982; see also Wilson and Farner 1960). Our data suggest that the opposite may be the case--low ambient temperature tends to suppress thyroxine (T4) during photostimulation (Fig. 4). In both sexes of Z. l. pugetensis and Z. l. oriantha, circulating T4 is reduced in birds at low temperatures and in some cases increased in birds at high temperatures (Fig. 4, C-F). The largest differences occur during the period from 10 to 30 days of photostimulation, which corresponds to the time of rapid gonadal growth. Temperature does not affect plasma T4 concentrations in male Z. l. gambelii (Fig. 4A); however, females held at a low temperature have suppressed levels 10 days after photostimulation (Fig. 4B).
Thyroid hormones may play a role in reproductive function in birds. In C. coturnix, administration of T4 during photostimulation inhibits gonadal maturation and plasma testosterone (Peczely 1985). However, conflicting results have been reported regarding the relationship between thyroid and gonadal function in birds. Whereas some studies indicate an inverse relationship (e.g. Oishi and Konishi 1978), others show a positive one (see Reinert and Wilson 1996). In Z. leucophrys, both triiodothyronine (data not shown) and thryroxine secretion are stimulated by increased day length (Fig. 3). This result is consistent with that reported for another passerine, the tree sparrow Spizella arborea (Reinert and Wilson, 1996).
Prolactin
The pituitary hormone prolactin (PRL) is affected by ambient temperature in many vertebrates and could play a role in gonadal maturation. Figuera et al. (1997) showed that ambient temperature affects PRL gene transcription in carp (Cyprinus carpio). In birds, El Halawani et al (1984) showed an effect of temperature on PRL secretion in the turkey (Meleagris gallopavo), and Silverin and Viebke (1994) found a similar effect in the willow tit (Parus montanus). PRL has specific effects on reproduction across vertebrate taxa, and plasma levels of PRL can be influenced by reproductive state. Concentrations of plasma PRL increase during the breeding season in many avian species, including Z. leucophrys (Hiatt et al. 1987), and PRL has been implicated in avian and mammalian gonadal recrudescence (Bex et al. 1978; Takase et al. 1990a; Das 1991) and termination of breeding (Goldsmith and Nicholls 1984; Rozenboim et al. 1993; Dawson and Sharp 1998).
Temperature cues affect photo-induced plasma PRL in a manner similar to that in which they affect gonadal development in the three species of Z. leucophrys tested. In Z. l. gambelii, neither males nor females alter their PRL profile in response to ambient temperature (Fig. 5, A-B). On the other hand, although PRL profiles do not differ among temperature treatments for male Z. l. pugetensis (Fig. 5C), females held at a low temperature have significantly lower plasma PRL than females held at warm temperatures on Days 20 and 30 of photostimulation (Fig. 5D). This PRL suppression could be responsible for a suppression of ovarian follicle size in the females at low temperature at Day 68 (Fig. 1D).
The effects of temperature on photo-induced PRL secretion in Z. l. oriantha support this hypothesis. Males held at a cool temperature have significantly lower plasma PRL than warmer males on Day 30 of photostimulation (Fig. 5E), a time when testis weight is also affected (Fig. 1E). By Day 96, the testes are completely regressed in males at warm temperatures whereas in males at a cooler temperature they remain enlarged. Thus, increased PRL secretion during the period of gonadal development is associated with enhanced testis weight at that time, as well as accelerated termination of reproduction at the end of the season. In female Z. l. oriantha, however, PRL secretion did not differ among temperature treatments at Day 30 (Fig. 5F) whereas follicle size was affected (Fig. 1F). By Day 68, plasma PRL in females held at cooler temperatures was suppressed compared with females at warmer temperatures; however, this appears not to have influenced follicle size (Fig. 1F).
PRL could transduce environmental information independently of LH by increasing the number of gonadotropin receptors at the gonad. For example, golden hamsters Mesocricetus auratus whose testes have been induced to regress by exposure to short days respond to PRL treatment by growing their testes and accessory reproductive glands (Bex et al. 1978). These changes appear to be caused by PRL-induced increases in both testicular LH receptors and circulating testosterone. They occur without affecting LH levels, however, and treatment with LH does not result in gonadal growth or increased plasma testosterone in hamsters housed under short photoperiods (Bartke et al. 1975). PRL increases LH receptors in mouse testis (Takase et al. 1990b) and rat ovary (Holt et al. 1976). Although fewer studies have investigated the pro-gonadal effect of PRL in birds (e.g. Das 1991), differences in PRL secretion could mediate gonadal maturation and activity independently of circulating LH.
