S04.3: Fat accumulation in pre-migratory roosting Barn Swallows in Europe
Andrea Pilastro1 & Fernando Spina2
1University of Padova, Biology Department, via U. Bassi 58/B, I-35131 Padova, Italy, fax 39 49 8276209, e-mail pilastro@civ.bio.unipd.it; 2Istituto Nazionale per la Fauna Selvatica, via Ca’ Fornacetta 9, I-40064 Ozzano Emilia (BO), Italy, e-mail infsmigr@iperbole.bologna.it
Pilastro, A. & Spina, F. 1999. Fat accumulation in pre-migratory roosting Barn Swallows in Europe. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: Johannesburg: BirdLife South Africa.
Energetic aspects related to the migration of the Barn Swallow Hirundo rustica have only recently started to be studied in detail. Swallows congregates in pre-migratory roosts where large numbers of birds can be ringed, allowing to study the variation of fat accumulation dynamics during the season and between different geographical areas. Previous analyses of body moult progress and fat accumulation across Italy suggest that fat accumulation starts only when body moult is completed; then, mean body mass and fat score increase rapidly from about 18 to 24 g. Daily body mass increase is in the range of those reported for other trans-Saharan passerine migrants, suggesting the potential for Swallows to cross the Mediterranean and Sahara without substantial refuelling. These results indicate that Swallows adopt the typical migration strategy of other long-distance migrant passerines. The pilot year of the EURING Swallow Project provided similar data from 5 European Countries. Analysis of body mass variation over a wider geographical range (range=37°N to 62°N; 6°W to 26°E) suggest that migration strategy may differ between western populations (namely UK and Spain) and those passing through Central and Eastern Europe (Finland, Switzerland and Italy).
INTRODUCTION
The Barn Swallow Hirundo rustica performs one of the longest migration trip among passerine and non-passerine migrants (Alerstam 1990; Cramp 1988). Despite the fact that is one of the most common birds in Europe, and most aspects of its biology have been thoroughly studied (e.g. Møller 1994), the energetic aspects of its migratory strategy are still poorly known (but see Ormerod 1989). Given that this species is able to feed on the wing, it has been long believed that, contrary to many other passerines, the Swallow adopt a fly-and-forage migration strategy, without the need of accumulating large amount of fat. The low estimated migration speed, as derived from recoveries (Ormerod 1991), may therefore be in relation with such strategy.
Recent studies of body mass variation of pre-migratory roosting Swallows in Italy seem to indicate that this view is, at least for these populations, not correct. In fact, it has been demonstrated that, in northern Italy, Swallows gain rapidly weight, starting from late August, when body moult has been terminated (Pilastro & Magnani 1997). At this time of the season, mean body mass and fat score increase rapidly from about 18 to 24 g. Daily body mass increase ranges between 1.5% (juveniles) and 2.1% (adults) of the lean body mass, a figure not far from that observed among other passerine trans-Saharan migrants (Loske 1990; Lindström 1991). Around mid-September, fat reserves of the 25% heaviest birds corresponded to 24.1% (juveniles) and 30.5% (adults) of the lean body mass, suggesting the potential for Swallows to cross the Mediterranean and Sahara without substantial refuelling (Pilastro & Magnani 1997). Estimates of potential flight range (Pennycuick 1989; Pennycuick et al. 1996) seems to corroborate this view. Mean daily body mass variation in response to different climatic and foraging conditions suggests that roosting pre-migratory Swallows are putting on fat at their maximum rate (Pilastro and Magnani 1997), as expected for migrants that are going to cross a large ecological barrier.
A survey of the geographical variation of fat reserves level was conducted in September 1996 in Italy by contemporary captures at 23 different roosts (Pilastro et al. 1998). The observed pattern of fat reserves suggests that, apparently with the only exclusion of most northern localities where no fattening was observed, Swallows accumulate significant amount of lipidic reserves all along the Italian peninsula, and only a slight north-south positive gradient of fat reserves was evident. On the other hand, it seems that British Swallows leave their breeding grounds in autumn without accumulating large amount of fat (Ormerod 1989). This would be in line with what is known for most trans-Saharan passerine migrants, in which northern populations start their migration with small reserves, and start to gain weight in Southern Europe (e.g. Bibby & Green 1983).
