S05.4: Information constraints in the timing of reproduction in arid-zone birds
Richard A. Zann
Department of Zoology, La Trobe University, Bundoora, 3083 Australia, fax 61 3 94791551, e-mail
zoorz@zoo.latrobe.edu.auZann, R. A. 1999. Information constraints in the timing of reproduction in arid-zone birds: Zebra Finches. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
265-278. Johannesburg: BirdLife South Africa.Regularity of annual breeding cycles vary widely across arid regions of the world but few are as irregular as those found in central Australia. Here Zebra Finches and other arid-adapted species are reputed to immediately exploit conditions favourable for breeding when and wherever they occur. A seven-year long study of Zebra Finch breeding patterns confirmed that breeding is opportunistic: flushes of grass seed produced immediate and proportionate flushes of breeding, irrespective of season. Zebra Finches are protein-limited breeders whose essential amino acids are provided by green grass seeds. Grass seed production was directly related to the unpredictable incidence of effective rainfall. However, breeding was constrained. It did not begin immediately it rained - a delay of two to four months occurred between effective rainfall and the production of grass seeds. In contrast, incidental observations of the same population 26 years earlier found no breeding delay after rain and it was hypothesised that the perception of rainfall provided the sole source of information on when breeding was possible. Clearly, cues other than perception of rain are necessary, and these must be identified and tracked by Zebra Finches.
INTRODUCTION
In order to make the necessary preparations needed for reproduction birds must anticipate the occurrence of favourable conditions by means of an internal circannual rhythm and predictive environmental cues (Lack 1968, Immelmann 1971, Wingfield et al. 1992). Optimal breeding conditions usually arise when the critical food resources for laying females and/or their young are abundant and accessible. In non-uniform environments this is often the period of rapid plant productivity so that a regular annual cycle occurs with breeding restricted to the same time each year, after which there is a set interval of non-breeding. In seasonal climates environmental cues allow birds to predict impending reproductive conditions with varying degrees of accuracy. By contrast, in some arid regions, those of Australia in particular, annual breeding conditions vary strongly in amplitude and periodicity (Serventy 1971). Consequently, many Australian species display irregular or flexible breeding patterns (Keast & Marshall 1954, Serventy & Marshall 1957, Braithwaite 1976, Davies 1977, 1979, Maclean 1976, Schodde 1982) and should be constrained in their use of initial predictive information (e.g. day length) which may not be reliable predictors in this environment.
The Australian subspecies of the Zebra Finch Taeniopygia guttata castanotis is the archetype of a flexible breeder with the potential to breed whenever suitable conditions arise. With exception of some populations in the extreme southern part of the range a recent survey of breeding periodicity at 12 localities/regions across the Australian-wide distribution showed that Zebra Finches are capable of breeding in any month of the year (Zann 1996). Despite the capacity for year-round breeding a rough annual pattern emerges when data from many years are amassed - a peak in spring/summer emerges in all localities and, with exception of one population from central Australia (site a Fig. 1), a lull falls in the winter months. Five populations had a second peak in breeding during the autumn months and in three cases these were greater than those in spring (sites g, i and k, Fig. 1).
Should good conditions persist Zebra Finches are capable of very protracted bouts of breeding. Length of the breeding season varied from 7.6 Equally Good Months (MacArthur 1964) in southern temperate regions (site f Fig. 1) to 10.6 for xeric populations in central Australia (site a Fig. 1). Controlling for latitude these values exceed those predicted for other Australian species (Wyndham 1986) and the latter value is one of the highest recorded for any bird at any latitude.
The breeding patterns displayed in Fig. 1 are closely coupled with pulses of maximum plant productivity in different climatic zones as predicted by Nix's (1976) plant response model which is based on inputs of solar radiation, temperature, precipitation and evaporation. The onset and peaks of breeding by granivores, such as Zebra Finches, lag behind those species that eat insects, nectar, and fruit, since seeds are produced later in the growth cycle - a pattern confirmed by Davies (1979) for birds in southwest Australia. The timing and extent of precipitation is the most variable factor in the plant response model and precipitation accounts for almost all the between year variations in breeding response at all locations, but this is not detectable in the compiled data in Fig. 1. In the central arid zone the incidence of precipitation is unpredictable in the extreme (Leeper 1970, Stafford Smith & Morton 1990), and there is no anticipated growing season (Slatyer 1962). Consequently, between year fluctuations in breeding activity of Zebra Finches at Alice Spring (Fig. 2) may be immense (Zann et al. 1995).
MINIMAL PREPARATIONS ARE NEEDED FOR BREEDING
In the laboratory it is possible for non-breeding Zebra Finches to initiate a clutch within approximately 14 days of the onset of appropriate breeding conditions. The reason for this remarkably swift response is that resting baseline levels of gonadal activity in non-breeding birds appear to be maintained at a high state of permanent preparedness such that minimal physiological changes are needed to attain thresholds for sperm production and laying. Farner and Serventy (1960) hypothesised that a unique hypothalamo-hypophysial system exists which maintains tonic levels of gonadotropins responsible for keeping gonads in near breeding condition. However, evidence from a range of wild populations is needed in order to verify this hypothesis.
