S05.3: Information constraints in the timing of reproduction in temperate zone birds: Great and Blue Tits.
Marcel E. Visser1 & Marcel M. Lambrechts2
1 Netherlands Institute of Ecology, P.O. Box 40, 6666 ZG Heteren, The Netherlands, fax 31 26 4723227
, e-mail m.visser@cto.nioo.knaw.nl; 2CEFE/CNRS, 1919 Route de Mende, 34293 Montpellier cedex 5, France, e-mail lambrech@cefe.cnrs-mop.frVisser, M.E. & Lambrechts, M.M. 1999 Information constraints in the timing of reproduction in temperate zone birds: Great and Blue Tits. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
249-264. Johannesburg: BirdLife South Africa.For the identification of cues used by birds to time their reproduction experiments in aviaries are essential. Only under controlled conditions the impact of different cues can be separated. However, also field data are needed because both the statistical analyses of date of egg laying and knowledge of the selection pressures in the field can be used to generate hypotheses about the impact of various cues. Variation in lay date of Great and Blue Tits in the field is well studied and recently also aviary experiments aiming to explain this variation are reported. There can either be variation in cues or in the way individuals respond to these cues, and variation between populations might have a different causation than variation within populations. Two basic approaches for experiments in aviaries are (1) keep individuals under conditions that differ in only one cue and measure differences in lay date, and (2) keep individuals from different populations (which possibly respond different to identical cues) under identical conditions and compare the difference in lay date with the differences between the natural populations. Both approaches will be illustrated.
INTRODUCTION
The temperate zone is characterised by strong fluctuations in the availability of food for organisms at the higher trophic levels, such as insectivorous birds. As it is crucial for animals to match the energy and nutrients requirements of their offspring with the availability of food, only a short period of the year is suitable for reproduction (Murton & Westwood 1974). The time within a year at which food availability peaks varies between areas and years, and as a consequence also the timing of reproduction will vary. This offers an adaptive explanation for why there is large intra-specific variation in the timing of reproduction between years and areas in temperate zone birds. It does however not answer the question which proximate factors are used in the timing of reproduction to enhance synchrony between offspring requirements and food availability. As reproduction is initiated much earlier than the time of maximum food requirement of the offspring, individuals should start reproduction in response to cues, available at the time of reproductive decision making, which predict the moment of maximum food abundance (Wingfield et al. 1992). We will term this process by which internal (condition, etc.) and external (photoperiod, temperature, etc.) cues are translated into a reproductive decision the response mechanism.
Because of the spatial and temporal variation in the time of maximum food abundance, the response mechanism should lead to different laying dates in different years and areas, and possibly different territories within areas. In other words, the response mechanism should lead to adaptive phenotypic plasticity, and the main selection pressure will be on the reaction norms (i.e. how the decisions changes along an environmental axis), not so much on the decision in a particular year or area. It is also well possible that there is intra-specific variation in response mechanisms in species that occur over a wide geographical range. Our knowledge of the response mechanism and the cues involved is limited but crucial for a full understanding of intra-specific variation in the timing of reproduction at different temporal and spatial scales.
Information constraints
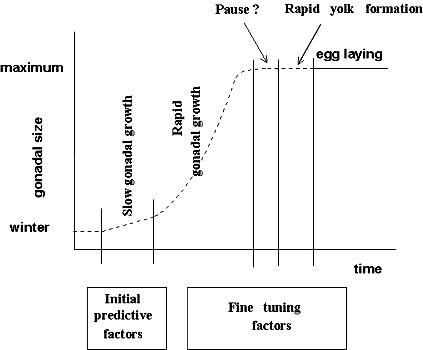
Before birds can start with egg laying they have to undergo a series of physiological, morphological and behavioural changes. This process can be divided in 3 phases: (a) a slow development of gonads, followed by (b) a rapid gonad development, and (c) rapid yolk formation (Murton & Westwood 1977; Silverin et al. 1989; Silverin et al. 1993; Meijer 1991; see Fig. 1). The start and the rate of the slow development may solely depend on an internal clock (Silverin et al. 1993; Wingfield 1993; Gwinner 1996), while so called initial predictive factors (sensu Wingfield et al. 1992) like photoperiod (or change in photoperiod) may determine the start of the rapid gonad development. Fine tuning factors, external stimuli such as temperature, the phenology of the vegetation, etc., may influence the development rate during the rapid phase and the onset of the rapid yolk formation. Whether the initial predictive or the fine tuning factors are more important will differ between species or even between populations, depending on the predictability of the environment (Wingfield et al. 1992; Wingfield et al. 1993). This will depend on the temporal or spatial variation in abiotic factors but also on the sensitivity of the food source for the birds to these abiotic factors. In many systems, the birds lay their eggs at a time that the peak in food abundance can still be affected by abiotic factors like temperature (van Noordwijk et al. 1995). For instance, temperatures after egg laying by Tits strongly affect the developmental time of caterpillars, their main prey species. The sensitivity of the prey for variation in an abiotic factor determines the potential degree of mistiming between food abundance and offspring needs. Birds can adjust their timing after start of egg laying to a limited extent, for instance by varying their clutch size, the interval between the last egg and the start of incubation or maybe the intensity of incubation. But their potential of speeding up or slowing down is typically much smaller than for their prey species.
Internal stimuli may also play a role in the timing of reproduction, even in the form of experiences from previous years. Laying dates of Great Tits are affected by the experienced asynchrony between food availability and nestling needs in the previous year (Nager & van Noordwijk 1995). Body condition will probably play a more important role, maybe primary during the rapid yolk formation (Meijer 1991). This makes it useful to distinguish ‘resources’ from ‘information’. We term the external variables that affect gonad growth ‘information cues’. Some of these cues are responsible for the fine tuning of the timing of reproduction, while others, like photoperiod, are initial predictive factors that do not vary between years. In this paper we will concentrate on the information cues used by temperate zone passerine species but we will first briefly address the role of resource constraints in the timing of reproduction.
