
S05.1 Fitness consequences of timing of reproduction
Jan-Åke Nilsson
Department of Animal Ecology, Lund University S-223 62 Lund, Sweden, fax 46 46 2224716, e-mail jan-ake.nilsson@zooekol.lu.se
Nilsson, J.-Å. 1999. Fitness consequences of timing of reproduction. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
234-247. Johannesburg: BirdLife South Africa.A general feature of birds reproducing in seasonal environments is a steady seasonal decline in reproductive success. The suggested causal mechanisms of this seasonal decline can be grouped under three main hypotheses; quality of the breeding situation, timing and chick quality. Seasonal variations in reproductive success are explained by parental and territory quality according to the ’quality of breeding situation’ hypothesis, by a seasonal decline in food availability or an increased competition between juveniles according to the timing hypothesis and by genetic and maternal factors according to the chick quality hypothesis. Experiments aiming at separating effects of these mechanisms on offspring survival, have generally resulted in manipulated delays in fledging date having negative effects irrespective of parental condition, especially when post-fledging survival has been analysed. This might be due to an over-representation of sedentary, non-migratory bird species in such studies. Sedentary species often have a dominance-structured social system during the non-breeding season. In such systems, dominance status is often determined by prior occupancy, favouring establishment success and survival of early-hatched young. However, ultimately it is the quality of parents and/or their territory that determine reproductive success since only parents in a high-quality breeding situation can start breed early. Manipulations that have either advanced or delayed the start of egg laying have produced results showing reduced parental survival as well as future reproductive success. This implies an individual optimisation process among breeding birds in relation to laying date. Thus, the decision of when to start breeding might be better viewed as a trade-off between current and future reproduction than as an absolute constraint on breeding time.
INTRODUCTION
The timing of reproduction is one of the reproductive traits with the largest impact on parental reproductive success and offspring survival (e.g. Clutton-Brock 1988). Although, components of fitness, e.g. clutch size, hatching success, growth rate, fledging success and recruitment rate differ, a general feature of birds reproducing in seasonal environments is a steady seasonal decline in reproductive success (e.g. Nilsson 1989a; Perrins & McCleery 1989; Daan et al. 1990; Hochachka 1990; Svensson 1997). In many populations, the average hatching date in the population occurs later than the optimal hatching date for the production of surviving young (Perrins 1970; Norris 1993; Svensson 1997). In this review, I will first outline the mechanisms that have been suggested as explanations for the seasonal decline in reproductive success. By using published manipulations of the temporal pattern of breeding, I will evaluate these explanations and suggest some generalisations from the experimental results. Furthermore, I will restrict myself to within-population mechanisms, thus ignoring the large body of data on effects of gene flow between habitats (e.g. Lambrechts et al. 1997).
MECHANISMS
Several mechanisms with the potential to explain the existence of a seasonally decreasing reproductive success have been suggested (Table 1). These mechanisms can be grouped under three general families of hypotheses, i.e. quality of the breeding situation (breeding quality), timing and chick quality hypotheses.
Breeding quality hypotheses
Parental quality
The quality of the breeding situation, i.e. the quality of the parents and/or the area where they breed, may cause the seasonal decline in reproductive success at the population level. Parental quality can potentially determine both timing of breeding and reproductive success (Price et al. 1988). According to this hypothesis, parents in good condition can start breeding early in the season and can also afford to invest much in reproductive effort, whereas parents of low quality reproduce late and invest less in reproductive effort. The result of this will be a decline in reproductive success with season in the population. Individual properties with the potential to explain a seasonal decline in reproductive success include e.g. age or breeding experience (Perrins & McCleery 1985; Murphy 1986; Hochachka 1990; De Forest & Gaston 1996), foraging success as evidenced by the level of glycosylated haemoglobin (Andersson & Gustafsson 1995) and prevalence of blood parasites (Korpimäki et al. 1993; Allander & Bennett 1995).
Territory quality
Quality differences in the breeding situation can also be mediated through variation in territory quality. Territories with high-quality foraging areas may permit the female to both start laying early and to attain high reproductive success (Cavé et al. 1989; Hughes et al. 1994). Variation in territory quality is not only related to varying foraging opportunities. The amount of ectoparasites has also been found to affect both laying date and reproductive success (Oppliger et al. 1994). Micro-climate may differ between territories and thereby influence the start of egg laying (Nager & van Noordwijk 1995). Also territory size has been found to cause a covariation between timing and reproductive success (Hunt & Hunt 1976). Parental and territory quality probably covary in many systems, resulting in high quality parents occupying high quality territories. This should be especially prevalent in migratory birds for which the date of arrival on the breeding grounds dictates the quality distribution of available territories. Delays in arrival time can be caused by e.g. infection of blood parasites (Rätti et al. 1993) or inexperience as evidenced by an age difference in arrival times (Hasselquist 1998).