Interactions of PRL and T4
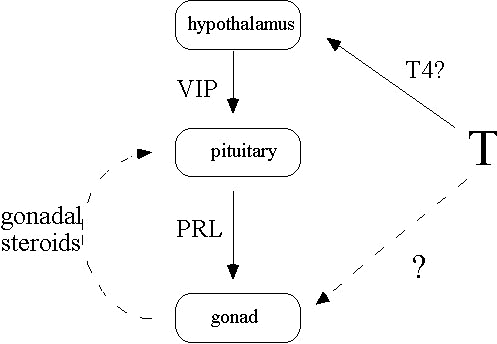
Plasma PRL appears to depend partly on T4 levels in passerines. Thyroidectomy prevents the photo-induced rise in PRL in starlings (Dawson et al. 1985), and treating thyroidectomized starlings with T4 results in a dose-dependent restoration of PRL secretion (Bentley et al. 1997). Perhaps a higher ambient temperature during gonadal development enhances the secretion of T4, which in turn may stimulate or allow PRL release (Fig. 6). In our studies, differences in PRL levels due to ambient temperature mirror differences in T4 secretion. In female Z. l. pugetensis, suppressed PRL levels in birds at a cool temperature (Fig. 5D) correspond temporally to reduced T4 levels (Fig. 4D). In male Z. l. oriantha held at a low temperature, low levels of T4 at day 10 (Fig. 4E) may be responsible for low levels of PRL found at day 30 (Fig. 5E). Finally, in female Z. l. oriantha held at a low temperature, ovarian development lags behind that of the warmer females for the duration of the experiment; PRL is low at Day 68 (Fig. 5F), possibly as a result of reduced T4 at Days 10 and 30 (Fig. 4F).
These results suggest that T4 may mediate temperature cues by affecting PRL secretion in Z. leucophrys. This hypothesis, however, is not entirely supported by the results of temperature experiments. In male Z. l. pugetensis, robust effects of temperature on T4 levels (Fig. 4C) did not cause differences in testis size (Fig. 1C) or plasma PRL (Fig. 5C). Similarly, in female Z. l. gambelii, T4 was significantly lower in the low temperature birds (Fig. 4B) with no affect on ovarian development by Day 30 (Fig. 1B). However, PRL and T4 were not both measured in the same Z. l. gambelii individuals. More experiments will be needed to assess the relationships among temperature, PRL, T4, and gonadal development.
Effects of gonadal steroids on PRL secretion
Temperature-mediated differences in plasma PRL may be a consequence, rather than a cause, of differences in gonadal maturation (Fig. 6). PRL secretion can be affected by gonadal steroids in some species. For example, in Bantam Cockerels Gallus domesticus reared on short days, castration at three weeks of age results in reduced PRL secretion in adulthood (Sreekumar and Sharp 1998). Turkeys ovariectomized during the laying stage of reproduction show a decrease in PRL levels, which is reversed by treatment with ovarian steroids (Mauro et al. 1992). Turkey anterior pituitary cells release more PRL in vitro when estradiol is present in the culture medium (Knapp et al. 1988). However, the degree to which PRL secretion depends on gonadal steroids may vary according to the stage of the reproductive cycle. Turkeys ovariectomized prior to photostimulation rather than during laying show the same photo-induced PRL response as intact females (El Halawani et al. 1983). These authors conclude that unlike the later rise in PRL at the completion of gonadal development, the photo-induced increase in PRL prior to gonadal development does not depend on ovarian hormones in the turkey.
Ovariectomized female White-crowned Sparrows that have experienced a winter-like period of short days show the same PRL response to photostimulation as intact females (Schwabl et al. 1988), suggesting that, like in turkeys, photo-induced PRL secretion occurs independently of gonadal steroids. Dawson and Goldsmith (1984) draw the same conclusion for starlings; captive gonadectomized males and females show PRL responses similar to intact birds. However, closer inspection of their data reveals that although the magnitude of the photo-induced PRL surge does not differ between ovariectomized and intact females, ovariectomy may delay the surge by several days. The effects of gonadal steroids on PRL secretion in male passerines is clearer. Although exogenous testosterone administration early during photostimulation can increase PRL, castration does not block or delay the photo-induced PRL increase in Great Tits or in starlings (Silverin and Goldsmith 1997; Dawson and Goldsmith 1984). Immunization against vasoactive intestinal peptide (VIP), a PRL releasing factor in passerines (Maney et al., unpublished manuscript; Vleck, C. M. and Patrick, D. J., Dept. Zoology and Genetics, Iowa State University, Ames, IA 50011, unpublished manuscript) may delay photo-induced testicular development in starlings (see Dawson and Sharp 1998). In the Song Sparrow Melospiza melodia, exogenous gonadal steroids did not affect PRL secretion (Wingfield et al. 1989); however, the steroids were administered late in the breeding season, after the gonads had matured. Future experiments must address the effects of gonadal steroids on photo-induced PRL secretion in White-crowned Sparrows before a role in the mediation of temperature cues can be shown.