Testes of Zebra Finches are usually held in a permanent functioning state once maturation is reached (Vleck & Priedkalns 1985), whereas the testes of most other species of birds so far investigated regress to a non-functioning conditioning during the non-breeding period (Lofts & Murton 1973, Wingfield & Farner 1993). Testis size varies little throughout the year (Vleck & Priedkalns 1985), but changes of 20-40% have been reported in wild birds from Western Australia, possibly in response to drought conditions (Davies 1977). These variations are insignificant compared with those of seasonally breeding species where the cycle of recrudescence and regression produces changes in testes mass of logarithmic proportions (Wingfield & Farner 1993). Surprisingly, spermatogenic activity in Zebra Finches is not closely related to testis size (Priedkalns et al. 1984, Vleck & Priedkalns 1985) nor is it influenced by day length (Marshall & Serventy 1958, Oksche et al. 1963), nor extremes of dehydration (Sossinka 1974, Vleck & Priedkalns 1985, Priedkalns et al. 1984). Nevertheless, Keast & Marshall (1954) found testicular tissues completely inactive in two populations sampled during a three year-long drought in the arid zone.
In the non-breeding season levels of plasma androgens in wild birds from southeastern Australia are about 50% lower than those in the breeding season (A. M. Dunn & R. A. Zann, unpublished manuscript). By contrast, in most periodic, temperate breeders plasma levels of androgens undergo extreme seasonal oscillations (Wingfield & Farner 1993).
The gonads of captive females also appear to be held in a state of permanent readiness for breeding. Ovarian follicles are held at a 'primary resting stage' and take less than two weeks to be yolked up for egg laying should a male and nest site become available (Sossinka 1980). Long days increase female mass and reduce the interval to laying in offspring of wild-caught birds (Meijer et al. 1996) and also increase clutch size in wild-caught ones (Sossinka 1970). Complete dehydration (two months without drinking water) reduces ovarian activity below the primary resting stage in captive breeding birds (Sossinka 1972, 1974), yet once rehydration begins it takes only two weeks for the onset of laying (Sossinka 1970). Nevertheless, prolonged drought in wild birds reduces the size of follicles below that of the primary resting stage (Keast & Marshall 1954).
In southeastern Australia females captured in winter were capable of laying within two weeks of relocation to aviary conditions (Zann 1996) and given the fact that Haywood (1993) found that active ovaries took a minimum of five days to make an egg it seems plausible that non-breeding females in reasonable condition can bring their semi-activated ovaries to breeding readiness within approximately 9-10 days. However, there is no information on the time it takes a female in poor physical condition to reach the primary resting stage of follicle development.
WHAT CRITICAL BREEDING RESOURCES SHOULD CUES PREDICT?
Unlike some species of estrildines that increase their consumption of insects during breeding (Immelmann 1962) Zebra Finches rarely eat insects at all (Davies 1977, Morton & Davies 1983, Zann & Straw 1984). Therefore, insect protein is not a requirement for breeding although it is a better source of essential amino acids than plant protein (Schultz 1987). Yet, a purely vegetarian diet means that breeding is not restricted by the timing and availability of insects and so allows Zebra Finches to be more opportunistic in tracking the seeding of grasses.
Does rain influence the timing of reproduction through its effects on seed abundance via the energy content or the nutrient content of the seed? A cheap source of energy, such as abundant shed seed, is essential for fledgling survival and rain may signal an increase in the prospects of such a supply. However, studies on wild and laboratory birds suggest that the protein content of seed is probably more important than energy. Despite the fact that wild-caught and domesticated Zebra Finches can breed on a diet of pure dry, ripe, commercial bird seed (Immelmann 1965a), this does not occur in wild birds. Provisioning of abundant and sustained supplies of commercial seed (12-14% protein) did not stimulate earlier and longer breeding in wild populations of Zebra Finches in southeast Australia (site f Fig. 1) where timing of breeding and the peaks and troughs in nesting persisted in the pre-supplement pattern (Zann & Straw 1984). Rather, the onset and peaks in laying over the eight-month long breeding season coincided with flushes of ripening seed heads of the principal species of wild grasses that constituted the bulk of the adult diet. Significantly, the crops of nestlings at this time contained much green food: green immature seeds, new leaf tips and the new heads of grasses.
A diet of pure, ripe seed may not provide sufficient protein needed for egg production and for feeding of young. In captive Zebra Finches a protein supplement of seven days duration produces an immediate increase in egg mass of the current clutch and exerts a beneficial effect on the mass of second and third clutches; female mass is also maintained during laying if there is a protein supplement (Williams 1996). Finally, when dry seed is supplemented with protein the growth rates of nestlings increase and the beneficial effects carry over to adulthood (Boag 1987).
IS FOOD ITSELF A SUPPLEMENTARY CUE?