Timing hypotheses
Absolute timing
Effects of the start of breeding per se on reproductive success can be explained by several mechanisms (Table 1). Parents may be constrained by a short breeding season, forcing late laying females to reduce clutch size to have time for moult and migration (Hamann & Cooke 1989; Verhulst et al. 1995; Ydenberg et al. 1995). In such cases, absolute calendar date when breeding can start, will be the important factor determining reproductive success. For species with sea-going chicks breeding in the far north, time of formation of sea ice may be an important time constraint leading to reduced reproductive success late in the season (Murphy 1995). A prediction from this hypothesis is that individuals breeding at northern latitudes should have a much stronger relation between timing and reproductive success than individuals of the same species breeding further south. This was actually found for House Wrens Troglodytes aedon breeding in Canada and in Costa Rica (Young 1994). However, the absolute timing hypothesis is not the only one which can account for such observations. If food availability is temporally more concentrated at northern latitudes, timing in relation to prey (see below) would give the same prediction.
Timing in relation to prey
In seasonal environments, the availability of some prey will follow a bell-shaped temporal distribution of short duration (e.g. Gibb 1950; Bryant 1975). Birds using such food types for breeding, must synchronise their breeding with this seasonally fluctuating prey population. Birds that start laying late in the season will experience reduced reproductive success due to declining food availability during the period of chick rearing (Norris 1993; Lepage et al. in press). The seasonal decline in clutch size is, however, hard to explain in proximate terms since laying dates are generally distributed on the upward slope of food availability (Daan et al. 1989). This indicates that late females make a strategic decision based on the number of young that can be adequately fed during the nestling or family flock periods. For late females, these energy demanding periods will be performed during the downward slope of food availability. Breeding birds may also themselves serve as food for other predators. This may potentially influence the timing of breeding if, for example, early breeders tend dependent young before the bulk of predators start feeding their own young.
Timing in relation to conspecifics
Early hatched young are often dominant over late hatched young due to their having a time advantage when getting established in new areas after becoming independent of their parents. This dominance advantage may be caused by a prior occupancy advantage (Tinbergen et al. 1985; Nilsson & Smith 1988; Nilsson 1989a; Nilsson 1989b; Sandell & Smith 1991; Verhulst 1992) or by increased experience of dominance interactions with age (Arcese & Smith 1985). In Marsh Tits Parus palustris, experimental manipulation of the time when juveniles could become established revealed that hatching date per se or some quality factor associated with early hatching was not responsible for the negative relationship between date and establishment success. Instead, the time when the young was allowed to try to get established determined its establishment success (Nilsson 1990). This means that timing of breeding is only important in relation to when conspecifics in the population start breeding. Also traits like clutch size may decline seasonally for the same reason. Females may trade a larger clutch against the offspring´s reduced chances to get established due to the delayed hatching stemming from the production of extra eggs (Norris 1993; Steeger & Ydenberg 1993).
Especially in colonial species, where conspecific adults are a cannibalistic threat to dependent young, timing may also be important in relation to conspecifics. In some cases it is advantageous to hatch early in the population and so avoid the time with the highest number of cannibalistic adults (Hatchwell 1991; Brouwer et al. 1995). In Glaucous-winged Gulls Larus glaucescens, early hatched young escape conspecific predation because potential cannibals are still incubating (Hunt & Hunt 1976). However, in relation to predation by both conspecifics and other species, the main selection may not be for early hatching. Rather, it may be advantageous to hatch in the middle of the season, when most young in the colony hatch (Parsons 1975; Hatchwell 1991). Thus, in this case synchronised hatching is more important than early hatching.
Variation in parental investment
All sub-hypotheses under the general timing hypothesis predict that young produced late during the breeding season will have reduced chances of recruiting into the breeding population. Thus, to the parents, these young are of lower reproductive value than young produced earlier during the breeding season (Daan et al. 1990). Since breeding is connected with costs, parents may not be willing to invest as much parental effort in late as in early young. Thus, parents may strategically reduce present reproductive effort and thus success, to gain an increased survival probability to the next breeding season (Brinkhof et al. 1993; Winkler & Allen 1996). In some cases this hypothesis will be close to the absolute timing hypothesis. It has been suggested that late-breeding Chinstrap Penguins Pygoscelis antarctica, voluntarily reduce parental effort, leading to a reduction in chick mass, and earlier neglect of the chicks. This results from a conflict between feeding chicks and storing body reserves for the postnuptial moult (Moreno et al. 1997). Such conflicts should be most common in environments with very short breeding seasons.
