S03.4: How cognitive processing and social interaction affect allospecific vocal learning in Grey Parrots
Irene Maxine Pepperberg
Department of Ecology & Evolutionary Biology, University of Arizona, Tucson, Arizona 85721, USA, fax 520 621 9190, e-mail impepper@u.arizona.edu
Pepperberg, I.M. 1999. How cognitive processing and social interaction affect allospecific vocal learning in Grey Parrots. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
177-192. Johannesburg: BirdLife South Africa.Although little is known about Grey Parrot Psittacus erithacus vocal learning in nature, in the laboratory these birds can learn allospecific (English) vocalisations in order to request objects vocally and correctly reply to queries about object identity, color, shape, material, relative size, quantity, and absence, and to queries involving various combinations of these dimensions (Pepperberg 1990, 1994a). Significantly, these skills appear to develop best in a social context (e.g. Pepperberg 1994b). The present paper provides additional evidence for the importance of social context for allospecific vocal learning and also suggests that parrots use cognitive processing to evaluate the quality of the training input and to made decisions about what to learn. Such cognitive processing is viewed as a selected trait and as evidence for the evolution of
INTRODUCTION
Numerous studies have examined how different types of input affect vocal learning in oscine songbirds (e.g. Baptista & Gaunt 1994; King, Freeberg, & West 1996). For some species, for example, socially interactive input merely facilitates or modifies the course of development (e.g. extent to which a male cowbird shifts to potent songs while singing to a female; West & King 1988); for other species, such interaction is necessary for acquiring exceptional vocalisations (Pepperberg 1985, 1986a,b) - communication characterised by vocal learning unlikely to occur in the normal course of development, such as acquisition of allospecific forms. Few studies, however, examine if birds actively process different types of input to make specific choices about what to learn. I study this problem for what may seem an unusual species - the mimetic Grey Parrot Psittacus erithacus.
Given that parrots, at least in captivity, reproduce everything from microwave beeps to the human voice, the prospect that they have some defined basis for deciding what should or should not be learned appears unlikely: ‘Parrot’ is a synonym for facile, mindless mimicry. Captivity, however, may cause natural processes to adapt in peculiar ways, and I propose that studying psittacine vocal learning from a cognitive perspective will explain how and why these birds choose to acquire and produce allospecific vocalisations, particularly utterances used in meaningful ways. I also suggest that this capacity for cognitive processing, and thus psittacine intelligence, has evolved in response to the need for such choices.
My studies are the first to tie cognition to psittacine allospecific vocal learning. Researchers have described allospecific vocalisations of wild Grey Parrots (Cruickshank et al. 1993) and Galahs Cacatua roseicapilla (Rowley & Chapman 1986), but little about how such utterances are acquired. Allospecific vocal learning in budgerigars Melopsittacus undulatus (Gramza 1970; Banta 1998) and Grey parrots (Todt 1975; Pepperberg 1981, 1993) has been studied in laboratories, but only recently have my students and I examined how cognitive processes might direct such learning (Pepperberg 1994a, 1997, 1998; Pepperberg & McLaughlin 1996; Pepperberg et al. 1998).
In this chapter, I review what I mean by a cognitive approach, what is currently known about psittacine vocal behaviour, and studies of allospecific vocal learning in Grey Parrots. I then propose a paradigm for adaptive vocal learning in these birds, based on a cognitive perspective. I close by suggesting avenues of future research.
A cognitive perspective
Definitions of cognition are as varied as researchers in the field (e.g. Kamil 1988; Zentall 1993). I define cognition in terms of information processing, and as the combination of two abilities, the first being a prerequisite for the second. The first is the ability to use experience to solve current problems. An organism faced with green and red fruits, for instance, might recall that red indicates ripe and tasty and green indicates unripe and bitter, and therefore choose red. The second is the ability to choose, from among many sets of acquired information, the set appropriate to the current problem (Pepperberg 1990b, 1998), e.g. to recognise conditions under which selection of green fruit might be wise (i.e. when red indicates spoilage). Thus the capacity must also exist to transfer skills learned in one situation or modality to another (Rozin 1976). An organism limited to the first ability can learn important associations and process some information, but lacks the flexibility that is a hallmark of my definition of cognitive processing. To learn about animal capacities, I believe we must hypothesise that animals engage in cognitive processing; that is, that at least to some degree, they do not react mindlessly to environmental stimuli, but instead process these stimuli and choose to react in certain ways (Pepperberg 1998).
I believe that a cognitive approach, as defined above, will explain many aspects of psittacine vocal behaviour. A cognitive approach asks us first to determine if behaviour is directed by simple associations (hide when others hide), by processing current information based on similar past events (use memory to recognise something threatening), or processing that also incorporates information from a variety of experiences (do I hide based on the number of storm clouds, or must I take other information into account?; Pepperberg 1998). The approach then asks us to use this knowledge to explain why a behaviour pattern occurs. I believe that the last process--a cognitive one--may help us understand how wild parrots use some species-specific vocalisations. I believe a connection also exists between cognitive processing and allospecific vocal learning in parrots, and that this connection can explain how, why, and when such learning occurs.