Apart from being the most effective ultimate cause of timing of breeding the food supply itself may have an immediate influence on the timing of egg laying through its effect on the reproductive physiology of the female Zebra Finch (cue #8, Fig. 3). Houston et al. (1995a,b) calculated that in order to produce a clutch of four eggs females require 540 mg of protein, 233 mg of lipids and 71 mg calcium. Fat comes from subcutaneous fat bodies and the protein from reserves in the pectoral muscles. Here scarce essential amino acids pass from the muscles to the eggs and this causes a decline of some 14 % dry weight in the muscles themselves (Houston et al. 1995c). Energy is not limiting in laboratory birds as there was no increase in daily seed consumption during laying, but it is limiting in wild birds (Zann & Straw 1984). Calcium requirements for the shells were not drawn from skeletal stores but were met by a four-fold increase in daily consumption of calcium carbonate during the days of laying. Therefore, female Zebra Finches require a minimum level of fat and protein reserves before breeding can commence, yet they still need to obtain their calcium requirements on a daily basis, so they may be considered both 'capital' and 'income' breeders (Drent & Daan 1980).
The protein condition of female Zebra Finches before the onset of egg formation can also have a significant effect on fecundity and attractiveness to males. A protein-enriched diet of 14 days duration provided 9-13 days before laying had a significant effect on reproductive output: larger eggs, larger clutches and greater hatching success were achieved in laboratory females given an egg-food supplement (Selman & Houston 1996). In mate-choice tests males preferred those females that had been fed the protein-rich diet over those kept on plain dry seed (Monaghan et al. 1996). Clearly, the pre-breeding protein condition of females has an immediate influence on the timing of egg laying and reproductive effort, and the sudden increase in dietary protein might be the only trigger necessary to stimulate follicular development.
SHOULD RAIN PREDICT GREEN GRASS SEEDS?
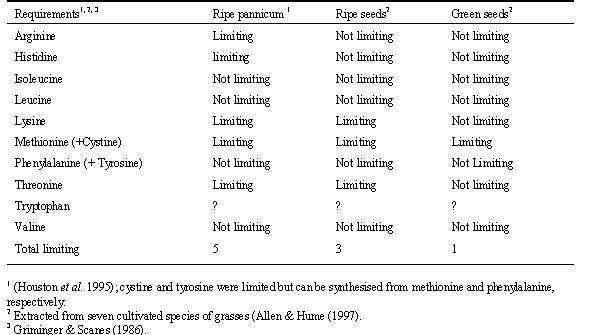
Houston et al. (1995a) found that ripe pannicum millet was deficient in five essential amino acids and Allen & Hume (1997) investigated the hypothesis that a better balance of amino acids for reproduction could be found in green , ripening grass seed because the balance of amino acids changes in the ripening process (Jennings & Morton 1963). Allen & Hume (1997) found that (1) the amino acid profiles of green seeds as a whole were closer to the egg standard than those of ripe seed; (2) three amino acids in ripe seed were in limited supply, but only one was limited in green seed, and (3) the softer, moister green seeds allowed a higher intake and throughput than ripe seed (Table 1). Therefore, green seed is a better food for egg production and the raising of young than ripe seed, and the one essential amino acid in short supply, methionine, is the one Houston et al. (1995c) found sequestered in the pectoral muscle. Methionine and phenylalanine can synthesise sulphur-rich proteins which are important in growth.
Germinating seed might also be an important source of amino acids, but this has not been investigated to date. Aviculturalists recommend its use as a nestling food (Immelmann 1965a) and Rozman (1998) has evidence that it stimulates egg laying and increases reproductive effort in laboratory Zebra Finches kept on dry seed. However, when seed is germinating in the wild it is often buried and difficult to access (Zann & Straw 1984), and for this reason food for granivores at the start of a new growing season is often in short supply in both tropical and temperate regions (Wiens & Johnston 1977).
DOES RAINFALL PREDICT BREEDING IN ZEBRA FINCHES?
Recruitment to populations in the arid zone is directly dependent on the amount of precipitation that falls in the previous year, but this is not the case in mesic regions (Zann 1996) where the interactive effects of both temperature and rainfall are the controlling variables which account for an underlying breeding seasonality (Frith & Tilt 1959, Immelmann 1965b, Kikkawa 1980, Zann & Straw 1984).
At a finer scale, incidental (e.g., Carter 1889, McGilp 1923) and short-term observers (e.g., Keast & Marshall 1954, Serventy & Marshall 1957, Immelmann 1963a,b, 1965a,b) in the arid zone have long been impressed by the immediate and direct breeding response of Zebra Finches, and other avian species, to drought breaking rain. Nevertheless, when dedicated studies of breeding periodicity were conducted in the arid region the direct links with rainfall became less obvious. For example, in a three year study in Western Australia Davies (1977) showed that there can be a delay between rainfall and breeding, and at certain times the response can be weak or non-existent. A shorter study by Maclean (1976) in northwestern New South Wales found birds bred after spring rains but not after autumn rains.