Chick quality hypotheses
Genetic origin
The observed seasonal decline in reproductive success would result if, for example, growth rate is genetically determined (Boag & van Noordwijk 1987) and early-hatched nestlings have a faster growth rate than late-hatched ones (Lepage et al. in press). Sedinger et al. (1997), however, failed to detect any genetic effects that could explain the seasonal variation in growth rate in Black Brant goslings Branta bernicla.
Maternal origin
Seasonally declining growth rate and fledging success might reflect differences in the initial quality between early and late hatchlings, which may be of maternal origin (Brinkhof 1997). The most commonly studied maternal effect is egg size. Although far from the rule, egg size has been shown to decrease with the season in some studies (e.g. Parsons 1975; Brinkhof et al. 1993; Hipfner et al. 1997) and to have an effect on hatchling size and survival of young nestlings (Nilsson & Svensson 1993; Perrins 1996).
EXPERIMENTAL TESTS
Procedure
Many of the suggested causal mechanisms of the hypotheses in Table 1 covary and it is difficult to judge their relative importance from observational studies calling for experiments. Two main types of experiments have been used to test the hypotheses: exchange and delaying experiments. The experimental protocol for these manipulations take advantage of the natural variation in laying dates and the natural seasonal trend of the reproductive trait under study. By exchanging clutches between early- and late-breeding parents or by delaying breeding for a random sample of parents, it is possible to distinguish between some of the different hypotheses in Table 1. If early-breeding parents receive, or are forced to produce, a late clutch, i.e. one hatching later than their original clutch would have done, the breeding quality hypotheses predict that their reproductive success will be as good as that of unmanipulated early-breeding parents (Fig. 1). According to the timing hypotheses, however, the reproductive success is predicted to conform to the seasonal trend and attain the same value as for originally late-breeding parents (Fig 1). In exchange experiments, also the effect on reproductive success of late-breeding parents receiving an early-hatching clutch can be studied.
The chick quality hypotheses predict different outcomes depending on which experiment is conducted. In exchange experiments, eggs are swapped and the success of young produced from these eggs should conform to the seasonal trend (as predicted from the timing hypotheses) irrespective of the breeding quality at the new location. However, in delaying experiments, where a female produces a new clutch, the chick quality hypotheses predict reproductive success of the replacement clutch to be equal to the original ones (as predicted from the breeding quality hypotheses) irrespective of date in the season.
The main difference between the two types of manipulations relates to the nature of unwanted side-effects of the manipulation. Females subjected to exchange experiments have to incubate either for a longer or a shorter period than naturally, whereas delayed females have to produce a new clutch of eggs.
Results
The results of experimental manipulations that I found in a survey of the literature are shown in Table 2 which also presents the explanation favoured by the authors. If more than one explanation was suggested, I have ranked them according to the authors if this was done in the original paper. Although the proposed mechanisms vary considerably, some general trends can be discerned.
In colonial species that suffer from heavy predation during the pre-fledging period, either synchronous breeding or an early timing of breeding seems to be advantageous. Since the predators in these cases often are conspecifics, escape from cannibalism is possible for those parents that lay early in relation to conspecifics and therefore have vulnerable young at a time when other adults are not yet a serious threat (Hunt & Hunt 1976). Several advantages of breeding at high densities have been proposed, e.g. improved defence, increased vigilance and swamping (Hatchwell 1991). In order to gain these advantages, parents have to synchronize their start of breeding. Thus, according to this explanation, there is a peak in reproductive success in the middle of the breeding period, with early and late breeders suffering a higher predation rate.
The various aspects of fitness used in the studies of Table 2 can be grouped into pre-fledging traits, e.g. clutch size, hatching success, growth rate and fledging success, and post-fledging traits, e.g. juvenile survival and recruitment. When pre-fledging traits have been evaluated, 73% (eight out of 11) of the analyses have concluded that breeding quality to some extent can explain the observed patterns. For post-fledging traits, only 20% (one out of five) of the analyses ascribed the result to variation in breeding quality; the rest of the cases conform to the timing hypotheses (Table 2). Thus, variation in parental or territory quality seems to have its largest impact on reproductive success during the pre-fledging period. However, even during this period, timing seems to be the most important factor. During the post-fledging period, effects of variation in parental or territory quality seem to have vanished in most cases (see also Hochachka (1990) for a similar conclusion from an observational study on Song Sparrows, Melospiza melodia). In an effort to assess the relative importance of breeding quality and timing for overall fitness, Spear & Nur (1994) working with Western Gulls Larus occidentalis and Verhulst et al. (1995) working with Great Tits Parus major, estimated timing per se to explain 75 and 87%, respectively, of the variation in overall fitness.