Resource constraints
Nutrients and energy are required for egg production, and both may be difficult to gather in early spring. For a long time it was thought that this limitation of resources set the earliest laying date possible within a year, and that birds did not lay earlier simply because they were unable to obtain enough resources (Perrins 1970). This resource limitation hypothesis can however also be viewed as a trade-off between current and future reproductive success (see also Nilsson 1994; Nilsson this volume). For each year and area there is a single laying date at which the fitness return from a clutch (the current reproductive value, CRV) is maximised: the most productive laying date (MPLD, Visser unpubl.). This is not necessarily the optimal laying date, which is the date at which fitness is maximised. When resources are limited early in the season, producing eggs at the MPLD (or incubating eggs that are laid on the MPLD) may be more costly (in terms of reduced future reproductive success) than later on. In those cases the optimal laying date will always be later than the MPLD (Visser unpubl.). From food provisioning experiments in the field it is known that laying date can be advanced by supplementing food but, in single brooded species, usually less than a week (Svensson 1995). This could indicate that there is a difference between the optimal laying date and the MPLD but usually not more than a few days.
We can now put resource and information constraints into a single framework using the optimality approach to the resource constraint hypothesis as outlined above. The birds will use cues to estimate the most productive laying date. In some years and areas the resource level will already be so high at this MPLD that this is also the optimal date to start laying. Thus, when the MPLD and the optimal laying date are the same, laying is timed to match offspring needs with food availability. However, in other situations resources will still be increasing at the MPLD and the optimal laying date will be later. In those situations laying is hampered because of low resource availability (which may result in a pause between full gonadal development and rapid yolk formation, see Fig. 1) and birds will have their young in the nest after the period with maximum food availability.
It is unknown how often the start of reproduction is hampered by limited resources and how often by the information cues. To complicate things further, there may well be an interaction between the two, with females producing eggs under low resource conditions when the information cues indicate that the optimal time of chick rearing is near. Also, we do not know whether food abundance of for instance small caterpillars are only a source of energy and nutrients or also act as an information cue.
Approaches
Studying information cues and the response mechanism used is only possible under controlled conditions. In the field many potentially important variables are correlated with each other, and many factors cannot or only to a limited extent be influenced in field experiments. One possibility is to keep females in small cages in the laboratory. Often, birds will not lay eggs under such conditions but by laparotomy or by analysing blood samples taken at regular intervals, it is possible to track the development of the gonads (see Maney et al., this volume). It is also possible to house pairs in aviaries with nesting opportunity and record the laying date, although it may be difficult to create the conditions under which animals will reproduce. It is this approach that we will concentrate on. To select potential cues to test, one approach is to determine which cues should give reliable information on the future abundance of nestlings’ food. Data for such an approach comes from correlative data from field studies or from field experiments. Much of what we know about cues that play a role in the onset of reproduction comes from field studies and we will summarise this first.
FIELD STUDIES
This review is skewed towards Great Tit Parus major and Blue Tit Parus caeruleus literature, as these species will also play a central role in the overview on aviary experiments. Both species are well studied and are one of the rare biological models in which laboratory studies of the timing of reproduction have been carried out within a framework of long-term studies in natural populations.
Photoperiod
Photoperiod is the most important initial predictive factor as it functions as a Zeitgeber for the internal clock, which controls the start of the slow gonadal growth (Silverin et al. 1993; Wingfield 1993; Gwinner 1996). Photoperiod also plays a role in the onset of the rapid gonadal growth. Great Tits from different parts of Europe differ in the critical photoperiod at which this rapid growth starts (Silverin et al. 1993).
Ambient temperature
Ambient temperature is possibly an important fine tuning factor as the rate of the rapid gonadal growth is affected by temperature (Farner & Mewaldt 1952; Engels & Jenner 1956; El Halawani et al. 1984; Silverin 1995). In Great Tits the effect of temperature seems to act in interaction with photoperiod and latitude. Temperature affected gonadal growth in Great Tits from Italy and, for extreme long photoperiods, from Sweden (Silverin 1995) but not from Finland (Suomalainen 1937). The increased rate of gonadal development at higher temperatures fits in with the common observation that many temperate zone species, including Great and Blue Tits, lay earlier in warm springs (Kluyver 1951; Dhondt 1970; van Balen 1973; Perrins & McCleery 1989). One complication with the correlation between spring temperature and laying date is the choice of the period over which the temperature has to be averaged. The common procedure is to tests several periods and calculated which of them gives the highest correlation (Kluyver 1951; van Balen 1973). A better solution, based on the results of Silverin et al. (1993), may be to use the date at which the threshold photoperiod for the rapid gonadal growth is reached as a starting point. But even in that case there is no a priori end date of the period over which the average temperature is calculated.
Apart from the effect of the average temperature in a relatively long period before egg laying, temperature also seems to affect laying at a much shorter time scale with birds laying 4-5 days after an increase in temperature (Kluyver 1951; Seel 1966; Perrins & McCleery 1989) or after a certain average temperature has been reached (Dhondt 1970). Sometimes night temperature seems to correlate better than the average temperature (Meijer et al. 1998). This finding is supported by natural variation in overnight temperature in nestboxes (O’Conner 1978, Dhondt & Eyckerman 1979) but not by experimentally altered night temperatures (Nager & van Noordwijk 1992). This effect could either be due to reduced energetic expenses for the females, which can then be used for egg production (but see Meijer et al. 1998). Alternatively, the increase in temperature is a reliable predictor for the appearance of insects, that can serve as food for the female during the egg laying and incubation periods.