The relative easiness of capturing Swallows at roosts makes this species a good study species for investigating migration strategy on a large geographical scale. Two questions raise from these first results on the strategy of Swallow for preparing their migration through Mediterranean sea and Sahara desert: i) do all European Swallow populations accumulate large amount of fat before leaving Europe, adopting the typical trans-Saharan passerine migrant strategy? If so, ii) at which latitude, or, in other words, at which distance from the Sahara do they start to accumulate fat? We report here the results of the pilot year of a wide international co-operative project on roosting Swallows that have been launched in 1997 by the EURING.
METHODS
Mist nets were set near the roost location during the day. Before the swallows arrival, about half hour before dusk, tape lure was turned on. According to the number of swallows caught, it was turned off when the roost was set or a sufficient number of swallows was caught. Using a flash light, when necessary, swallows were taken out from the nets and put into paper boxes or cotton bags until ringed and measured. swallows were then set free at next dawn. For each individual ringed, when possible, age and sex were determined according to Svensson (1992) and Jenni & Winkler (1994). Feather-length of the third outermost primary was taken, to the nearest 0.5 mm, as the measurement of size (Berthold & Friedrich 1979). Body moult intensity was scored as follows: 0 = no body feathers growing; 1 = up to about 20 growing feathers; 2 = more than 20 body feathers growing, including feathers growing due to the completion of the juvenile plumage in birds just after fledging. The amount of visible subcutaneous fat was determined by scoring two fat deposits: (a) the furcular (intraclavicular depression, 'tracheal pit') and (b) the abdominal. A 9-grade score (0 - 8) was used, based on Kaiser (1993). Birds were weighed using a Pesola spring balance to the nearest 0.5 g or an electronic scale to the nearest 0.1 g. more details can be found on the Swallow Project Field Manual (Jenni 1998) and on the ESF-Network Field Manual (Bairlein 1995). When the number of swallows captured per session was high, some of the biometrics were not taken, and therefore sample sizes can differ slightly among analyses.
RESULTS
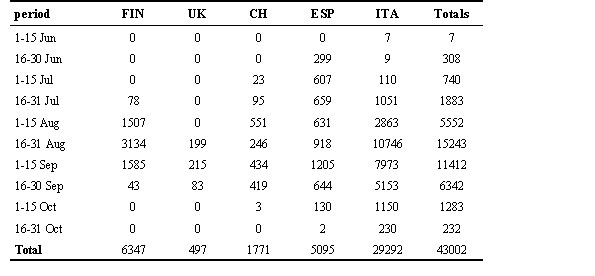
Eleven Countries joined the Swallow Project, and more than 85000 swallows have been ringed in more than 80 different roosts, providing a valuable amount of information. Here we analysed the data concerning 43000 swallows from 40 roosts in five different countries, that were already digitised by the end of April 1998 (Table 1). In total, 720 capture sessions were run, with an average capture of 59.7 swallows per session (median = 31, S.D. = 103.4). In order to analyse the geographical variation of fattening strategy, we excluded from the analyses swallows heavily moulting on the body (body moult < 2). In Italy, swallows start to gain weight at the end of August – beginning of September (Pilastro & Magnani 1997). We therefore divided our data, for some analyses, into two periods, summer (July and August) and autumn (September and October).
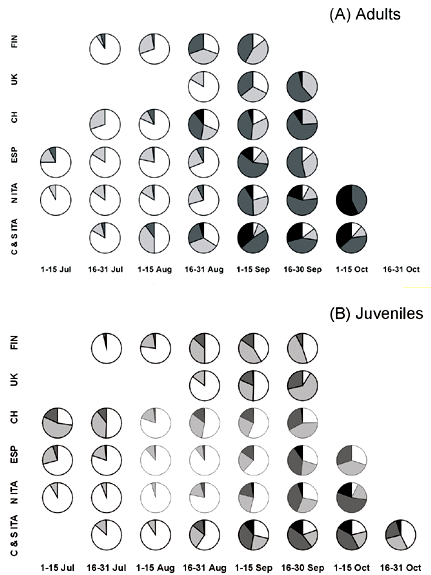
As it can be seen from Fig. 1, body mass increase started the second half of August. There is no clear north-south gradient in the starting of body mass increase, although differences existed, with some areas apparently showing an earlier start of fattening (e.g. juveniles in UK, adults in Finland and southern Italy). From the end of August body mass increased in all the European Countries studied here. Southern Countries, as Italy and Spain, showed higher mean values. In the second half of September, we found adult swallows with body mass values greater than 24 g in UK, Switzerland, Spain and Italy, but the frequency of these very fat birds was highest in Italy, south of 43° N. In one of these southern roosts, more than 75% of the captured adults weighed more than 24 g (Table 2). In autumn, the frequency of birds with fat score > 3 is more than 50% in Italy, and slightly lower in the other countries. Very fat individuals are found mainly in Italy, where almost 10% of the captured individuals (ages pooled) weigh more than 24 g. Considering only the adults, this frequency raised up to 22.6%. In principle, these body mass values would allow a flight of more than 3,000 km (see below). Apparently, swallows migrating through the Italian peninsula therefore accumulated more fat than those following the Iberian peninsula, as also indicated by the results of the multiple regression of body mass on geographical position (latitude and longitude), and month and time of capture (Table 3). Similar results were obtained when we used the mean values for each roost instead of the original data (not shown).