PROLACTIN AND THE TERMINATION OF BREEDING
Both thyroxine and PRL have been implicated in shutting down reproductive effort at the end of the breeding season (e.g., Bentley et al., 1997), thus the termination of breeding in passerines may be influenced by temperature cues. In the three subspecies of Z. leucophrys we have tested, elevated ambient temperature accelerates the postnuptial moult (Fig. 2; cf. Lewis and Farner 1973), which normally occurs at the end of the breeding season and coincides with increased PRL secretion (Goldsmith and Nicholls 1984). Z. l. oriantha males held at warm temperatures regressed their testes sooner than males held at lower temperatures (Fig. 1E). Lewis and Farner (1973) showed a significant effect of ambient temperature on Zugenruhe in Z. l. gambelii; birds held at higher temperatures experienced this migration-related increase in activity sooner than did birds held at lower temperatures. Higher levels of T4 and PRL during the onset of breeding may accelerate the entire process such that breeding terminates sooner. In our experiments, birds experienced the same temperature throughout the breeding season; it would be interesting to test whether changes in temperature later in the season affect the timing of gonadal regression.
It may seem paradoxical to suggest that PRL enhances gonadal development and is also involved in the termination of breeding. However, the effects of PRL may depend on reproductive stage and overall level of secretion or may influence the rate of the cycle. El Halawani et al. (1984) divided photo-induced PRL secretion in turkeys into three distinct stages. First, a small initial increase appears to be a direct result of photostimulation and is not sensitive to temperature cues. A second increase coincides with the onset of sexual maturity and may be modified by ambient temperature; gonadal development is enhanced in female turkeys at warmer temperatures. A third and robust rise is associated with incubation behaviour and may also be sensitive to ambient temperature. Whereas low PRL levels such as those during the first 30 days of photostimulation in Z. leucophrys may enhance gonadal development, the maximal levels after Day 70 may be related to termination of reproduction, photorefractoriness, and gonadal regression.
Most studies that show an inverse relationship between plasma PRL and LH have been conducted using individuals with high levels of PRL (analogous to the third stage of PRL secretion described by El Halawani et al. 1984), such as incubating birds (Goldsmith et al. 1981; 1984; Dawson and Goldsmith 1982; Hiatt et al. 1987) and lactating rats (Lu et al. 1976). High systemic doses of exogenous PRL inhibit plasma LH and estradiol as well as hypothalamic GnRH content in laying turkey hens (Camper and Burke 1977; Rozenboim et al. 1993), and intracranial injection of PRL reduces plasma LH and testicular weight in Ring Doves Streptopelia risoria ( Buntin et al. 1988). Vasoactive intestinal peptide (VIP), a potent releaser of PRL in domestic fowl (Macnamee et al. 1986; El Halawani et al. 1990), ring doves, (Lea and Vowles 1990) and passerines (Maney et al., unpublished manuscript; Vleck and Patrick, unpublished manuscript), also inhibits plasma LH when infused into the cerebral ventricular system in turkeys (Pitts et al. 1994). These studies indicate that PRL and related peptides are associated with termination of reproduction, and may exert their effects by inhibiting GnRH at the level of the hypothalamus.
SEXUAL DIMORPHISM IN THE RESPONSE TO TEMPERATURE CUES
Higher female sensitivity
In our studies, we saw robust effects of temperature on PRL secretion only in females. Because females invest more than males in reproduction, it follows that they would be more responsive to environmental conditions. Female gonadal maturation involves two distinct stages that may have unique control mechanisms. During the first stage, the immature ovary develops until follicles are about 2-3 mm in diameter. At this time, growth pauses and generally does not progress unless the environment is conducive to breeding (King et al. 1966). The second stage involves the rapid deposition of yolk, followed by ovulation and oviposition within 3-7 days (Wingfield and Farner 1980). Yolk deposition is an energetically expensive process that should correspond with favorable conditions. In contrast to the first stage, which may depend primarily on photoperiod (Farner and Follett 1979), the second phase of gonadal maturation can be expected to be more sensitive to a variety of environmental cues than the first. In addition to laying the eggs, it is the female that performs all of the incubation. This behavior is thought to be highly PRL-dependent, and in species with asymmetrical parental care, the sex which provides more care generally secretes more PRL (Buntin 1996). Female Z. l. pugetensis have higher levels of circulating PRL during the breeding season than males (Hiatt et al. 1987). These sex differences in reproductive strategy, physiology and behavior may help to explain the disparity between the male and female responses to temperature cues in Z. l. pugetensis and Z. l. oriantha . It would be interesting to look at the PRL responses to temperature cues in other species, especially those with high male parental investment.