Food abundance at the time of egg production
As always with correlative relationships, it is well possible that spring temperatures not directly affect laying date but that the causal pathway is via a third variable. A possible candidate is food abundance at the time of egg reproduction, which may affect the condition of the parents or may be a good predictor for the time of abundant food for nestlings later. An example of the latter is the Piñon Jay in which reproduction is triggered by green cones of the Piñon pine (Ligon 1974; Ligon 1978).
Possible evidence for a role of food abundance as a cue comes from food provisioning experiments, which often show that laying date is advanced by providing extra food (Daan et al. 1989; see Svensson 1995 and Nager et al. 1997 for reviews). Nilsson (1994) provided food (live mealworms) to Blue Tits until they had laid their second egg. The experimental animals laid eggs earlier than the non-fed controls, had a longer interval between the last egg and the onset of incubation but still hatched their chicks earlier. Total brood loss, which occurred at the time the chicks were between 5 and 10 days old, was found more frequent in the experimental group. Also, the fed females had a lower survival to the next season. The explanation favoured by Nilsson (1994) is that the birds use food abundance as a cue, and that providing food has tricked them into laying their eggs too early with respect to the peak in food availability for the offspring. It is well possible that encountering live immature insects, like mealworms or caterpillars, acts as an information cue, indicating that the peak in food abundance is near. An alternative explanation, also given by Nilsson (1994), is that because the food provisioning was stopped after the second egg, females were exhausted from egg laying and incubation at the time of chick rearing. This would then have caused the brood loss, not a mismatch between food abundance and offspring demands. The difficulty with food supplementation experiments is that it affects the optimal laying date (as Nilsson’s experiment shows) because it reduces the costs of producing eggs early, i.e. the optimal laying date shifts towards the most productive laying date. This effect masks any potential effect food has as a cue.
Tree phenology
Another candidate for a causal pathway through which temperature can play a role is the development of the vegetation; especially bud burst of deciduous trees. Slagsvold (1976) demonstrated that the laying date of Great Tits in Norway is closely correlated with the bud burst of birch trees. In the Netherlands, the mean laying date of the Hoge Veluwe Great Tit populations correlates well with the mean date of Oak bud burst (Visser unpubl.). This correlation is better than the correlation with the mean temperature between the first of March and the 15th of April (the best correlating period with laying date for this population; van Balen 1973).
A possible causal pathway by which bud burst plays a role is via a chemical substance 6-MBOA. This chemical is formed from its precursor, DIMBOA, when predators damage plants. DIMBOA is particularly abundant in vegetatively growing seedlings. In both field en laboratory experiments it has been shown that 6-MBOA is a strong reproductive stimulant for Montane Voles Microtus mantanus ( Berger et al. 1981; Berger et al. 1987). Although not yet shown, it is well possible that also buds of trees contain substantial amounts of DIMBOA. As Blue and, to a lesser extent, Great Tits eat buds in early spring (Betts 1955), they can thus potentially come in contact with 6-MBOA which then may affect the reproductive system.
AVIARY EXPERIMENTS
Variation in laying date can either be due to a single response mechanism being affected by variation in internal or external cues (phenotypic plasticity) or due to variation in the response mechanism (genetic differences). The former can explain variation in laying date between years and, if areas provide different cues, between areas. If cues are very local, it is even possible that territories differ in their cues and that phenotypic plasticity can explain within population variation in laying date (Nager & van Noordwijk 1995). Variation in response mechanism can account for differences in areas, which differ in such a way that similar cues are used as predictors for different times of food abundance. If there is a mixture of response mechanisms within populations, variation in response mechanisms could also explain within population variation in timing of reproduction (van Noordwijk et al. 1981). This is not an unlikely scenario since response mechanisms will not be flexible enough to lead to complete phenotypic plasticity. There may therefore be a set of response mechanisms within a population that do equally well when evaluated over a large number of years, but lead to different laying dates in most of the years.
For experiments on phenotypic plasticity a commonly used experimental set up is to keep pairs that are as similar as possible with respect to area of origin, age, health etc. under two conditions that differ in only one respect: the cue that is tested. Differences in laying date between these groups must be due to this cue. In experiments on variation in response mechanism individuals with possible different mechanisms (for instance from different areas) are kept under identical conditions. Any difference in laying date between these groups is due to genetic differences in the response mechanism.
Testing phenotypic plasticity
The first step in experiments that test phenotypic plasticity is the choice of the cue that is tested. Results from correlative studies can be used, in combination with considerations whether the cue is a good predictor for favourable conditions and whether there is evidence for a causal pathway.
A potential important cue for Blue and Great Tits is the time of bud burst of Oak trees (Blondel et al. 1993). In the Great Tit population at the Hoge Veluwe (The Netherlands), the average laying date is correlated with the date of bud burst of Oak trees (Visser, unpubl.). Caterpillars on Oak, like the Winter Moth Operophtera brumata, form the main source of food for the nestlings. These caterpillars can only start their development just after bud burst, as earlier there is no food available and later in the season leaf quality rapidly declines (Feeney 1970; Holliday 1985). Caterpillar development is temperature dependent, and for average spring temperatures it takes about 40 days until pupation (Topp & Kirsten, 1991), the time at which they are no longer available to the birds. This period more or less matches the time needed for the birds from the onset of rapid yolk formation to 10-day-old chicks. Thus, bud burst is a reliable cue for the time of maximum food abundance, although temperatures after laying can advance or delay caterpillar development, resulting in a mismatch between nestlings’ requirements and food abundance (van Noordwijk et al. 1995).