In southern Europe swallows accumulated enough fat to cross in a single flight Mediterranean sea and Sahara desert. Body mass of swallows with no body moult and fat score = 0 was equal to 17.8 g (S.D. = 1.53, n = 372). If one takes this as an estimation of lean body mass, Pennycuick’s model (1986) and recent corrections (Pennycuick et al. 1996) give the impressive figure of more than 5000 km of potential flight range for the maximum body mass observed during this study (29.5 g). More details on flight range estimates are given on Table 4.
DISCUSSION
Data collected during the first, pilot year of the EURING Swallow Project gave us an impressive amount of information on the preparation to migration in this migrant species. Results presented here concern only some aspects of the problem, whereas others (e.g. moult strategy, phenology, age-related differential migration, etc.) are still to be investigated. Despite the fact that more information from other routes and Countries will be available in the next future, some interesting results are already coming out from this first year of study. In particular, previous finding about the migration strategy of swallows in western Europe, i.e. pronounced fattening as other trans-saharan passerine migrants (Pilastro & Magnani 1997; Pilastro et al., 1998) are confirmed by this survey of body mass values. According to the previous study by Ormerod (1989) on UK Swallows, a certain fattening is also observe in northern Europe, and, at least in the year of study, it started nearly simultaneously at all latitudes. The slightly higher body mass values observed in July in Switzerland and Spain may due to the capture of recently fledged young that often carry some fat when leaving the nest.
The observed body mass values correspond to fat reserves of 30-40% of the lean body mass. Such fat levels, according to the new estimates of potential flight range, would allow many of these to safely cross the Mediterranean sea and the Sahara desert without the need to refuel. This is confirmed by the recovery of a bird ringed in northern Italy in September with a body mass of 26 g and recovered seven days later in Niger, after having covered 3028 km at an average daily distance of 433 km (A. Magnani, F. Spina & P. Micheloni, unpublished result). It can therefore be confirmed that this species, as many other passerines, in autumn accumulate in southern Europe sufficient lipid reserves to cross the Sahara without en route refuelling. This observation has an important implication for models of optimal migration (Alerstam & Lindstrom 1990). As said before, at least part of the European Swallows do not need to refuel in north Africa before crossing the Sahara. In principle, the best strategy for migrants should be to accumulate fat just before barrier crossing (Weber & Houston 1997), but apparently this is not the case for swallows, as well as for other migrant species (e.g. Spina & Massi 1992). Similar pattern has been suggested also for the geographical pattern of fattening in the sub-Sahara for spring migration (Pilastro & Spina 1997). Long distance migrant, that need to rapidly accumulate great fat reserves, are probably constrained to do so in particular habitats that meet the species specific ecological requirements, and these habitats are not necessarily found close to the barrier they have to cross. For what Swallow is concerned, it is probable that the most important component of its ecological needs when preparing for migration is represented by large reed beds where they can roost during the fattening period.
Our results suggest that, along the Iberian peninsula, swallows accumulate lower fat reserve levels compared to those observed, at comparable latitude, in Italy. This result may suggest that the strategy of direct Sahara crossing is mainly adopted by central Europe populations, whereas those migrating along more western routes more often adopt a migration strategy based on shorter flights and stopovers (i.e. an energy minimising strategy). Given the variability of body condition observed at different roosts in Italy (Pilastro et al. 1998; this study), it may also be that the observed differences between Spain and Italy may just be due to the fact that in the Iberian Peninsula less roost have been studied in 1997 and a better geographical coverage may reduce or annul these differences. A wider geographical coverage is therefore necessary to confirm these findings.