A seven year-long study of monthly breeding activity of Zebra Finches near Alice Springs (23o40'S 133o50'E) in central Australia revealed its erratic, discontinuous, and non-annual characteristics (Fig. 2) (Zann et al. 1995). Four breeding episodes were detected and while two of them continued uninterrupted for 13 and 15 months respectively, an uninterrupted period of 12 months also elapsed when no breeding was detected at all. The timing and extent of breeding at this location was linked to the timing and amount of rainfall. Peaks of breeding activity followed those of rainfall events, and the lag between the first rains and the onset of breeding varied from one month in summer to three months in winter. Breeding did not follow every rain, but beyond a threshold, the greater the rainfall event, the longer and more intense the breeding episode. A statistical model was fitted to the monthly breeding data and it explained a significant amount of the variation (R2 = 0.67) - seven of 14 regressors had significant effects in a multiple regression, but those of rainfall were the most significant. Rainfall recorded four months previously had the most positive effect on nesting while rainfall in the month of hatching actually had a negative effect. The model accurately predicted breeding events, especially in the first four years (Fig. 2a). A periodic rainfall probably accounted for the absence of significant seasonal effects in the model and for the absence of any significant autocorrelation. This unique long-term study confirms the unpredictable nature of Zebra Finch breeding in the arid zone and the results, taken together, indicate that rainfall has a significant effect on the onset and extent of breeding, but the relationship is complex. The study failed to confirm observations made by incidental observers that any rain event will automatically trigger immediate breeding. This is not surprising because rain does not always mean imminent breeding food. Therefore, when arid zone Zebra Finches attempt to use a single rain event as a predictor of breeding conditions their response is constrained by other factors that qualify the accuracy of this most important environmental signal.
How quickly can Zebra Finches make a breeding response to rainfall?
Latencies between first rains and the onset of breeding in Zebra Finches range from a few hours to three months. In November 1959 at Kununurra (15o42'S, 128o36'E), northwest Australia, Immelmann (1963a) saw courtship with the first showers of the monsoon, and copulation the next day, with the first eggs laid 14 -16 days later. There was great individual variation in responsiveness. However, in this part of the tropics most breeding by Zebra Finches occurs at the end of the monsoon, some three to four months after the first rains, hence environmental cues other than the 'sight of falling rain' must be responsible for this main breeding response.
In Alice Springs in May 1960 Immelmann was again fortunate to witness the first rains in over two months. He saw courtship during the downpour and the first copulations soon afterwards with the first eggs laid 13 days later. On 19 June 1986 I witnessed the first rains at Alice Springs that interrupted a prolonged dry period of 17 months duration. However, there was no courtship or mating in the following days, or weeks, and the first eggs did not appear until 18 August, an interval of two months from the initial downpour (Zann et al. 1995). Subsequent breeding episodes and resurgences over the following seven years had laying latencies between one and three months from the first rains.
In the Gascoyne River region (25o10'S 116o5'E) of Western Australia Carnaby (1954) documented the breeding response of some 58 species to good falls of aseasonal rain that first fell at the end of March 1934. Zebra Finches had their first eggs within three to four weeks, but some other 22 species, all insectivores and/or nectarivores, laid earlier than this.
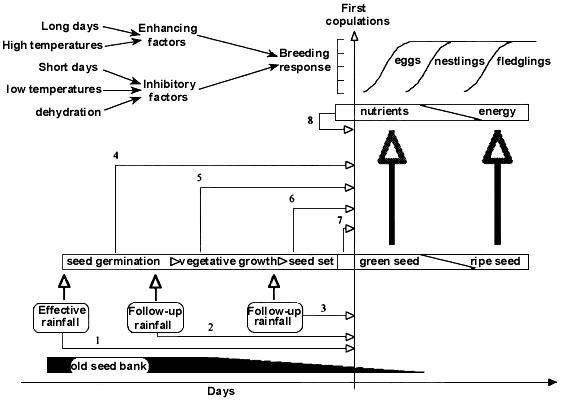
Clearly, populations of Zebra Finches across the arid zone and beyond, have great flexibility in breeding responsiveness to rainfall. Why should this be so? There are at least three possibilities: a) the genetic predispositions of the individuals in the populations may have led to different mechanisms and the use of different environmental cues, b) their health or condition may have reduced the swiftness of response, and c) the accuracy of the information provided by a rain may need to be calibrated with respect to other sources of local environmental information that affect the timing and occurrence of the trophic resources critical for breeding. The physiological evidence presented above suggests that even if completely debilitated after prolonged drought birds will need only a few weeks of favourable conditions to restore the gonads to an active or semi-active state. High levels of mobility and dispersal (Zann & Runciman 1994, Zann et al. 1995) may largely account for the individual variation in responsiveness within populations. However, the principal reason for the range of latencies in breeding response to rain is that not all rain events have equal amounts of information - some rains (e.g. cue #3) are more accurate predictors than others (e.g. cues #1 & 2, Fig. 3).
TRACKING GREEN GRASS SEEDS IN TIME AND SPACE
The green seed hypothesis predicts that in order to breed Zebra Finches must track the occurrence of green grass seeds. These are not only a source of limiting essential amino acids but they are also a sign of an impending increase in the abundance, quality and accessibility of shed, ripe seed that is needed by the fledglings to ensure survival. New tips of leaves may also be a source of limiting amino acids (cues #4 & 5, Fig. 3) but their occurrence is a less reliable indication of impending supplies of seed although they could provide mid- to long-range supplementary information in tracking seeding grass heads.