One way by which variation in parental or territory quality could have lasting effects on fitness would be through the size of fledglings. However, as indicated by the analysis above, fledgling size seems to be of less importance than time of independence (see also e.g. Nilsson & Smith 1988; Hochachka & Smith 1991; Verboven & Visser 1998). However, a bias in the kind of species studied can potentially be responsible for this result. Most studies of post-fledging survival have been performed on sedentary species. The reason for this is easy to understand, only in such populations is it conceivable to monitor the survival of juveniles after independence from their parents. Sedentary species often have a dominance-structured social system during the non-breeding season, emphasizing the importance of prior occupancy and, thus, timing in relation to conspecifics as a determinant of survival and recruitment. Of the five studies in Table 2 that measured post-fledging success, four were conducted on tits, in agreement with the above argument. It is of some interest that the single one that favoured the breeding quality hypotheses as explanations for the variation in juvenile survival, was conducted on the Coot Fulica atra. The coot is the only one of these species in which prior occupancy can be assumed to have little importance for post-fledging survival (Brinkhof et al. 1997). Furthermore, juvenile survival was found to be determined by fledgling size in the migratory Collared Flycatcher Ficedula albicollis, whereas it was determined by hatching time in the sedentary Great Tit (Lindén et al. 1992). Thus, in species where prior occupancy is of minor importance, hatching date would be less important and other asymmetries, such as in fledgling size, might have a larger impact on juvenile survival.
The paucity of studies ascribing variation in reproductive success to maternal effects may depend on the same bias in the kind of species studied as discussed above. However, the influence of factors like egg size is considered to be of very short duration (e.g. Schifferli 1973; Ricklefs 1984; Smith et al. 1995). Thus, maternal effects, at least those acting via egg size, will probably only affect pre-fledging traits.
Besides the sedentary – migratory continuum, variation in life expectancy may influence the mechanisms of seasonal decline in reproductive success. Individuals of long-lived species, finding themselves to breed late during a season, may voluntarily restrict their effort in the present reproductive event to increase their chances of early breeding during the next breeding season (Brinkhof et al. 1993; Winkler & Allen 1996; Moreno et al. 1997). Such a trade-off, based on the existence of a cost of reproduction, should be of less importance to short-lived species. Small passerines have also been shown to invest at least as much energy in a replacement brood as in an earlier, first brood (Hails & Bryant 1979), in some cases resulting in a higher reproductive cost of late parents (Nilsson & Svensson 1996). Furthermore, both Collared Flycatchers and Blue Tits Parus caeruleus produce an optimistic replacement clutch size, resulting in more nestlings dying in such broods than in first broods (Wiggins et al. 1994; J.-Å. Nilsson, unpubl.). In short-lived species, a trade-off between current and future reproduction may instead affect the decision whether or not to produce a replacement or second brood. Individuals facing the decision to produce a new clutch late in the season usually refrain from doing so (Brinkhof et al. 1993; Verhulst et al 1995; Verboven & Verhulst 1996; Bauchau & Seinen 1997), probably because the reproductive value of the young produced cannot outweigh the reproductive cost of producing them.
Timing of reproduction as a life-history trade-off
Many studies (including this one) have tried to separate the effects of breeding quality and timing on reproductive success. However, ultimately it is the quality of parents and/or their territory that determines reproductive success, either directly (breeding and chick quality hypotheses) or indirectly because only parents of high quality, breeding on high quality territories can start breeding early with its associated advantages according to the timing hypotheses (Verhulst et al. 1995; Winkler & Allen 1996; Aparicio 1998).
Thus, the start of breeding can be viewed as being under selective pressure for earlier breeding through positive effects of absolute timing, timing in relation to prey or timing in relation to conspecifics. However, only parents of high quality and/or breeding on high-quality territories can produce eggs very early (Perrins 1970) or are able to adequately feed young early during the season (Nilsson 1994). I think that the decision to start breeding should be viewed not as an absolute constraint (sensu Perrins 1970), but instead as a trade-off between present and future reproductive success (Daan et al. 1990). Energy intake or perceived food availability can nonetheless serve as cues for when it should be possible, given parental and territory quality, to perform energy demanding activities such as incubation and feeding of nestlings and fledglings (Svensson & Nilsson 1995). Such a relation of initiation of breeding to parental and territory quality was termed the "individual optimal date hypothesis" by Brinkhof et al. (1993). Some evidence for this view comes from food supplementation experiments in which manipulation of food availability tricked parents to start breeding too early, with detrimental effects on offspring survival (Davies & Lundberg 1985; Clamens & Isenmann 1989; Nilsson 1994). Furthermore, European Kestrels Falco tinnunculus receiving clutches hatching either earlier or later than their original clutch would have done, produced fewer fledglings than control pairs (Aparicio 1998).