Social cues
Social cues have dramatic effects on gonadal development in Z. leucophrys (Moore 1983) and may play a permissive role in the transduction of temperature cues. The Z. leucophrys in the experiments described here were housed singly in cages. However, each environmental chamber contained both males and females, providing ample opportunity for visual and auditory interaction with the opposite sex. Female Z. l. pugetensis isolated from males show no effects of ambient temperature on gonadal development or T4 secretion, unlike males housed in chambers apart from females (Wingfield et al. 1997). Similar kinds of cues may also play a role. In turkeys, ambient temperature affects gonadal development and plasma PRL levels only in birds with access to nests. In nest-deprived birds, these effects of temperature disappear (El Halawani et al. 1984). Thus, whereas temperature can be an important factor in the timing of reproduction, other variables such as the presence of a mate and a nest site should and do take precedence. These results raise interesting questions about the perception and transduction of environmental signals. The hormonal mechanisms underlying these interrelationships await investigation.
CONCLUSIONS
It is clear that many vertebrates use ambient temperature as a cue to time their reproductive effort. The extent to which conditions in the breeding environment vary from season to season determines the relative importance of cues such as temperature (Wingfield et al. 1992). Birds breeding in variable environments respond to elevated ambient temperature with accelerated gonadal maturation, whereas reduced ambient temperature inhibits gonadal growth (El Halawani et al. 1984; Fig. 1). However, this effect may not mediated by differences in LH secretion. Silverin and Viebke (1994) report significant effects of temperature on LH secretion in Great Tits, but these changes do not translate into effects at the gonad. In the Willow Tit, low ambient temperature suppresses gonadal development without affecting plasma LH (Silverin and Viebke 1994), which is consistent with our results in the White-crowned Sparrow (Fig. 3). We have shown that temperature modulates the secretion of PRL, which is thought to enhance gonadal development early in the breeding season, subsequently mediate initiation of incubation, and finally induce gonadal regression. Future experiments should focus on the relationships among PRL secretion, gonadal steroids, and gonadal development.
ACKNOWLEDGEMENTS
We thank Lynn Erckmann for animal care and Lee Astheimer, Tom Hahn, Marty Morton, Marilyn Ramenofsky, Troy Smith, Mark Stanback, Masaru Wada, and Pete Wilson for technical assistance. We are also grateful to A.F. Parlow (Pituitary Hormones and Antisera Center, UCLA-Harbor Medical Center, Torrance, CA) for his generous donation of chicken prolactin antiserum and standard, and to Tom Hahn and Peter Sharp for thoughtful comments on sections of the manuscript. This work was supported by NSF grants DCB-9005081, IBN-9408013, and IBN-9631350 to JCW.
REFERENCES
Bentley, G. E., Goldsmith, A. R., Dawson, A., Glennie, L. M., Talbot, R. T., & Sharp, P. J. 1997. Photorefractoriness in European Starlings Sturnus vulgaris is not dependent upon the long-day-induced rise in plasma thyroxine. General and Comparative Endocrinology 107: 428-438.
Bex, F., Bartke, A., Goldman, B. D., & Dalterio, S. 1978. Prolactin, growth hormone, luteinizing hormone receptors, and seasonal changes in testicular activity in the golden hamster. Endocrinology 103: 2069-2080.
Buntin, J. D. 1996. Neural and hormonal control of parental behavior in birds. In: Rosenblatt, J. S. & Snowdon, C. T. (eds) Advances in the Study of Behavior. Vol. 25; New York; Academic press: 161-213.
Buntin, J. D., Lea, R. W., & Figge, G. R. 1988. Reductions in plasma LH concentrations and testicular weight in ring doves following intracranial injection of prolactin or growth hormone. Journal of Endocrinology 118: 33-40.
Cain, J. R. & Lien, R. J. 1985. A model for drought inhibition of Bobwhite Quail Colinus virginianus reproductive systems. Comparative Biochemistry and Physiology 82A: 925-930.
Cohen, D. 1967. Optimizing reproduction in a varying environment. Journal of Theoretical Biology 16: 1-14.
Colwell, R. K. 1974. Predictability, constancy, and contingency of periodic phenomena. Ecology 55: 1148-1153.
Das, K. 1991. Effects of testosterone proprionate, prolactin and photoperiod on feeding behaviours of Indian male weaver birds. Indian Journal of Experimental Biololy 29: 1104-1108.