ACKNOWLEDGEMENTS
This research could not have been possible without the enthusiastic contribution of many people, both ringers and helpers. Although it is impossible to name all of them, we would like to try to thank here at least the ringers that provided us with their ringing data for this study: Aalto T., Amann Fritz, Anderegg Kurt, Andersag Karl, Antoniazza Michel, Baader Edi, Barcenilla Mario, Barragan Alfonso, Bassin Albert, Baudraz Michel, Béguin Daniel, Benassi Romano, Benoit Francis, Bermejo Ana, Berner René, Beyeler Marianne, Blattner Martin R., Boto Alberto, Brahier Jean-Luc, Brüngger Hans, Bruni Eros, Buchli Jachen, Bürkli Wolfram, Calesini Luciano, Calleja Juan A., Castelli Francesco, Cavaliere Vincenzo, Cercadillo Eduardo, Chautems Christophe, Corti Walter, Cortone Pino, Cuti Natalino, Dall’Antonia Paolo, Di Lauro Federica, Dominguez Francisco, Droll Beat, Eggenschwiler Martina, Ehrenbold Sämi, Ekblom H., Fernandez Jesus, Ferreiro Ernesto, Ferro Mimmo, Fischer Luzius, Fleissner Martin, Fliri Rico, Fliri Urs, Frara Patrick, Frias Oscar, Froidevaux Laurent, Furler Martin, Galardini Andrea, Galvez Miguel, Garcia Eladio L. de la, Gardiazabal Andrea, Gargallo G., Ghisleni Onofrio, Giannella Carlo, Gianom Gian Peider, Gilliéron Georges, Giusini Umberto, Glauser Christa, Gonzalez Gumersindo, Good Albert, Good Niklaus, Good-Roth Albert, Gremaud Jérôme, Group Català d’Anillament, Guzzon Carlo, Hersberger Heinz, Hokkanen T., Huber Matthias, Hüni Max, Hüppin Leo, Juillerat Laurent, Kälin Hansruedi, Kestenholz Matthias, Kiviharju E., Kohler Michel, Korhonen J., Kurmann Silvio, La Gioia Giuseppe, Lampérth Elisabeth, Landucci Giuseppe, Lanz Ueli, Lardelli Roberto, Laurenti Stefano, Le Nédic Christophe, Lehti M., Leuenberger Max & Yvonne, Leuzinger Hans, Lo Valvo Fabio, Lokkki H., Lotti Claudio, Lyytinen S., Magnani Ariele, Magro Jose R., Martignoli Veronika, Marzano Giacomo, Matalon Guy, Maumary Lionel, Maurer-Lehmann Bruno, Micheloni Pierfrancesco, Molina Blas, Monnerat Paul, Moral Juan C. del, Mosca Carl, Muff Sepp, Müller Mathis, Mundwiler Kurt, Muzzatti Mario, Negra Osvaldo, Nievergelt Fränzi, Nissardi Sergio, Oberhänsli Hans, Ochsner Werner, Pasanen E., Pérez Javier, Pinilla Jesus, Politi Paolo, Polt Heidi, Poo Christian, Porro Marzio, Pouttu P., Pouttu R., Pritchard Jan, Puente Javier de la, Ravussin Pierre-Alain, Ritter Markus, Rondelli Marzio, Ruben Chasper, Rusticali Renzo, Sartori Rito, Saunier Alain, Savo Enzo, Scebba Sergio, Schadegg Max, Schaffner Werner, Schaub-Schneider Christoph & Susi, Schäublin Adrian, Scheidegger Tobias, Schuler Urs, Seoane Javier, Serpellini Walter, Simon Richard, Spence Ian, Spiess Martin, Stalling Thomas, Steidinger P., Suter-Tague Vreni, Thévoz Jacques, Thiel Dominik, Tijera Raul E., Trolliet Daniel, Trüb Jacques, Turrian François, Vallotton Laurent, Velatta Francesco, Venemies J., Vigano’ Enrico, von Deschwanden Peter, Vonwil Peter, Willenegger Laurent, Wiprächtiger Peter, Zollinger Jean-Luc, Zucca Carla, Zufferey Michael. We wish to thank here also the staff of the ringing schemes that coordinated the activity of the ringers and in many cases digitized the ringing data. Colin Pennycuick kindly provided the upgraded version of his program for flight range estimation.