Considering the time to start breeding as a trade-off between present and future reproduction suggests survival differences between parents adopting different strategies. Generally no relationship between breeding time and parental survival has been found in unmanipulated situations (Newton & Marquiss 1984; Daan et al. 1990; Verhulst et al. 1995; Winkler & Allen 1996). However, quality differences between breeding individuals could be responsible for this result (cf. Noordwijk & Jong 1986), calling for manipulations to break the quality-based correlation between present reproductive success and survival.
Female survival was lower among experimentally delayed Great Tits than controls during one of four years (Verhulst & Tinbergen 1991; Verhulst et al. 1995). In delayed Blue Tits, both males and females had reduced survival during one of two years (Nilsson & Svensson 1996). The winter affecting experimental and control birds differently was more severe than the other winter, suggesting that the effect on adults of a delayed breeding is expressed only during cold winters. During both years studied, delayed Blue Tit females started to breed later in the subsequent breeding season and the females of delayed males laid smaller clutches compared to control pairs (Nilsson & Svensson 1996). Thus, there is some evidence for reduced future survival and reproductive success of parents manipulated to breed later than they had set out to do. Such a reproductive cost is probably mediated through reduced moulting effort, leading to the production of feathers of low insulating capacity (Nilsson & Svensson 1996).
Blue Tit parents were tricked into breeding earlier than they had set out to do by supplemental feeding before the start of egg laying. Females that advanced breeding, showed reduced survival rates to the next breeding season (Nilsson 1994). Thus, also breeding earlier than normal was detrimental to female survival.
Conclusion
My aim in presenting the different mechanisms and their support in experiments, has not been to favour or refute some of them. A literature survey such as this one, likely suffers from a bias stemming from the kinds of species that have been studied. I would like to stress that all of the mechanisms suggested under the different hypotheses have support, their impact depending on the ecological setting and social system of the species under study. The underlying mechanism for an observed seasonal decline in reproductive success may, however, be of some importance. The trade-off between timing of breeding on one hand and adult and offspring survival on the other, will be influenced by the mechanism explaining the seasonal decline in reproductive success in the specific case. Furthermore, viewing an individual’s timing of breeding as a trade-off between present and future reproduction, suggests that different optimal solutions may exist, depending on e.g. age, residual reproductive value and quality of mates.
ACKNOWLEDGMENTS
I am grateful to H. Källander, M. Lambrechts, L. Råberg and M. Visser for their comments on the manuscript.
REFERENCES
Allander, K. & Bennett, G.F. 1995. Retardation of breeding onset in Great Tits Parus major by blood parasites. Functional Ecology 9: 677-682.
Andersson, M. & Gustafsson, L. 1995. Glycosylated haemoglobin – a new measure of condition in birds. Proceedings of the Royal Society of London B 260: 299-303.
Aparicio, J.M. 1998. Individual optimisation may explain differences in breeding time in the European Kestrel Falco tinnunculus. Journal of Avian Biology 29: 121-128.
Arcese, P. & Smith, J.N.M. 1985. Phenotypic correlates and ecological consequences of dominance in Song Sparrows. Journal of Animal Ecology 54: 817-830.
Bauchau, V. & Seinen, I. 1997. Clutch desertion and re-nesting in Pied Flycatchers: an experiment with progressive clutch removal. Animal Behaviour 54: 153-161.
Boag, P.T. & van Noordwijk, A.J. 1987. Quantitative genetics in wild bird populations. In: Cooke, F. & Buckley, P.A. (eds) Avian genetics. London; Academic Press: 45-78.
Brinkhof, M.W.G. 1997. Seasonal decline in body size of Coot chicks. Journal Avian Biology 28: 117-131.
Brinkhof, M.W.G., Cavé, A.J., Hage, F.J. & Verhulst, S. 1993. Timing of reproduction and fledging success in the Coot Fulica atra: evidence for a causal relationship. Journal Animal Ecology 62: 577-587.
Brinkhof, M.W.G., Cavé, A.J. & Perdeck, A.C. 1997. The seasonal decline in the first-year survival of juvenile Coots: an experimental approach. Journal Animal Ecology 66: 73-82.