The bud burst hypothesis was tested by keeping 16 pairs of Great Tits in closed aviaries under artificial light (Visser unpubl). Photoperiod was increased following the natural increase in day length. Wild caught birds were introduced in the aviaries at the end of January (photoperiod of less than 9 h), well before the critical photoperiod for the start of the rapid gonadal growth (between 11 and 12 h., Silverin et al. 1993). When the photoperiod reached 14.25 h large branches of Oak trees were introduced in the aviaries. The two treatments only differed in the stage of the buds of the branches, ranging from zero (winter rest) to three (fully unfolded leaves; Merle & Mazet 1983). Branches were replaced weekly, and bud burst advanced over time. For as long as possible a difference in development of buds was preserved. In most cases, birds stripped the branches of all their buds within a few days. Only fully closed buds and the fully unfolded leaves were less attractive. Laying started in both years and for both treatments about a week after the first introduction of the branches (Fig. 2). There was no significant difference in laying date between the two treatments. Thus, from these data we have to conclude that birds do not respond differently to different stages of bud burst. The data suggest that the presence of branches, independent of the state of their buds, do stimulate birds to lay but additional experiments are needed to test this.
Another cue for which correlative evidence exists is a direct effect of temperature on the start of egg production (Kluyver 1951; Seel 1966; Perrins & McCleery 1989). This cue may not be a predictor for the time of food abundance for the offspring (for which probably the average temperature over a longer period before laying is more important) but it may predict an almost instantaneous increase in resources that females can use for egg production (Ojanen et al. 1981; Meijer et al. 1998). To test whether temperature may act as a cue, Meijer et al. (1998) kept Starlings Sturnus vulgaris in three groups of four males and seven females in climate chambers with artificial light, following the natural increase in photoperiod. All groups were kept at a day temperature of 9°C and a night temperature of 5°C, and these temperatures were raised with 5°C after three, four or five weeks. In all three groups the median laying date was 7-8 days after the increase in temperature, and thus the absolute date of laying clearly differed between groups. These results show that temperature has a direct effect on egg production.
An increase in food availability can also affect egg production. A group of Starlings kept at 80% of the amount of food eaten by another group of Starlings laid significantly later than this ad libitum fed group. Most females in the food rationed group only started laying after the amount of food was increased to 90% of the food eaten by the ad libitum group (Meijer & Langer 1995). The most likely explanation for the effect of food availability is not that this increase predicts the time of food abundance for the offspring, but that it lifts the resource constraint on egg production. Food availability just prior to laying is therefore rather a resource than information to the birds (but see the section ‘Field studies’).
Testing variation in response mechanism
There is clear geographical variation in the date at which the availability of food for offspring peaks. This variation may be due to differences in latitude, altitude but also in vegetation composition. As a consequence, there is geographical variation in optimal laying dates. Provided that the populations are to some extent isolated, it can therefore be expected that there is also spatial variation in the response mechanism within species. Aviary experiments can be used to show that populations differ in their response mechanism.
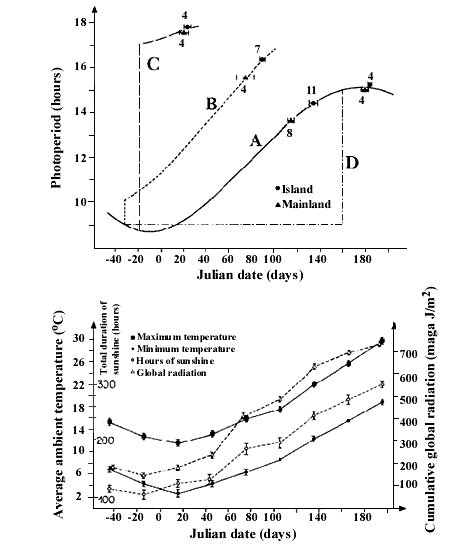
Blue Tits in the Mediterranean region are exposed to large variation in the peak date of caterpillar availability, the primary food source for their offspring. There is a consistent 3-5 week difference in peak caterpillar availability between broad-leaved deciduous oak and evergreen woodlands. In southern France, the deciduous woodland is the preferred breeding habitat for Blue Tits while on Corsica evergreen woodland is dominant. After controlling for altitude, latitude and habitat type, Blue Tits on Corsica Parus caeruleus ogliastrae lay on average three weeks later than the Blue Tits in southern France P. c. caeruleus (Blondel et al. 1993). Birds from the two study areas were housed in pairs in outdoor aviaries and the date of the first egg was recorded. Under these identical conditions the difference in laying date between the two populations was preserved, showing that there is a genetic difference in the response mechanism (Blondel et al. 1990; Lambrechts & Dias 1993). Subsequently, the exact nature of this difference was disentangled in experiments varying the photoperiod. Birds were either given a long day from mid-December onwards (treatment C) or where given an extra hour of day length from the end of November, after which the photoperiod was increased with 15 minutes a week (treatment B). There was no difference in timing of reproduction between the two populations when photoperiod was increased in a single step, but the differences remained when an additional hour of day length was give on top of the normal increase in day length (Fig. 3). These experiments therefore showed that the difference in laying date in the aviaries is due to differences in the responsiveness to photoperiod, and not to differences in gonadal growth rates (Lambrechts et al. 1996). In yet another light treatment birds were kept under short day lengths until the beginning of June, after which they were introduced in the outdoor aviaries. Again, there was no difference in the laying dates of the two populations, although the temperatures before and during laying were much higher than in the treatment where photoperiod was increased in mid-December (Fig. 3). This shows that the two populations do not differ in their response to temperature (Lambrechts et al. 1997a). These experiments show that there are differences in the response mechanism between the two populations, and the only way they differ seems to be the responsiveness to photoperiod. The differences are adaptive as the two populations are exposed to consistent, and therefore highly predictable, year to year differences in the peak date of caterpillar abundance between broad-leaved deciduous and evergreen forests.