In arid Australia rain does not always mean immediate grass seed production and the possibilities of breeding by Zebra Finches. The extent and timing of rainfall, the prevailing nutrients, soil temperatures and amount of rain in previous seasons all affect germination, growth and seeding of grasses (Slatyer 1962). Precise timing of the seeding response also depends on the species of grasses involved and whether they simply regrow from remnant tufts or from germinating seed. To be effective, 'initial' rains must be followed up by 'carry-over' rains and it is likely the latter will be a more precise cue than the former. Hence, not all falls of rain will be equal in their ability to reduce uncertainty about the timing of seed set by grasses. Somehow Zebra Finches must calibrate their sensitivity to rainfall cues by means of previous events or their effects on the local environment in such a way that greens seeds are available just in time for nestlings (cue #7) or possibly even earlier for ovulating females (cue #8, Fig. 3).
In addition to temporal uncertainty there is spatial heterogeneity with some sites more productive and responsive to rain than others. For example, 'flow-on' sites accumulate more useful rain and have more soil nutrients than others and grasses here respond more rapidly and require less direct precipitation (Davies 1986, Stafford Smith & Morton 1990). Thunderstorms often provide significant rainfall in the arid zone, but their impact is highly localised so that patches or oases of green lushness occur in a sea of barrenness. It is highly likely that Zebra Finches, which are highly mobile in this region (Zann et al. 1995), could find and exploit these breeding conditions without experiencing the precipitation responsible. Therefore, in many instances, rainfall cannot provide accurate supplementary information (Wingfield et al. 1992) on when breeding can begin because of its uncertain effects in space and time. Rain is important for quickly rehydrating drought-affected birds and returning their gonads to breeding readiness, but in many instances it cannot provide precise information on when egg formation should begin. This inability to accurately predict breeding conditions has probably been the principal factor leading to the selection of individuals that can maintain the gonads in a state of near readiness on a year-round, permanent basis. The fitness payoffs of this adaptation must be considerable. This fundamental shift in the reproductive physiology probably arose in the Quaternary period during episodes of extreme aridity, and it has also ensured that extant populations in more mesic and periodic environments on the periphery of the Australian continent continue to maintain great flexibility in the timing of breeding. Investigations of the metabolic costs and ecological risks of maintaining gonads in a permanent state of semi-activation would be informative.
Despite the uncertainty in predicting when breeding conditions occur in response to rain Immelmann (1963a) did observe Zebra Finches apparently responding in an immediate and direct way to rain as it fell in central Australia in 1960. Yet 26 years later rain at the same time of year at a site 35 km away had no immediate effect on breeding and if a cycle had been initiated young would have starved (Zann et al. 1995). Why should rain act as an immediate trigger for breeding at one time and not another? A number of explanations are possible. First, rainfall records suggest that it is likely Immelmann described a simple resurgence in breeding activity after a brief lull in a long bout of breeding that began six months earlier, whereas in 1986 no breeding had probably occurred for the previous 17 months. Response may have been rapid in Immelmann's case (cue #3, Fig. 3) because some birds were still in breeding condition and tufts of grasses were able to regrow rapidly and set seed within about four weeks of the downpour - this would have provided food for newly-hatched young. Second, it is conceivable that we are dealing with two geographically distinct populations with different breeding sensitivities to rain in a situation comparable to that described by Lambrechts et al. (1996) for photoperiod responsiveness in Blue Tits. However, this hypothesis is unlikely given the high vagility of Zebra Finches in the arid zone and the absence of any physical barriers between the locations. Third, the composition of grass species has changed in the region since 1960; short-lived native annuals with rapid seeding latencies have been gradually replaced by an exotic perennial whose seeding phenology is slower and timing of seed set is different (Zann et al. 1995). This event could have rapidly selected for individuals with longer response latencies to rain so that the onset of breeding would still coincide with the onset of seeding.
DISCUSSION
Taken together, the physiological evidence from laboratory birds and the observational evidence from the wild suggest that Zebra Finches have adapted to their uncertain, aperiodic environment by means of a major change in the physiological machinery that permits the maintenance of the gonads in a semi-active state on a year-round basis. With this adaptation long-range warnings ('initial predictive information (Wingfield & Farner 1993) are not needed to make the physiological and behavioural preparations required for breeding and selection has focused on those cues ('essential supplementary information') needed for the fine-tuning of the response to local conditions. Consequently, rain cues #1-3 (Fig. 3) may simply have a priming effect through their influence on the vegetation, and the energetic and nutrient benefits of the first flush of green seeds (cue #6) may be all that is required to stimulate the rapid phase of follicular development and the onset of laying. In situations where rain does appear to be a direct trigger (cue #3) it is likely that the accuracy of its information has been enhanced by earlier environmental cues such as previous seed germination (cue #4) and vegetative growth (cue #5) from earlier rains, and the swiftness of the response is likely to be modulated by previous experience of the population to local conditions. Given the short lifespan of Zebra Finches (Zann & Runciman 1994) it is unlikely any individual experiences of past latencies between cues and breeding conditions will be possible.