Brouwer, A., Spaans, A.L. & de Wit, A.A.N. 1995. Survival of Herring Gull Larus argentatus chicks: an experimental analysis of the need for early breeding. Ibis 137: 272-278.
Bryant, D.M. 1975. Breeding biology of House Martins Delichon urbica in relation to aerial insect abundance. Ibis 117: 180-216.
Cavé, A.J., Visser, J. & Perdeck, A.C. 1989. Size and quality of the Coot Fulica atra territory in relation to age of its tenants and neighbours. Ardea 77: 87-98.
Clamens, A. & Isenmann, P. 1989. Effects of supplemental food on the breeding of Blue and Great Tits in Mediterranean habitats. Ornis Scandinavica 20: 36-42.
Clutton-Brock, T.H. 1988. Reproductive success. Chicago; University of Chicago Press.
Daan, S., Dijkstra, C., Drent, R. & Meijer, T. 1989. Food supply and the annual timing of avian reproduction. In: Quellet, H. (ed) Proceedings of the XIX International Ornithological Congress. Ottawa; University of Ottawa Press: 392-407.
Daan, S., Dijkstra, C. & Tinbergen, J.M. 1990. Family planning in the Kestrel Falco tinnunculus: the ultimate control of covariation of laying date and clutch size. Behaviour 114: 83-116.
Davies, N.B. & Lundberg, A. 1985. The influence on food on time budgets and timing of breeding of the Dunnock Prunella modularis. Ibis 127: 100-110.
De Forest, L.N. & Gaston, A.J. 1996. The effect of age on timing of breeding and reproductive success in the Thick-billed Murre. Ecology 77: 1501-1511.
Gibb, J. 1950. The breeding biology of the Great and Blue Titmice. Ibis: 92: 507-539.
Hails, C.J. & Bryant, D.M. 1979. Reproductive energetics of a free-living bird. Journal of Animal Ecology 48: 471-482.
Hamann, J. & Cooke, F. 1989. Intra-seasonal decline of clutch size in Lesser Snow Geese. Oecologia 79: 83-90.
Hasselquist, D. 1998. Polygyny in Great Reed Warblers: a long-term study of factors contributing to male fitness. Ecology 79: 2376-2390.
Hatchwell, B.J. 1991. An experimental study of the effects of timing of breeding on the reproductive success of Common Guillemots Uria aalge. Journal of Animal Ecology 60: 721-736.
Hipfner, J.M., Gaston, A.J. & De Forest, L.N. 1997. The role of female age in determining egg size and laying date of Thick-billed Murres. Journal of Avian Biology 28: 271-278.
Hochachka, W. 1990. Seasonal decline in reproductive performance of Song Sparrows. Ecology 71: 1279-1288.
Hochachka, W & Smith, J.N.M. 1991. Determinants and consequences of nestling condition in Song Sparrows. Journal of Animal Ecology 60: 995-1008.
Hughes, R.J., Reed, A. & Gauthier, G. 1994. Space and habitat use by Greater Snow Geese broods on Bylot Island, Northwest Territories. Journal of Wildlife Management 58: 536-545.
Hunt, G.L., Jr & Hunt, M.W. 1976. Gull chick survival: the significance of growth rates, timing of breeding and territory size. Ecology 57: 62-75.
Korpimäki, E., Hakkarainen, H. & Bennett, G.F. 1993. Blood parasites and reproductive success of Tengmalm´s Owls: detrimental effects on females but not on males? Functional Ecology 7: 420-426.
Lambrechts, M.M., Blondel, J., Maistre, M. & Perret, P. 1997. A single response mechanism is responsible for evolutionary adaptive variation in a bird´s laying date. Proceedings of the National Academy of Sciences of the United States of America 94: 5153-5155.
Lepage, D., Desrochers, A. & Gauthier, G. Seasonal decline of growth and fledging success in Snow Geese, Anser caerulescens: an effect of date or parental quality? Journal of Avian Biology in press.
Lindén, M., Gustafsson, L. & Pärt, T. 1992. Selection on fledging mass in the Collared Flycatcher and the Great Tit. Ecology 73: 336-343.
Moreno, J., Barbosa, A., Potti, J. & Merino, S. 1997. The effects of hatching date and parental quality on chick growth and creching age in the Chinstrap Penguin Pygoscelis antarctica: a field experiment. Auk 114: 47-54.
Murphy, E.C. 1995. Seasonal declines in duration of incubation and chick periods of Common Murres at Bluff, Alaska in 1987-1991. Auk 112: 982-993.