On Corsica evergreen woodland is dominant but in some valleys Blue Tits breeding in deciduous woodland are found. The distance between the evergreen population and the deciduous woodland population is small (about 25 km) but there are clear differences in the timing of reproduction. Both populations are well synchronised with the local variation in caterpillar abundance, with the deciduous woodland population laying about four weeks earlier than the evergreen population (Lambrechts et al. 1997b). The intriguing question that Lambrechts and co-workers are currently testing is whether there are also differences in response mechanism between these populations that are so close to one another. Birds from both populations are kept in pairs in aviaries in southern France and their laying dates are recorded. In a 5 year aviary study, the individuals from the deciduous woodland population laid about two weeks later than the individuals from the evergreen population (Lambrechts et al. unpubl.), while in the field they lay about 4 weeks earlier (Lambrechts et al. 1997b). This indicates that the two Corsican populations differ in their response mechanism, but that the conditions before laying in the field differ from the aviary conditions such that the deciduous woodland population lays earlier under field conditions. The specific cues used by these birds are yet unknown but aviary experiments are under way to find them (Lambrechts et al. unpubl.).
DISCUSSION
Timing of reproduction in temperate-zone birds is affected by a whole range of cues (Wingfield et al. 1992). Different cues affect different stages of gonadal development. Photoperiod and an internal clock are the main initial predictive factors that open the reproductive window. After the start of the rapid gonadal growth fine tuning factors come in play, of which temperature and the phenology of vegetation may be important. For the final stage, the rapid yolk formation, again other factors, like food availability, play a role. Also the ability of the birds to control reproductive events via behavioural responses to cues increases. For instance, we can imagine that birds cannot control the triggering of reproduction by long photoperiods, or cannot control the speed of gonad development in response to ambient temperature, but that the bird has the capacity to decide whether it invests in reproduction, or not. This means that the study of proximate mechanisms become more complex when later stages of the reproductive cycle are studied.
Advantages and pitfalls of aviary experiments
We have advocated aviary experiments where birds are kept in pairs or social groups (depending on their breeding system) with nesting opportunity. Only under these conditions we can modify each of the cues that play a role in timing of reproduction. However, if research is directed at the initial predictive factors, it may not be needed to keep birds with mates and nesting material assuming that these factors only affect the last stages of egg production. Having said that, we want to point out that there are interactions between variables like for instance temperature and photoperiod (Engels & Jenner 1956; Silverin 1995), but maybe also between temperature or photoperiod and the presence of a mate or a nest (El Halawani et al. 1984; Silverin & Westin 1995; Maney et al. this volume) on gonadal development.
One of the lessons we have learned from the experiments on differences in the response mechanism is that it is important not to just compare local populations but to chose populations for which the selection pressures are different, as for instance the evergreen and deciduous woodlands in the Blue Tit study (Lambrechts and co-workers). For the choice of which cue to test in experiments on phenotypic plasticity it is important to use correlative field data and to have knowledge of selection pressures in the field as this is essential to asses the predictive value of cues. Thus, both types of aviary experiments should be carried out in combination with field studies.
There are many potential problems with aviary experiments, especially if the goal is to observe actual laying dates. If wild caught animals are used, stress may hamper the gonadal development and consequently laying. A possible solution is to breed animals and use birds born in captivity. This is however only possible if offspring of a reasonable quality can be raised in captivity. Another way to reduce stress is to use large aviaries. This however makes them more expensive to build, especially if they are temperature and photoperiod controlled. More expensive cages will usually mean fewer cages, and another disadvantage of aviary experiments is that only one data point per year is obtained from an aviary. A possible solution is to keep a second group of animals under short photoperiod (shorter than the critical value for the start of rapid gonadal growth) during the breeding season. Birds can then be placed in the aviaries after the first group has laid, either at the prevailing photoperiod (Lambrechts et al. 1997a) or, if photoperiod is controlled, at day lengths corresponding with early spring (Visser unpubl.). One needs to be careful when comparing laying dates between the two groups as the internal clock will differ between the groups, even if all other cues are kept the same.
Another common problem with experiments under controlled conditions is how to treat the pairs that do not lay in the statistical analysis. One can analyse the effect of the treatment on the proportion of pairs that do not lay but usually samples sizes are too small to draw conclusions. One way to avoid this problem is to let photoperiod increase (or improve conditions in any other way) over time so that all birds will lay during the experiment. Testing two distributions of laying dates is statistically preferred over testing zero-one data (did or did not lay). If birds have not laid under the conditions of the experiment it is important to assess whether or not they can lay. This can be done by improving conditions (see for instance Meijer & Langer 1995; Meijer et al. 1998). If birds start laying after this alteration one can strictly no longer include them in the comparison of treatments by using their laying date but they can be included as censored data points (Haccou & Meelis 1992).
The treatment in phenotypic plasticity experiments will generally be the presence or absence of a cue or the value of a cue (i.e. temperature). It may be important to distinguish between treatments that animals have to undergo (photoperiod, temperature, etc.) and treatments in which animals have a choice, like presenting them with different types of food. An additional problem with food is that it can serve as a cue but is also as a resource. The availability of resources affects female condition, and therefore also influences laying date. To minimise variation within treatments it is preferred to have ad libitum food in aviary experiment aiming at identification of information cues, thus separating resource and information constraints. But obviously experiments altering both types of constraints will be needed to enable the translation from laboratory experiments to field conditions. When studying the role of resources as a potential cue it is difficult to have a proper control group that gets the same amount of energy and nutrients but in a form that holds no information. In experiments with Great Tits, Visser (unpubl.) therefore used fly pupae as a source of proteins as there is no clear increase in fly pupae abundance during spring.