Dawson, A. & Sharp, P. J. 1998. The role of prolactin in the development of reproductive photorefractoriness and postnuptial molt in the European starling (Sturnus vulgaris). Endocrinology 139: 485-490.
Dawson, A. & Goldsmith, A. R. 1984. Effects of gonadectomy on seasonal changes in plasma LH and prolactin concentrations in male and female starlings (Sturnus vulgaris). Journal of Endocrinology 100: 213-218.
Dawson, A. & Goldsmith, A. R. 1982. Prolactin and gonadotrophin secretion in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to nesting, incubation and rearing young. General and Comparative Endocrinology 48: 213-221.
Dawson, A., Goldsmith, A. R., & Nicholls, T. J. 1985. Development of photorefractoriness in intact and castrated male starlings (Sturnus vulgaris) exposed to different periods of long-day lengths. Physiological Zoology 58: 253-261.
El Halawani, M. E., Silsby, J. L., & Mauro., L. J. 1990. Vasoactive intestinal peptide is a hypothalamic prolactin-releasing neuropeptide in the turkey (Meleagris gallopavo). General and Comparative Endocrinology 78: 66-73.
El Halawani, M. E., Silsby, J. L., Behnke, E. J., & Fehrer, S. C. 1984. Effect of ambient temperature on serum prolactin and luteinizing hormone levels during the reproductive life cycle of the female turkey (Meleagris gallopavo). Biology of Reproduction 30: 809-815.
El Halawani, M. E., Silsby, J. L., Fehrer, S. C., & Behnke, E. J. 1983. Effects of estrogen and progesterone on serum prolactin and luteinizing hormone levels in ovariectomized turkeys (Meleagris gallopavo). General and Comparative Endocrinology 52: 67-78.
Farner, D. S. & Follett, B. K. 1979. Reproductive plasticity in birds. In: Barrington, E. J. W. (ed) Hormones and Evolution. New York: Academic Press: 829-872.
Goldsmith, A. R., Burke, S., & Prosser, J. M. 1984. Inverse changes in plasma prolactin and LH concentrations in female canaries after disruption and reinitiation of incubation. Journal of Endocrinology 103: 251-256.
Goldsmith, A. R., Edwards, C., Koprucu, M., & Silver, R. 1981. Concentrations of prolactin and luteinizing hormone in plasma of doves in relation to incubation and development of the crop gland. Journal of Endocrinology 90: 437-443.
Goldsmith, A. R. & Nicholls, T. J. 1984. Prolactin is associated with the development of photorefractoriness in intact, castrated and testosterone-implanted starlings. General and Comparative Endocrinology 54, 247-255.
Hiatt, E. S., Goldsmith, A. R., & Farner, D. S. 1987. Plasma levels of prolactin and gonadotropins during the reproductive cycle of White-crowned Sparrows (Zonotrichia leucophrys). Auk 104: 208-217.
Holt, J. A., Richards, J. S., Midgley, A. R., Jr., & Reichert., L. E., Jr. 1976. Effect of prolactin on LH receptor number in rat luteal cells. Endocrinology 98: 1005-1013.
King, J. R., Follett, B. K., Farner, D. S., & Morton, M. L. 1966. Annual gonadal cycles and pituitary gonadotropin in Zonotrichia leucophrys gambelii. Condor 68: 476-487.
Knapp, T. R., Fehrer, S. C., Silsby, J. L., Porter, T. E., Behnke, E. J., & El Halawani, M. E. 1988. Gonadal steroid modulation of basal and vasoactive intestinal polypeptide-stimulated prolactin release by turkey anterior pituitary cells. General and Comparative Endocrinology 72: 226-236.
Lea, R. W. & Vowles, D. M. 1986. Vasoactive intestinal polypeptide stimulates prolactin release in vivo in the ring dove (Streptopelia risoria). Experientia 42: 420-422.
Lewis, R. A. & Farner, D. S. 1973. Temperature modulation of photoperiodically induced vernal phenomena in White-crowned Sparrows (Zonotrichia leucophrys). Condor 75: 279-286.
Lu, K. H., Chen, H. T., Huang, H. H., Grandison, L., Marshall, S., & Meites, J. 1976. Relation between prolactin and gonadotrophin secretion in post-partum lactating rats. Journal of Endocrinology 68: 241-250.
Marshall, A. J. 1959. Internal and environmental control of breeding. Ibis 101: 456-478.
Marshall, A. J. 1970. Environmental factors other than light involved in the control of sexual cycles in birds and mammals. In: Benoit, J. & Assenmacher, I. (eds) La Photoregulation de la Reproduction Chez les Oiseaux et les Mammiferes. Paris; Presses de CNRS: 53-64.