REFERENCES
Alerstam, T. 1990. Bird Migration. Cambridge: Cambridge University Press.
Alerstam, T. & Lindström, Å. 1990. Optimal bird migration: the relative importance of time, energy and safety. In: Gwinner, E (ed.). Bird Migration: Physiology and Ecophysiology. Springer-Verlag, Berlin, pp. 331-351.
Bairlein, F. 1995. ESF-Network Manual of Field Methods. Whilelmshaven, Germany.
Berthold, P. & Friedrich, W. 1979. Die Federlänge. Ein neues nützliches Flügelmass. Die Vogelwarte 30: 11-21.
Bibby, C.J. & Green, R.E. 1983. Food and fattening of migratoing warblers in some French marshlands. Ringing & Migration. 4: 175-184.
Cramp, S. 1988. The birds of Western Palearctic. Oxford University Press, Oxford.
Jenni, L. & Winkler, R. 1994. Moult and Ageing of European Passerines. New York: Academic Press.
Jenni, L. 1998. EURING Swallow Project. Manual. Sempach: Schweizerische Vogelwarte.
Kaiser, A. 1993. A new multi-category classification of subcutaneous fat deposits on songbirds. Journal of Field Ornithology 64: 246-255.
Lindström, Å. 1991. Maximum fat deposition rates in migrating birds. Ornis Scandinavic 22: 12-19.
Loske, K.-H. 1990. Spring weights and fat deposition of Palearctic passerine migrants in Senegal. Ringing & Migration 11: 23-30.
Møller, A.P. 1994. Sexual selection in the barn Swallow. Oxford: Oxford University Press.
Ormerod, S.J. 1989. The influence of weather on the body mass of migrating Swallows Hirundo rustica in south Wales. Ringing & Migration 10: 65-74.
Ormerod, S.J. 1991. Pre-migratory and migratory movements of Swallows Hirundo rustica in Britain and Ireland. Bird Study 38: 170-178.
Pennycuick, C.J. 1989. Bird flight performance: a practical calculation manual. Oxford: Oxford University Press.
Pennycuick, C.J., Klaassen, M., Kvist, A. & Lindström, Å. 1996. Wingbeat frequency and the body drag anomaly: Wind-tunnel observations on a thrush nightingale (Luscinia luscinia) and a teal (Anas crecca). Journal of Experimental Biology 199: 2757-2765.
Pilastro, A. & Magnani, A. 1997. Weather conditions and fat accuymulation dynamics in pre-migratory roosting Barn Swallows Hirundo rustica. Journal of Avian Biology 28: 338-344.
Pilastro, A. & Spina, F. 1998. Ecological and morphological correlates of residual fat reserves in passerine migrants at their spring arrival in southern Europe. Journal of Avian Biology 28: 309-318.
Pilastro A., Spina, F. & Micheloni, P. 1998. Geographical variation in pre-migratory conditions of Swallows Hirundo rustica in Italy. Ringing & Migration, in press.
Spina, F. & Massi, A. 1992. Post-nuptial moult and fat accumulation of the Ashy-headed Wagtail (Motacilla flava cinereocapilla) in Northern Italy. Die Vogelwarte 36: 211-220.
Svensson, L. 1992. Identification guide to European passerines (forth edn.). Tring: British Trust for Ornithology.
Table 1. Number of Swallows captured during the EURING Swallow Project during 1997, according to Country and period of capture. Capture effort and periods differed between roosts, and frequencies reported here do not necessarily correspond to the abundance of Swallows, but only represent the available data set.

Table 2. Frequency of capture of young and adults, mean body mass and body moult of Swallows ringed at roost in 1997 (ranked on decreasing latitude). In some cases, data from roosts close to each other were pooled and mean latitude and longitude were used. Small roosts were pooled with large, nearby roosts.