Selection on response mechanisms
Tinbergen (1963) formulated four questions about animal behaviour, among which the questions on the causation and on the adaptive value, in terms of reproduction and survival, of behaviour. Studies on the response mechanism clearly address the former of these two but we want to argue that knowledge of the response mechanism is also essential for the question on the adaptive value of behaviour. Natural selection will act on this response mechanism, rather than on the single decisions in any particular year or area. It is well possible that in some years or areas birds lay too early or too late because the response mechanism may not be completely plastic (Visser et al. 1998). But there need not be overall selection against these genotypes as the underlying response mechanism leads to accurate timing decisions in many other years or areas.
The notion that response mechanisms do not lead to complete phenotypic plasticity and that there will be therefore selection on these mechanisms is also important when individuals are faced with a new or changing environment. In France, habitats have been heavily modified by humans, for instance by evergreen oak plantations. In these habitats Blue Tits lay their eggs too early in relation to the optimal time of breeding. The birds in the less productive evergreen oak woods lay at the same time as in the deciduous woodlands because gene flow from the latter to the former prevents them from evolving a more suitable photoperiodic response that would enable them to lay at the best time to raise their young (Lambrechts et al. 1997a).
In the past decades the phenology of the vegetation has advanced due to higher spring temperatures (Myneni et al. 1997). It has been shown that many bird species in the UK have advanced their date of egg laying over the last 25 years (Crick et al. 1997). This pattern is confirmed by some long-term studies of a few bird populations (Winkel & Hudde 1997; McCleery & Perrins 1998) but not in all. No advancement of laying date was found for a Great Tit population in the Netherlands, despite the advancement of the peak date of caterpillar abundance. As a consequence, selection for early laying has increased over the 23 year period (Visser et al. 1998). One of the two explanations they give is that the increase in spring temperature affects the food peak but not the cues used by the birds. This is because the increase in temperature is not significant between the first of March and the 15th of April, but has increased in the subsequent 30 days, thereby affecting the developmental rate of the caterpillars. The response mechanism the birds use has evolved in an environment where there is a certain relationship between the temperatures before laying (affecting the cues) and after laying (affecting the change in food availability over time). When this relationship between food availability and the cues used for timing of breeding has changed, there will be selection on the reaction norm relating these two variables. However, the response to such selection may be slow (van Tienderen & Koelewijn 1994).
When assessing the adaptive value of a response mechanism it is necessary to evaluate the mechanisms over many years and the different types of landscapes within dispersal distance, as any particular mechanism is expected to result in maladaptive decisions in particular years or landscapes. These years or areas with a poor synchrony with the food peak may however be compensated with other years or areas where the same mechanism leads to accurate laying dates. It is therefore crucial to have knowledge on the year to year or area to area variation in selection pressures. It is therefore important not only to follow populations of the bird species studied but also, as reproduction is ultimately timed with respect to food abundance, of their main prey species. The final test to determine whether all selection pressures and constraints on the response mechanism are known is to compare the calculated long term fitness of individuals using the mechanism observed against the calculated fitness of any other mechanism, for a realistic environment.
Final conclusions
Understanding adaptation in the time of egg laying requires besides the identification on the main selection pressures also the studies on the proximate factors. To predict how and how fast laying dates adapt to habitat heterogeneity and change we must understand the proximate basis of reaction norms of laying dates. Reaction norms of laying dates are proximately determined by responses to specific cues and by processes prior to egg laying, including gonad and egg development. These different processes of development in response to specific cues can only be identified and quantified using a multidisciplinary approach in which different specialists (ecologists, physiologists, etc.) collaborate to solve a single evolutionary problem.
Studies limited to long term ecological studies in single local study plots are often not enough for a full understanding of adaptive variations in life history decisions, such as laying dates. First, we assume that selection acts on reaction norms that have been selected in environments with between-year and between-area variations, both within and across generations (e.g. parent vs. offspring after natal dispersal). Second, factors determining between-population variation may differ from those that determine within-population variation. Working at different temporal and spatial scales is therefore essential.
The best way to identify response mechanisms and cues is to combine long term field studies that generate realistic hypotheses with laboratory studies. Laboratory studies of laying dates may be complicated because of practical or financial reasons. However, these laboratory tests are often the only way to examine hypotheses that cannot be tested in the field, and should therefore be encouraged.
ACKNOWLEDGEMENTS
We like to thank Theo Meijer for discussion and the opportunity to cite an unpublished manuscript, and Theo Meijer and Christiaan Both for comments on a previous version of this paper.
REFERENCES
Balen, J.H. van 1973. A comparative study of the breeding ecology of the Great Tit Parus major in different habitats. Ardea 61: 1-93.
Berger, P.J., Negus, N.C., Sanders, E.H., Gardner, P.D. 1981. Chemical triggering of reproduction in Microtus montanus. Science 214: 69-70.
Berger, P.J., Negus, N.C., Rowsemitt, C.N. 1987. Effect of 6-methoxybenzoxazolinone on sex ratio and breeding performance in Microtus montanus. Biology of Reproduction 36: 255-260.
Betts, M.M. 1955. The food of titmice in oak woodland. Journal of Animal Ecology 24: 282-323.
Blondel, J.,Perret, P. & Maistre, M. 1990. On the genetical basis of the laying date in an island population of blue tits. Journal of Evolutionary Biology 3: 469-475.
Blondel, J., Dias, P.C., Maistre, M. & Perret, Ph. 1993. Habitat heterogeneity and life-history variation of Mediterranean Blue Tits. Auk 110: 511-520.
Crick, H.Q.P., Dudley, C. & Glue, D.E. 1997. Long-term trends towards earlier egg-laying by UK birds. Nature 388: 526.