Mauro, L. J., Youngren, O. M., Proudman, J. A., Phillips, R. E., & El Halawani, M. E. 1992. Effects of reproductive status, ovariectomy, and photoperiod on vasoactive intestinal peptide in the female turkey hypothalamus. General and Comparative Endocrinology 87: 481-493.
Macnamee, M. C., Sharp, P. J., Lea, R. W., Sterling, R. J., & Harvey, S. 1986. Evidence that vasoactive intestinal polypeptide is a physiological prolactin-releasing factor in the Bantam hen. General and Comparative Endocrinology 62: 470-478.
Moore, M. C. 1983. Effect of female sexual displays on the endocrine physiology and behavior of male White-crowned Sparrows, Zonotrichia leucophrys. Journal of Zoology London 199: 137-148.
Morton, M. L. & Allan, N. 1990. Effects of snowpack and age on reproductive schedules and testosterone levels in male White-crowned Sparrows in a montane environment. In: M. Wada, S. Ishii, & C. G. Scanes (eds) Endocrinology of Birds: Molecular to Behavioral. Berlin: Springer-Verlag: 235-249.
Oishi, T. and Konishi, T. 1978. Effects of photoperiod and temperature on testicular and thyroid activity of the Japanese quail. General and Comparative Endocrinology 36: 250-254.
Peczely, P. 1985. The role of thyoid and adrenal cotical hormones in the modulation of the gonadal function in birds. Acta Biologica Hungaria 36: 45-70.
Pitts, G. R., Youngren, O. M., Silsby, J. L., Rozenboim, I., Chaiseha, Y., Phillips, R. E., Foster, D. N., & El Halawani, M. E. 1994. Role of vasoactive intestinal peptide in the control of prolactin-induced turkey incubation behavior. II. Chronic infusion of vasoactive intestinal peptide. Biology of Reproduction 50: 1350-1356.
Reinert, B. D. and Wilson, F. E. 1996. The thyroid and the hypothalamus-pituitary-ovarian axis in American tree sparrows (Spizella arborea). General and Comparative Endocrinology 103: 60-70.
Rozenboim, I., Tabibzadeh, C., Silsby, J. L., & El Halawani, M. E. 1993. Effect of ovine prolactin administration on hypothalamic vasoactive intestinal peptide (VIP), gonadotropin releasing hormone I and II content, and anterior pituitary VIP receptors in laying turkey hens. Biology of Reproduction 48: 1246-1250.
Schwabl, H., Schwabl-Benzinger, I., Goldsmith, A. R., & Farner, D. S. 1988. Effects of ovariectomy on long-day-induced premigratory fat deposition, plasma levels of luteinizing hormone and prolactin, and molt in White-crowned Sparrows, Zonotrichia leucophrys gambelii. General and Comparative Endocrinology 71: 398-405.
Silverin, B. & Viebke, P. A. 1994. Low temperatures affect the photoperiodically induced LH and testicular cycles differently in closely related species of tits (Parus spp.). Hormones and Behavior 28: 199-206.
Silverin, B. & Goldsmith, A. R. 1997. Natural and photoperiodically induced changes in plasma prolactin levels in male great tits. General and Comparative Endocrinology 105: 145-157.
Smith, J. P. 1982. Changes in blood levels of thryoid hormones in two species of passerine birds. Condor 84: 160-167.
Sreekumar, K. P. & Sharp, P. J. 1998. Effect of photostimulation on concentrations of plasma prolactin in castrated bantams (Gallus domesticus). Journal of Neuroendocrinology 10: 147-154.
Takase, M., Tsutsui, K., and Kawashima, S. 1990a. Effects of prolactin and bromocryptine on the regulation of testicular luteinizing hormone receptors in mice. Journal of Experimental Zoology 256: 200-209.
Takase, M., Tsutsui, K., & Kawashima, S. 1990b. Effects of PRL and FSH on LH binding and number of Leydig cells in hypophysectomized mice. Endocrinology Japon 37: 193-203.
Wilson, A. C. and Farner, D. S. 1960. The annual cycle of thyroid activity in White-crowned Sparrows of eastern Washington. Condor 62: 414-425.
Wingfield, J. C. 1983. Environmental and endocrine control of reproduction: an ecological approach. In: Mikami, S. I. & Wada, W. (eds) Avian Endocrinology: Environmental and Ecological Aspects. Tokyo and Berlin; Japanese Scientific Press and Springer-Verlag: 205-288.
Wingfield, J. C. 1980. Fine temporal adjustment of reproductive functions. In: Epple, A. & Stetson, M. H. (eds) Avian Endocrinology. New York; Academic Press: 367-389.