FUTURE RESEARCH
The green seed model for opportunistic breeding in Zebra Finches is still highly speculative. Most mechanisms and causal connections are far from clear and investigations have been mainly descriptive, not experimental. So far, laboratory studies have examined factors that enhance the breeding response rather than those that stimulate its onset in the first place. This is because laboratory Zebra Finches have no constraints on breeding situation that does not occur in the wild. While many questions remain to be answered on the endocrinology, energetics and nutrition of reproduction n laboratory Zebra Finches there have been no physiological investigations of wild birds at all. Year round profiles of hormonal (gonadotropins and sex steroids) and gonadal activity are needed from populations of comparable latitude in xeric and mesic environments to confirm the state of their breeding readiness and to find any phenotypic variation that will enable the fitness consequences to be tested. At the same time protein condition and availability of ripe and green grass seeds need to be measured and the focus should be on individual condition and variation in the timing of initiation and cessation of breeding and how this relates to fitness (fecundity and survival). Investigations should also consider what physiological events terminate breeding. Finally, on the ecology side, protein supplementation experiments are unlikely to be successful; consequently, large scale irrigation experiments may be the only way to verify the green seed hypothesis.
ACKNOWLEDGMENTS
I thank Marcel Visser, Jan Rozman and Marcel Lambrechts for helpful comments on a draft of the manuscript.
REFERENCES
Allen, L.R. & Hume, I.D. 1997. The importance of green seed in the nitrogen nutrition of the Zebra Finch Taeniopygia guttata. Australian Journal of Ecology 22: 412-418.
Boag, P.T. 1987. Effects of nestling diet on growth and adult size of Zebra Finches (Poephila guttata). Auk 104: 155-166.
Braithwaite, L.W. 1976. Environment and timing of reproduction and flightlessness in two species of Australian ducks. Proc. 16 Int. Ornithol. Congr. Canberra: 489-501.
Carnaby, I.C. 1954. Nesting seasons of West Australian birds. Western Australian Naturalist 4: 149-156.
Carter, T. 1889. Notes from Western Australia. Zoologist Series 3: 267-268.
Davies, S.J.J.F. 1977. The timing of breeding of the Zebra Finch Taeniopygia guttata at Mileura, Western Australia. Ibis 119: 369-372.
Davies, S.J.J.F. 1979. The breeding seasons of birds in southwestern Australia. Journal of the Royal Society of Western Australia 62: 53-64.
Davies, S.J.J.F. 1986. A biology of the desert fringeópresidential address 1984. Journal of the Royal Society of Western Australia. 62: 53-64.
Drent, P.J. & Daan, S. 1980. The prudent parent, energetic adjustments in avian breeding. Ardea 68: 225-252.
Farner, D.S. & Serventy, D.L. 1960. The timing of reproduction in birds in the arid region of Australia. Anatomical Record 137: 354.
Frith, H.J. & Tilt, R.A. 1959. Breeding of the Zebra Finch in the Murrumbidgee irrigation area. Emu 59: 289-295.
Griminger, P. & Scanes, C.P. 1986. Protein metabolism. In: Sturkie, P. D. (ed) Avian physiology; New York; Springer-Verlag: 326-344.
Haywood, S. 1993. Sensory control of clutch size in Zebra Finches (Taeniopygia guttata). Auk 110: 778-786.
Houston, D.C., Donnan, D. & Jones, P.J. 1995a. The sources of the nutrients required for egg production in Zebra Finches Poephila guttata. Journal of the Zoological Society of London 235: 469-483.
Houston, D.C., Donnan, D., Jones, P., Hamilton, I. & Osborne, D. 1995b. Changes in the muscle condition of female Zebra Finches Poephila guttata during egg laying and the role of protein storage in bird skeletal muscle. Ibis 137: 322-328.
Houston, D.C., Donnan, D. & Jones, P.J. 1995c. Use of labelled methionine to investigate the contribution of muscle proteins to egg production in Zebra Finches. Journal of Comparative Physiology B 165: 161-164.
Immelmann, K. 1962. Beitr'ge zu einer vergleichenden Biologie australischer Prachtfinken (Spermestidae). Zoologische Jahrb¸cher Abteilung f¸r Systematik ÷kologie und Geographie der Tiere 90: 1-196.
Immelmann, K. 1963a. Tierische Jahresperiodik in –kologischer Sicht. Zoologische Jahrb¸cher, Abteilung f¸r Systematik ÷kologie und Geographie der Tiere 91: 91-200.
Immelmann, K. 1963b. Drought adaptations in Australian desert birds. Proc. 13 Int. Ornithol. Congr. Ithaca: 649-657.
Immelmann, K. 1965a. Australian finches in bush and aviary. Sydney; Angus & Robertson: 216pp.
Immelmann, K. 1965b. Versuch einer –kologischen Verbreitungsanalyse beim australischen Zebrafinken, Taeniopygia guttata castanotis (Gould). Journal f¸r Ornithologie 196: 415-430.
Immelmann, K. 1971. Ecological aspects of periodic reproduction. In Farner, D. S. & King, J. R. (eds) Avian Biology. Vol. 1; New York; Academic Press: 342-389.
Jennings, A.C. & Morton, R.K. 1963. Changes in carbohydrate, protein, and non-protein nitrogenous compounds of developing wheat grain. Australian Journal of Biological Science 16: 318-331.
Keast, A.J. & Marshall, A.J. 1954. The influence of drought and rainfall on reproduction of Australian desert birds. Proceedings of the Zoological Society of London 124: 393-499.