Daan, S., Dijkstra, C., Drent, R. & Meijer, T. 1989. Food supply and the annual timing of avian reproduction. In: H. Ouellet (ed.) Proceedings of the XIX International Ornithological Congress, University of Ottawa Press, Ottawa: 392-407.
Dhondt, A.A. 1970. De regulatie der aantallen in Gentse koolmeespopulaties (Parus m. major L.). PhD Thesis, University of Gent, Gent, Belgium.
Dhondt, A.A. & Eyckerman, R. 1979. Temperature and date of laying by Tits Parus spp. Ibis 121: 329-331.
El Halawani, M.E., Silsby, J.L., Behnke, E.J. & Fehrer, S.C. 1984. Effect of ambient temperature on serum prolactine and luteinizing hormone levels during the reproductive life cycle of the female turkey Meleagris gallopavo. Biology of Reproduction 30: 809-815.
Engels, W.L. & Jenner, C.E. 1956. The effect of temperature on testicular recrudescence in Juncos at different photoperiods. Biological Bulletin 110: 129-137.
Farner, D.S. & Mewaldt, L.R. 1952. The relative roles of photoperiod and temperature in gonadal recrudescence in male Zonotrichia leucophrys gambelii. Anatomical Record 113: 612-613.
Feeny, P. 1970. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51: 565-581.
Gwinner, E. 1996. Circannual clocks in avian reproduction and migration. Ibis 138: 47-63.
Haccou P. & Meelis, E. 1992. Statistical analysis of behavioural data. Oxford, Oxford University Press: 396 pp.
Holliday, N.J. 1985. Maintenance of the phenology of the winter moth (Lepidoptera: Geometridae). Biological Journal of the Linnean Society 25: 221-234.
Kluyver, H.N. 1951. The population ecology of the Great Tit, Parus m. major L. Ardea 39: 1-135.
Lambrechts, M.M. & Dias, P.C. 1993. Differences in the onset of laying between island and mainland Mediterranean Blue Tits Parus cearuleus: phenotypic plasticity or genetic differences? Ibis 135: 451-455.
Lambrechts, M.M., Perret, P. & Blondel, J. 1996. Adaptive differences in the timing of egg laying between different populations of birds result from variation in photoresponsiveness. Proceedings of the Royal Society London series B 263: 19-22.
Lambrechts, M.M., Blondel, J., Maistre, M. & Perret, P. 1997a. A single response mechanism is responsible for evolutionary adaptive variation is a bird's laying date. Proceedings of the National Academy of Sciences USA 94: 5153-5155.
Lambrechts, M.M., Blondel, J., Hurtrez-Boussès, S., Maistre, M. & Perret, P. 1997b. Adaptive inter-population differences in blue tit life-history traits on Corsica. Evolutionary Ecology 11: 599-612.
Ligon, J.D. 1974. Green cones of the Piñon pine stimulates late summer breeding in the Piñon Jay. Nature 250: 80-82.
Ligon, J.D. 1978. Reproductive interdependence of Piñon Jays and Piñon pines. Ecological Monographs 48: 111-126.
McCleery, R.H. & Perrins, C.M. 1998. … temperature and egg-laying trends. Nature 391: 30-31.
Merle, P. du & Mazet, R. 1983. Stades phenologiques et infestation par Tortrix viridana L. (Lep., Tortricidae) des bourgeons du chêne pubescent et du chene vert. Acta Oecologica 4: 47-53.
Meijer, T. 1991. The effect of a period of food restriction on gonad size and moult of male and female Starlings Sturnus vulgaris under constant photoperiod. Ibis 133: 80-84.
Meijer, T. & Langer, U. 1995. Food availability and egg-laying of captive European Starlings. The Condor 97: 718-728.
Meijer, T., Nienaber, U., Langer, U & Trillmich, F. 1998. Temperature and timing of egg-laying of European Starlings. The Condor, in press.
Murton, R.K. & Westwood, N.J. 1977 Avian breeding cycles. London; Oxford University Press: 594 pp.
Myneni, R.B., Keeling, C.D., Tucker, C.J., Asrar, G. & Nemani, R.R. 1997. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386: 698-702.
Nager, R.G. & Noordwijk, A.J. van 1992. Energetic limitation in the egg-laying period of Great Tits. Proceedings of the Royal Society London series B 249: 259-263.
Nager, R.G. & Noordwijk, A.J. van 1995. Proximate and ultimate aspects of phenotypic plasticity in timing of Great Tit breeding in a heterogeneous environment. American Naturalist 146: 454-474.
Nager, R.G., Rüegger, C. & Noordwijk, A.J. van 1997. Nutrient or energy limitation on egg formation: a feeding experiment in Great Tits. Journal of Animal Ecology 66: 493-507.
Nilsson, J.-Å. 1994. Energetic bottle-necks during breeding and the reproductive cost of being too early. Journal of Animal Ecology 63: 200-208.
Noordwijk, A.J. van, Balen, J.H. van & Scharloo, W. 1981. Genetic variation in the timing of reproduction in the Great Tit. Oecologia 49: 158-166.
Noordwijk, A.J. van, McCleery, R.H. & Perrins, C.M. 1995. Selection of timing of Great Tit Parus major breeding in relation to caterpillar growth and temperature. Journal of Animal Ecology 64: 451-458.
O'Connor, R.J. 1978. Nest-box insulation and the timing of laying in the Wytham woods population of Great Tits Parus major. Ibis 120: 534-537.
Ojanen, M., Orell, M. & Väisänen, R.A. 1981. Egg size variation within passerine clutches: effects of ambient temperature and laying sequence. Ornis Fennica 58: 93-108.
Perrins, C.M. 1970. The timing of birds' breeding season. Ibis 112: 242-255.