Wingfield, J. C. and Farner, D. S. 1993. Endocrinology of reproduction in wild species. In: D. S. Farner, J. R. King, and K. C. Parkes (eds) Avian Biology. Vol. 9; New York; Academic Press: 163-327.
Wingfield, J. C. & Farner, D. S. 1980. Environmental and endocrine control of seasonal reproduction in temperate zone birds. Progress in Reproductive Biology 5: 62-101.
Wingfield, J. C. & Kenagy, G. J. 1991. Natural control of reproduction. In: Pang, P. K. T. & Schriebman, M. P. (eds) Vertebrate Endocrinology: Fundamentals and Biomedical Implications. Vol. 4B; New York; Academic Press: 181-242.
Wingfield, J. C., Hahn, T. P., Wada, M., & Schoech, S. J. 1997. Effects of day length and temperature on gonadal development, body mass, and fat depots in White-crowned Sparrows, Zonotrichia leucophrys pugetensis. General and Comparative Endocrinology 107: 44-62.
Wingfield, J. C., Hahn, T. P., Wada. M., Astheimer, L. B., & Schoech, S. J. 1996. Interrelationship of day length and temperature on the control of gonadal development, body mass, and fat score in White-crowned Sparrows, Zonotrichia leucophrys gambelii. General and Comparative Endocrinology 101: 242-255.
Wingfield, J. C., Hahn, T. P., Levin, R., & Honey, P. 1992. Environmental predictability and control of gonadal cycles in birds. Journal of Experimental Zoology. 261: 214-231.
Wingfield, J. C., Ronchi, E., Marler, C., and Goldsmith, A. R. 1989. Interactions of steroids and prolactin during the reproductive cycle of the song sparrow (Melospiza melodia). Physiological Zoology 62: 11-24.
Wingfield, J. C., Smith, J. P., and Farner, D. S. 1982. Endocrine responses of White-crowned Sparrows to environmental stress. Condor 84: 399-409.
Fig. 1. Effects of temperature and photoperiod on testicular and ovarian follicle development in Z. leucophrys (means ± SE). VLOF: Volume of largest ovarian follicle. (A) male (N = 11, 7, and 9 for the 5, 20, and 30 C groups, respectively) and (B) female (N = 12, 9, and 7) Z. l. gambelii at Day 30 of photostimulation; after Wingfield et al., 1996. (C) male (N = 10, 8, and 8) and (D) female (N = 10, 8, and 8) Z. l. pugetensis; after Wingfield et al., 1997. (E) male (N = 6, 7, and 8) and (F) female (N = 9, 10, and 8) Z. l. oriantha; after Wingfield, unpublished manuscript. Method: male and female Z. leucophrys were captured in Japanese mist nets or potter traps during the post-breeding season. All subjects were transported to Seattle, Washington, where they were held on natural photoperiod for at least three weeks. For each subspecies, individuals were transferred to individual cages in three identical light- and temperature-controlled environmental chambers at 20°C. Photoperiod was set at 8 hours of light per day (8L: 16D) for Z. l. gambelii, and 9 hours of light per day (9L:15D) for Z. l. pugetensis and Z. l. oriantha. Birds were exposed to this short-day protocol for at least 10 weeks. Each chamber contained both males and females. At Day 0, all environmental chambers were transferred to a day length representative of the breeding season for each subspecies: 20L:4D for Z. l. gambelii, 16L:8D for Z. l. pugetensis, and 15L:9D for Z. l. oriantha. Also at this time, one of the three environmental chambers was set to 5°C, the second maintained at 20°C, and the third set to 30°C. These temperatures were selected after examination of weather records at locations along migratory routes for each subspecies.

Fig. 2. Effects of temperature and photoperiod on prebasic moult in Z. leucophrys (means ± SE). Moult score was determined by noting the position of absent or growing primary feathers on each wing. The higher the score, the more advanced the moult stage. (A) male and (B) female Z. l. gambelii at Day 75 of photostimulation; after Wingfield, unpublished manuscript. (C) male and (D) female Z. l. pugetensis at Day 114 of photostimulation; after Wingfield et al., 1997; n.b. these birds were housed in single-sex groups rather than mixed sexed groups; no data on mixed sex groups are available. (E) male and (F) female Z. l. oriantha at Day 96 of photostimulation; after Wingfield, unpublished manuscript.