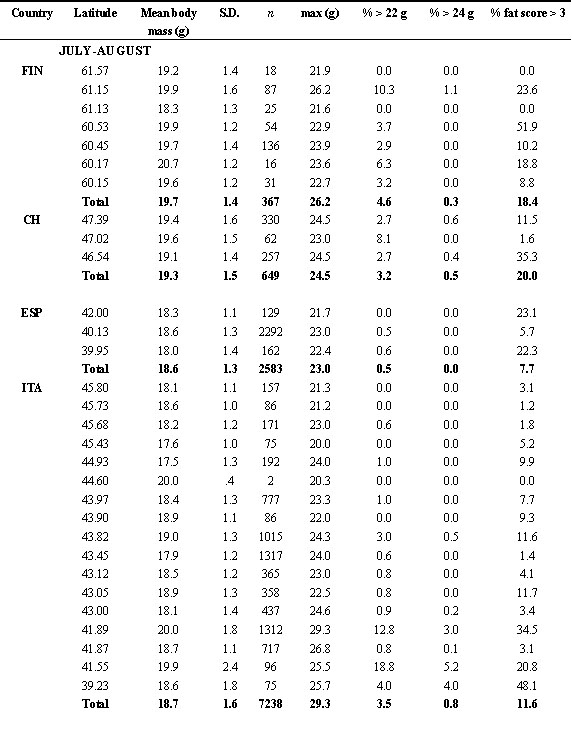
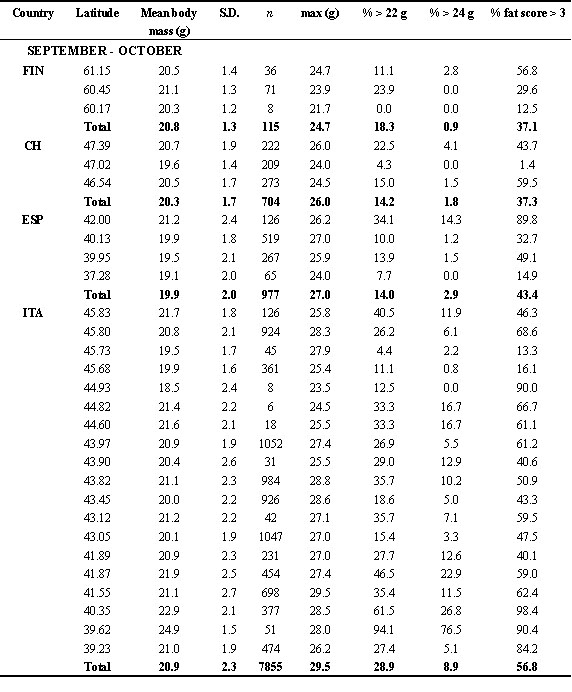
Table 3. Body mass variation according to the geographical position of the roost of capture (latitude and longitude). Since data from different roosts varied for the period sampled and the time of capture, we used a multiple regression to statistically control these possible confounding factors. Only individuals not intensely moulting (body moult score < 2) and captured between 1700 and 0600 hours were considered. Capture period = July-October.
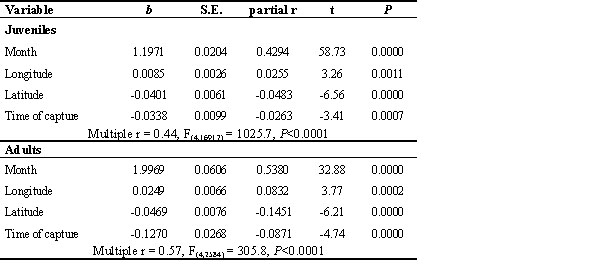
Table 4. Estimated flight range of Swallows according to fat load.
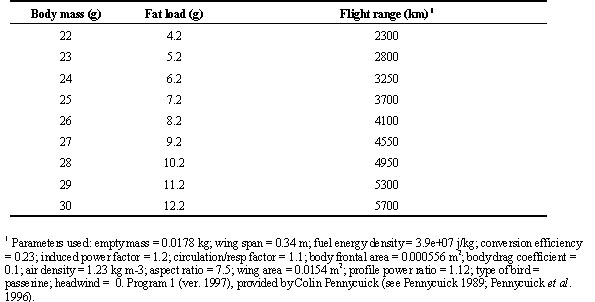
Fig. 1. Body mass frequency of roosting adult (A) and juvenile (B) Swallows according to period and Country of capture. Body mass categories were: <19.5, 19.5-21, 21-24, >24 g (from white to black, respectively). Only individuals with no body moult were included. Roosts in Italy have been subdivided into two groups: north of 43° N (N ITA) and south of 43° N (C & S ITA). All Spanish roosts were located south of 43° N. Sample sizes are listed on Table 1.