Murphy, M.T. 1986. Temporal components of reproductive variability in Eastern Kingbirds Tyrannus tyrannus. Ecology 67: 1483-1492.
Nager, R.G. & van Noordwijk, A.J. 1995. Proximate and ultimate aspects of phenotypic plasticity in timing of Great Tit breeding in a heterogeneous environment. American Naturalist 146: 454-474.
Newton, I. & Marquiss, M. 1984. Seasonal trend in the breeding performance of Sparrowhawks Accipiter nisus. Journal of Animal Ecology 53: 809-830.
Nilsson, J.-Å. 1989a. Causes and consequences of natal dispersal in the Marsh Tit Parus palustris. Journal of Animal Ecology 58: 619-636.
Nilsson, J.-Å. 1989b. Establishment of juvenile Marsh Tits in winter flocks: an experimental study. Animal Behaviour 38: 586-595.
Nilsson, J.-Å. 1990. Establishment success of experimentally delayed juvenile Marsh Tits Parus palustris. Ethology 85: 73-79.
Nilsson, J.-Å. 1994. Energetic bottle-necks during breeding and reproductive costs of being too early. Journal of Animal Ecology 63: 200-208.
Nilsson, J.-Å. & Smith. H.G. 1988. Effects of dispersal date on winter flock establishment and social dominance in Marsh Tits Parus palustris. Journal of Animal Ecology 57: 917-928.
Nilsson, J.-Å. & Svensson, E. 1993. Causes and consequences of egg mass variation between and within Blue Tit clutches. Journal of Zoology, London 230: 469-481.
Nilsson, J.-Å. & Svensson, E. 1996. The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proceedings of the Royal Society of London B 263: 711-714.
Noordwijk, A.J. van & Jong, G. de 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. American Naturalist 128: 137-142.
Norris, K. 1993. Seasonal variation in the reproductive success of Blue Tits: an experimental study. Journal of Animal Ecology 62: 287-294.
Oppliger, A., Richner, H. & Christe, P. 1994. Effect of an ectoparasite on lay date, nest-site choice, desertion, and hatching success in the Great Tit (Parus major). Behavioral Ecology 5: 130-134.
Parsons, J. 1975. Seasonal variation in the breeding success of the Herring Gull: an experimental approach to pre-fledging success. Journal of Animal Ecology 44: 553-573.
Perrins, C.M. 1970. The timing of birds` breeding seasons. Ibis 112: 242-255.
Perrins, C.M. 1996. Eggs, egg formation and the timing of breeding. Ibis 138: 2-15.
Perrins, C.M. & McCleery, R.H. 1985. The effect of age and pair bond on the breeding success of Great Tits Parus major. Ibis 127: 306-315.
Perrins, C.M. & McCleery, R.H. 1989. Laying dates and clutch size in the Great Tit. Wilson Bulletin 101: 236-253.
Price, T., Kirkpatrick, M. & Arnold, S.J. 1988. Directional selection and the evolution of breeding date in birds. Science 240: 798-799.
Ricklefs, R.E. 1984. Components of variance in measurements of nestling European Starlings (Sturnus vulgaris) in southeastern Pennsylvania. Auk 101: 319-333.
Rätti, O., Dufva, R. & Alatalo, R.V. 1993. Blood parasites and male fitness in the Pied Flycatcher. Oecologia 96: 410-414.
Sandell, M. & Smith, H.G. 1991. Dominance, prior occupancy, and winter residency in the Great Tit (Parus major). Behavioral Ecology and Sociobiology 29: 147-152.
Schifferli, L. 1973. The effect of egg weight on the subsequent growth of nestling Great Tits Parus major. Ibis 115: 549-558.
Sedinger, J.S., Lindberg, M.S., Eichholz, M. & Chelgren, N. 1997. Influence of hatch date versus maternal and genetic effects on growth of Black Brant goslings. Auk 114: 129-132.
Smith, H.G., Ohlsson, T. & Wettermark, K.-J. 1995. Adaptive significance of egg size in the European Starling: experimental tests. Ecology 76: 1-7.
Spear, L. & Nur, N. 1994. Brood size, hatching order and hatching date: effects on four life-history stages from hatching to recruitment in Western Gulls. Journal of Animal Ecology 63: 283-298.
Steeger, C. & Ydenberg, R.C. 1993. Clutch size and initiation date of Ospreys: natural patterns and the effect of a natural delay. Canadian Journal of Zoology 71: 2141-2146.
Svensson, E. 1997. Natural selection on avian breeding time: causality, fecundity-dependent, and fecundity-independent selection. Evolution 51: 1276-1283.