Perrins, C.M. & McCleery, R.H. 1989. Laying dates and clutch size in the Great Tit. Wilson Bulletin 101: 236-253.
Seel, D.C. 1966. Breeding seasons of the House Sparrow and the Tree Sparrow Passer spp. at Oxford. Ibis 110: 129-144.
Silverin, B. 1978. Circannual rhythms in gonads and endocrine organs of the Great Tit Parus major in south-west Sweden. Ornis Scandinavica 9: 207-213.
Silverin, B. 1995. Reproductive adaptations to breeding in the north. American Zoologist 35: 191-202.
Silverin, B. , Massa, R. & Stokkan, K.A. 1993. Photoperiodic adaptation to breeding at different latitudes in Great Tits. General and Comparative Endocrinology 90: 14-22.
Silverin, B.,Veibke, P.A. & Westin, J. 1989. An artificial simulation of the vernal increase in day length and its effects on the reproductive system in three species of tits (Parus spp.), and modifying effects of environmental factors - a field experiment. The Condor 91: 598-608.
Silverin, B. & Westin, J. 1995. Influence of the opposite sex on photoperiodically induced LH and gonadal cycles in the Willow Tit (Parus montanus). Hormones and Behavior 29: 207-215.
Suomalainen, H. 1937. The effect of temperature on the sexual activity of non-migratory birds, stimulated by artificial lighting. Ornis Fennica 14: 108-112.
Svensson, E. 1995. Avian reproductive timing: when should parents be prudent? Animal Behaviour 49: 1569-1575.
Tienderen, P.H. van & Koelewijn, H.P. 1994. Selection on reaction norms, genetic correlations and constraints. Genetic Research, Cambridge 64: 115-125.
Tinbergen, N. 1963. On aims and methods of ethology. Zeitschrift fur Tierpsychologie 20: 410-433.
Topp, W. & Kirsten, K. 1991. Synchronisation of pre-imaginal development and reproductive success in the Winter Moth, Operophtera brumata L. Journal of Applied Entomology 111: 137-146.
Visser, M.E., Noordwijk, A.J. van, Tinbergen, J.M. & Lessells, C.M. 1998. Warmer springs lead to mis-timed reproduction in Great Tits (Parus major). Proceedings of the Royal Society London series B: 265: 1867-1870.
Wingfield, J.C. 1993. Control of testicular cycles in the Song Sparrow, Melospiza melodia melodia: interaction of photoperiod and an endogenous program? General and Comparative. Endocrinology 92: 388-401.
Wingfield, J.C., Hahn, T.P., Levin, R. & Honey, P. 1992. Environmental predictability and control of gonadal cycles in birds. Journal of Experimental Zoology 261: 214-231.
Wingfield, J.C., Hahn, T.P. & Doak, D. 1993. Integration of environmental factors regulating transitions of physiological state, morphology and behaviour. Journal of Endocrinology XX: 111-122.
Winkel, W. & Hudde, H. 1997. Long-term trends in reproductive traits of Tits (Parus major, P. caeruleus) and Pied Flycatchers Ficedula hypoleuca. Journal of Avian Biology 28: 187-190.
Fig. 1. A conceptual diagram of gonadal development in female Great and Blue Tits. Slow gonadal growth starts in December and is likely due to an endogenous factor (Silverin et al., 1993) for which photoperiod acts as a Zeitgeber. The onset of the rapid gonadal growth is related to photoperiod (Silverin et al. 1993). Under normal temperatures it takes 6 to 8 weeks to reach the maximal gonadal size in the Great Tit (Silverin 1978) but the growth rate of the gonads depend on a number of fine tuning factors. These factors may include temperature, tree phenology and nesting material, possibly in interaction with photoperiod. The final phase is the rapid yolk formation (sensu Meijer 1991) which takes 4 days in Great Tit (Kluyver 1951). Food availability, temperature, nesting material and the male may affect the onset of this phase. At present it is unclear whether a pause can occur between the time of maximal gonadal size and the rapid yolk formation, maybe as a result of low resource availability at the time maximal gonadal size is reached.

Fig. 2. Laying dates of wild caught Great Tits kept in pairs in aviaries under artificial light (Visser, unpubl.). Birds were introduced in the aviaries before the onset of the rapid gonadal growth and photoperiod was increased following the natural pattern (every 2 tic marks on the x-axis is a week; outside a photoperiod of 14 hours is reached on the 13th of April, 15 hours on the 1st of May, 16 hours on the 22nd of May). Branches of Oak trees were introduced (Ñ ) differing in the stage of bud burst (0 = winter rest, 3 = fully unfolded leaves; Merle & Mazet 1983). Laying dates of birds in an ‘early spring environment’ (E) are indicated with a l (1997: n=7, 1998: n=7) those in a ‘late spring environment’ (L) with a n (1997: n=4, 1998: n=5).
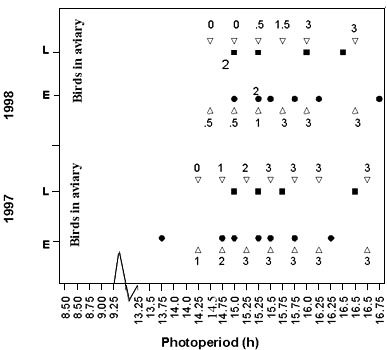
Fig. 3. Onset of first clutches (average ± SE in Julian dates; January 1 =1, February 1 = 32) of captive mainland and island Blue Tits of the Mediterranean region breeding in outdoor aviaries at Montpellier, Southern France presented with four different changes in photoperiod (upper) and the monthly change in local climate (average ± SE, 1985 – 1995) to which the birds were exposed (lower). Taken from Lambrechts et al. 1997a.