Kikkawa, J. 1980. Seasonality in the nesting season of Zebra Finches at Armidale, N. S. W. Emu 80: 13-20.
Lack, D. 1968. Ecological adaptations for breeding in birds. London; Methuen: 404pp.
Lambrechts, M.M., Perret, P. & Blondel, J. 1996. Adaptive differences in the timing of egg laying between different populations of birds result from variation in photoresponsiveness. Proceedings of the Royal Society (London) Series B 263: 16-22.
Leeper, G.W. 1970. The Australian environment. Melbourne; CSIRO & Melbourne University Press: 151pp.
Lofts, B. & Murton, R.K. 1973. Reproduction in birds. In: D. S. Farner & Kings, J. R. (eds.) Avian Biology. Vol. 3; New York; Academic Press: 1-107.
Maclean, G.L. 1976. Rainfall and avian breeding seasons in northeastern New South Wales in spring and summer 1974-75. Emu 76: 139-142.
Marshall, A.J. & Serventy, D.L. 1958. The internal rhythm of reproduction of xerophilous birds under conditions of illumination and darkness. Journal of Experimental Biology 35: 777-670.
MacArthur, R.H. 1964. Environmental factors affecting species diversity. American Naturalist 98: 387-397.
McGilp, J.N. 1923. Birds of Lake Frome district, South Australia. Emu 22: 274-287.
Meijer, T., Rozman, J., Schulte, M. & Stach-Dreesmann, C. 1996. New findings in body mass regulation in Zebra Finches (Taeniopygia guttata) in response to photoperiod and temperature. Journal of the Zoological Society of London 240: 717-734.
Monaghan, P., Metcalfe, N.B. & Houston, D.C. 1996. Male finches selectively pair with fecund females. Proceedings of the Royal Society of London Series B 263: 1183-1186.
Morton, S.R. & Davies, P.H. 1983. Food of the Zebra Finch (Poephila guttata), and an examination of granivory in birds of the Australian arid zone. Australian Journal of ecology 8: 235-243.
Nix, H.A. 1976. Environmental control of breeding, post-breeding dispersal and migration of birds in the Australian region. Proc. 16 Int. Ornithol. Congr. Canberra: 272-305.
Priedkalns, J., Oksche, A., Vleck, C. & Bennet, B.K. 1984. The response of the hypothalamo-gonadal system to environmental factors in the Zebra Finch, Poephila guttata castanotis. Cell and Tissue Research 238: 23-35.
Oksche, A., Farner, D.S., Serventy, D.L. , Wollf, F. & Nicholls, C.A. 1963. The hypothalamo-hypophysial neurosecretory system of the Zebra Finch, Taeniopygia castanotis. Zeitschrift für Zellforschung 58: 846-914.
Rozman, R. 1998. Regulation of body mass and reproduction in Australian Zebra Finches (Taeniopygia guttata castanotis)óeffects of photoperiod, temperature and diet. Ph. D Thesis, Bielefeld University, Bielefeld, Germany.
Schodde, R. 1982. Origin, adaptation and evolution of birds in arid Australia. In: Barker, W. R. & Greenslade, P. J. M. (eds) Evolution of flora and fauna of arid Australia. Adelaide; Peacock Publications: 191-224.
Schultz, D. 1987. Nutrition and orphan rearing in young birds. In MacWhirter (ed.) Everybird - a guide to bird health; Melbourne; Inkarta Press: 30-34.
Selman, R.G. & Houston, D.C. 1996. The effects of prebreeding diet on reproductive output in Zebra Finches. Proceedings of the Royal Society of London Series B 263: 1585-1588.
Serventy, D.L. 1971. Biology of desert birds. In: Farner, D.S. & King, J. R. (eds) Avian Biology Vol. 9; New York; Academic Press: 287-339.
Serventy, D.L. & Marshall, A.J. 1957. Breeding periodicity in Western Australian birds: an account of unseasonal nesting in 1953 and 1955. Emu 57: 99-126.
Slatyer, R.O. 1962. Climate of the Alice Springs area. In: Perry, A. (ed.) General report of the lands of Alice Springs Area, Northern Territory, 1956-57. Melbourne; CSIRO Land Research Series: 109-128.
Sossinka, R. 1970. Domestikationserscheinungen beim Zebrafinken Taeniopygia guttata castanotis (Gould). Jahrbücher Abteilung für Systematik Ökologie und Geographie der Tier 97: 455-521.
Sossinka, R. 1972. Besonderheiten in der sexuellen Entwicklung des Zebrafinken Taeniopygia guttata castanotis (Gould). Journal für Ornithologie 113: 29-36.
Sossinka, R. 1974. Der Einfluss von Durstperioden auf die Schilddrüse und Gonadenaktivit't und ihre Bedeutung für Brutperiodik des Zebrafinken (Taeniopygia castanotis Gould). Journal für Ornithologie 115: 128-140.
Sossinka, R. 1980. Ovarian development in an opportunistic breeder, the Zebra Finch Poephila guttata. Journal for Experimental Zoology 211: 225-230.
Stafford Smith, D.M. & Morton, S.R. 1990. A framework for the ecology of arid Australia. Journal of Arid Environments 18: 255-278.