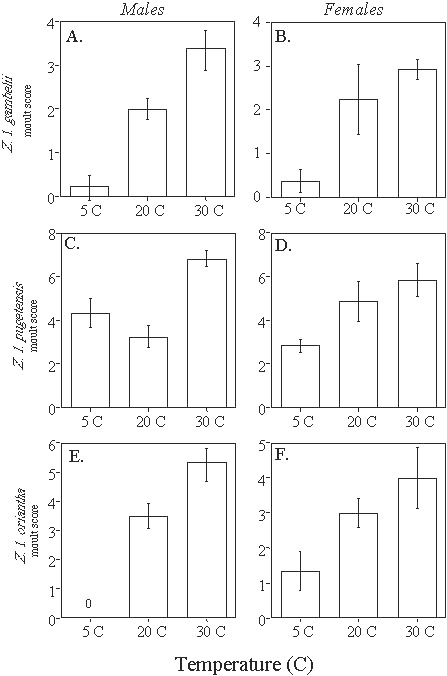
Fig. 3. Effects of temperature and photoperiod on plasma levels of luteinizing hormone (LH) in Z. leucophrys (means ± SE). (A) male and (B) female Z. l. gambelii; after Wingfield et al., 1996. (C) male and (D) female Z. l. pugetensis; after Wingfield et al., 1997. (E) male and (F) female Z. l. oriantha; after Wingfield, unpublished manuscript.
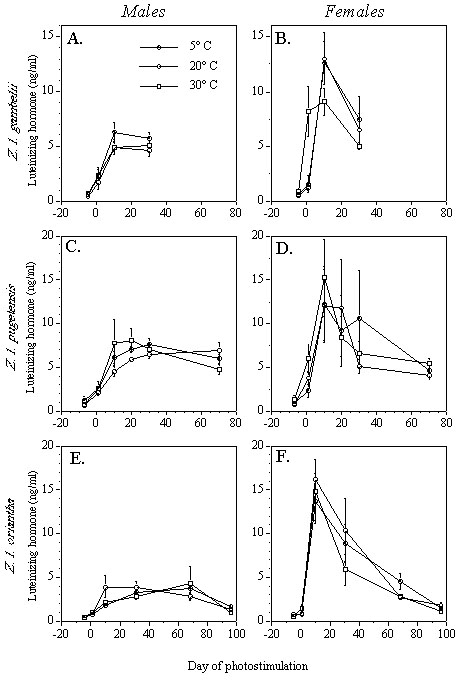
Fig. 4. Effects of temperature and photoperiod on plasma levels of thyroxine (T4) in Z. leucophrys (means ± SE). (A) male and (B) female Z. l. gambelii; after Wingfield et al. 1996. (C) male and (D) female Z. l. pugetensis; after Wingfield et al. 1997. (E) male and (F) female Z. l. oriantha; after Wingfield, unpublished manuscript.
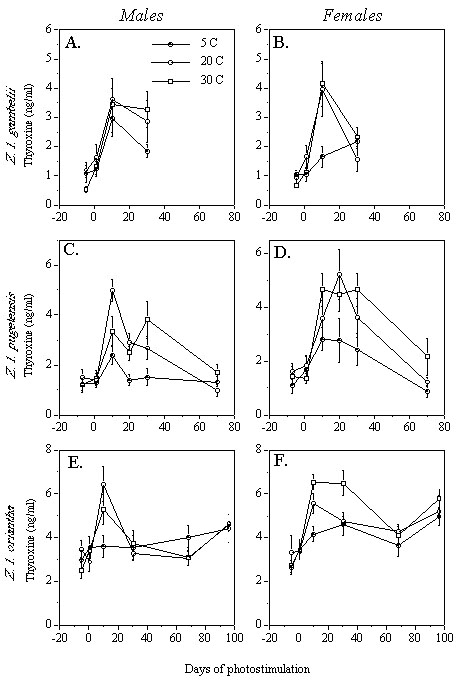
Fig. 5. Effects of temperature and photoperiod on plasma prolactin (PRL) in Z. leucophrys; after Maney, unpublished manuscript (means ± SE). (A) male and (B) female Z. l. gambelii, RSP-IR: recombinant starling prolactin-like immunoreactivity; (C) male and (D) female Z. l. pugetensis, chPRL-IR: chicken prolactin-like immunoreactivity; (E) male and (F) female Z. l. oriantha.
Fig. 6. Possible mechanisms of the hormonal transduction of temperature cues. Solid lines indicate a hypothesis that increased ambient temperature stimulates vasoactive intestinal peptide (VIP) cells in the hypothalamus, possibly via increased secretion of thyroxine (T4). VIP is secreted at the median eminence and travels to the pituitary, eliciting prolactin (PRL) release. PRL then may then stimulate gonadal development. Dashed lines represent an alternative hypothesis explaining temperature-dependent PRL secretion: increased temperature stimulates gonadal development via another mechanism, causing an increase in circulating gonadal steroids which in turn stimulate prolactin release.