Svensson, E. & Nilsson, J.-Å. 1995. Food supply, territory quality, and reproductive timing in the Blue Tit (Parus caeruleus). Ecology 76: 1804-1812.
Tinbergen, J.M., van Balen, J.H. & van Eck, H.M. 1985. Density-dependent survival in an isolated Great Tit population: Kluyvers´ data re-analysed. Ardea 73: 38-48.
Verboven, N & Verhulst, S. 1996. Seasonal variation in the incidence of double broods: the date hypothesis fits better than the quality hypothesis. Journal of Animal Ecology 65: 264-273.
Verboven, N. & Visser, M.E. 1998. Seasonal variation in local recruitment of Great Tits: the importance of being early. Oikos 81: 511-524.
Verhulst, S. 1992. Effects of density, beech crop and winter feeding on survival of juvenile Great Tits; an analysis of Kluyver´s removal experiment. Ardea 80: 285-292.
Verhulst, S. & Tinbergen, J.M. 1991. Experimental evidence for a causal relationship between timing and success of reproduction in the Great Tit Parus m. major. Journal of Animal Ecology 60: 269-282.
Verhulst, S., van Balen, J.H. & Tinbergen, J.M. 1995. Seasonal decline in reproductive success of the Great Tit: variation in time or quality? Ecology 76: 2392-2403.
Wiggins, D.A., Pärt, T. & Gustafsson, L. 1994. Seasonal decline in Collared Flycatcher Ficedula albicollis reproductive success: an experimental approach. Oikos 70: 359-364.
Winkler, D.W. & Allen, P.E. 1996. The seasonal decline in Tree Swallow clutch size: physiological constraint or strategic adjustment? Ecology 77: 922-932.
Ydenberg, R.C., Clark, C.W. & Harfenist, A. 1995. Intraspecific fledging mass variation in the Alcidae, with special reference to the seasonal fledging mass decline. American Naturalist 145: 412-433.
Young, B.E. 1994. Geographic and seasonal patterns of clutch-size variation in House Wrens. Auk 111: 545-555.
Table 1. Suggested mechanisms with the potential to cause the seasonal decline in reproductive success commonly observed in field studies.

Table 2. Overview of exchange and delay experiments from the literature. Type of experiment refers to exchange (E) or delay (D). Mechanisms refer to the numbered hypotheses in Table 1. Stated are the most important mechanism and lower-ranked mechanisms (in parentheses) as assessed by the authors.
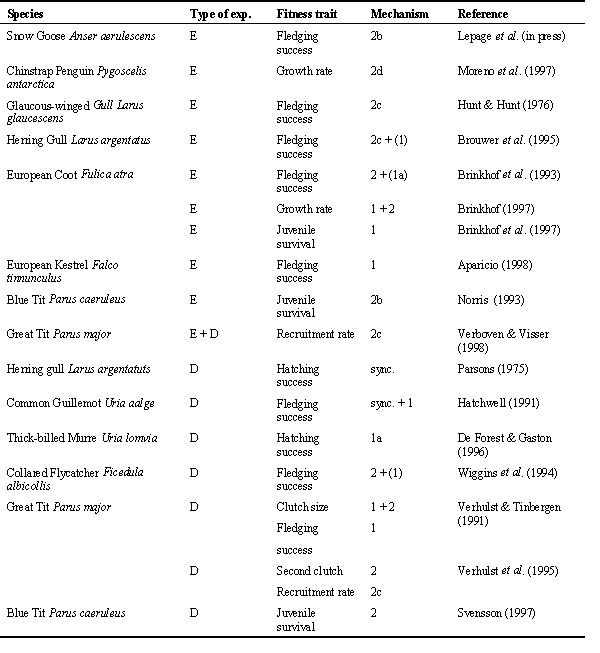
Fig. 1. Predicted relation between a hypothetical reproductive trait and date during the breeding season in exchange and delay experiments according to the breeding quality, timing and chick quality hypotheses. The solid line represents the natural seasonal decline of reproductive success in the population. The dotted line represents the swapping of clutches in an exchange experiment. Only one direction of the exchange experiment is shown. Parents setting out to breed at date A will receive a clutch from parents breeding at date B. Their new clutch will therefore hatch later than their original clutch would have done. In the delay experiment, parents laying at A are forced to lay a repeat clutch at date B. Predictions are shown as broken lines. In the exchange experiment, the breeding quality hypotheses predict reproductive success to have a value of C and the timing and chick quality hypotheses to have a value of D. In the delay experiment, the breeding and chick quality hypotheses predict reproductive success to have a value of C and the timing hypotheses to have a value of D.