Vleck, C.M. & Priedkalns, J. 1985. Reproduction in Zebra Finches: hormone levels and effect of dehydration. Condor 87: 37-46.
Wiens, J.A. & Johnstone, R.F. 1977. Adaptive correlates of granivory in birds. In Pinowski, J. & Kendeigh, S. C. (eds). Granivorous birds in ecosystems; Cambridge; Cambridge University Press: 301-340.
Williams, T.D. 1996. Variation in reproductive effort in female Zebra Finches (Taeniopygia guttata) in relation to nutrients-specific dietary supplements during egg laying. Physiological Zoology 69: 1255-1275.
Wingfield, J.C., Hahn, T.P., Levin, R., & Honey, P. 1992. Environmental predictability and control of gonadal cycles in birds. Journal of Experimental Zoology 261:214-231.
Wingfield, J.C. & Farner, D.S. 1993. Endocrinology of reproduction in wild species. In: D. S. Farner & King, J. R. (eds) Avian Biology Vol. 9; New York; Academic Press: 163-327.
Wyndham, E. 1986. Lengths of birds' breeding seasons. American Naturalist 128: 115-164.
Zann, R.A. 1996. The Zebra Finch: a synthesis of field and laboratory studies. Oxford; Oxford University Press: 335pp.
Zann, R.A., Morton, S.R., Jones, K.R. & Burley, N. 1995. The timing of breeding of Zebra Finches in relation to rainfall at Alice Springs, central Australia. Emu 95: 208-222.
Zann, R. & Runciman, D. 1994. Survivorship, dispersal and sex ratios of Zebra Finches Taeniopygia guttata in southeast Australia. Ibis 136: 136-146.
Zann, R. & Straw, B. 1984. Feeding ecology and breeding of Zebra Finches in farmland in northern Victoria. Australian Wildlife Research 11: 533-552.
Table 1. Ten essential amino acids required for egg production and nestling growth in the Zebra Finch and whether sources available from ripe and green seeds are limited or not.

Fig. 1. Timing of breeding of Australian Zebra Finches by month at 12 locations. All sites are located in the arid zone except peripheral locations d, f, g, and i, which are mesic to semi-arid in climate (From Zann 1996 with permission of Oxford University Press).
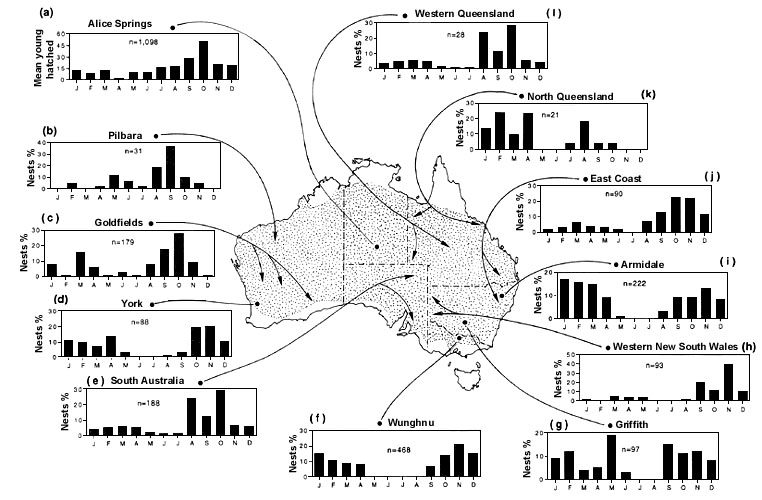
Fig. 2. Breeding activity (number hatched) of Zebra Finches and climatic data by month at Alice Springs, central Australia from 1986-1989 (a) and 1989-1992 (b). No data were available for months with a question mark. The line with open diamonds shows values predicted by the following multiple regression: log10 number hatched month-1 = + 2.9 +0.01 (rainfall 4 months previously) -2.3 (year) -0.01 (rain current month )(From Zann et al. 1995).
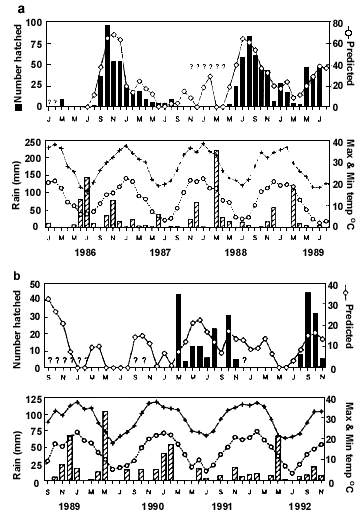
Fig. 3. A schematic portrayal of the range of possible environmental cues (#1- 8) used by Zebra Finches to predict the onset of breeding conditions following rainfall in arid Australia. The first copulations occur about 10 days before the first egg is laid. Assuming that the gonads are maintained in a near active state on a permanent, year-round basis initial predictive information is probably unnecessary and cues #1- 7 provide a range of supplementary cues of varying latencies that depend on local conditions. The 'green seed' hypothesis predicts that only cues #7 and 8 are reliable and necessary, although cue #3 will produce a rapid response if the population has been sensitised by earlier cues.