A cognitive basis for vocal learning in Psittacids?
Although limited, data on psittacine vocal communication present a complex picture. At the least, simple information processing capacities are likely involved in sentinel behaviour, individual vocal recognition, and possibly duetting; quite likely cognitive processing (i.e. flexibility to adapt one’s knowledge to varying conditions) is also involved (see Pepperberg 1998 for a review). Sentinel behaviour, wherein one member of a flock perches in an exposed position and calls at the approach of danger, reflects some cognitive capacity for categorisation and using information flexibly: A predator observed routinely in one site, for example, must be recognised when seen elsewhere. Even if alarm calls do not refer to specific predators, the nature of each call must be specific. Individual recognition, which can occur between members of mated pairs, members of a flock, and between parents and offspring, requires cognitive processing: the ability to learn, memorise, and, most importantly, the flexibility to update information and transfer it among situations. Duetting also appears to require considerable plasticity and learning capacity: When dispersed in a flock, birds may use different duets than when in close contact; such behaviour might mediate interactions among flock members and the appropriate set of vocalisations must be chosen for a given situation.
Are cognitive capacities also involved in how parrots select and use allospecific sounds? Does the ability to decode on-going speech - a cognitive similarity I have found between Greys and humans (Pepperberg 1990b, 1992b; Patterson & Pepperberg 1994; cf. Lieberman 1984) - enable production not just of speech but also other allospecific vocalisations? Is the decoding process a by-product of, or prerequisite for, cognitive capacities? Why do captive pet psittacids apparently mimic indiscriminately (Amsler 1947; Hensley 1980), whereas birds in operant psychology studies usually fail to learn targeted speech (e.g. Mowrer 1958)? I cannot yet answer these questions, but believe answers can be found by examining the extent to which such behaviour requires cognitive processing: I suggest that allospecific learning is affected by a constellation of conditions, and that a cognitive approach will identify the different conditions and their various effects.
FACTORS INVOLVED IN ACQUIRING AN ALLOSPECIFIC CODE
If much of psittacine mimicry in captivity is indeed aberrant, we must assume that wild parrots’ imitative capacities are tempered to prevent maladaptive mimicry and thus that a mechanism exists to prevent widespread mimicry in nature. Presumably, selection would work against wild parrots that, for example, reproduced environmental noises indistinguishable from background sounds. Birds that could, in contrast, mimic - and thus maybe join or repel sympatric species might fare quite well. What constraints might direct useful learning and screen out irrelevant sounds, and why do constraints that appear to exist in the wild apparently fail under some types of captivity? My students and I believe we have found at least some of these constraints for Grey Parrots. The following sections (adapted from Pepperberg 1998) describe the proposed constraints and our experimental results, and the possible reasons why parrots in different situations exhibit different types of learning.
Constraints on Grey Parrot mimicry: rationale for testing effects of input
I suggest that some of the same social conditions that constrain allospecific learning in other birds exist for Grey Parrots. For some oscines, the extent, timing, and occurrence of allospecific vocal learning are influenced by the input they receive (e.g. Baptista & Petrinovich 1984, 1986). Some species may learn even their conspecific songs most readily if given visual and vocal interaction with a live tutor (e.g. Zebra Finches, Taeniopygia guttata: Price 1979, see Slater et al. 1988; cf. Adret 1993; ten Cate & Houx, this volume). That social interaction may affect allospecific vocal learning in psittacids is, in fact, suggested by several studies earlier than my own. My work, however, focuses on how such social interaction and cognitive processing might be linked.
Such a link can be demonstrated only by determining which aspects of input are important for learning, whether these aspects involve social interaction or cognitive processing - or both - and also their relative importance. First, however, I review studies that failed to engender allospecific vocal learning: Comparing unsuccessful and successful procedures may suggest which aspects of input are necessary for successful acquisition.
Prior to my studies, several researchers attempted to teach psittacids to reproduce human speech (see Pepperberg 1990a, 1998). Little or nothing was learned by Budgerigars, a Yellow- headed Amazon Amazona ochrocephala and a Grey Parrot that heard repetitive human phrases and could receive food rewards for successful mimicry (Mowrer 1954, 1958). In contrast, Greys that observed two humans interactively model specific vocal dialogues (Todt 1975; Pepperberg 1981, 1990a, 1993, 1994a,b) acquired targeted speech patterns.
Given reports of extensive speech mimicry by captive psittacids (see Amsler 1947), such data on effects of differential input were surprising. Arguably, the findings could be a consequence of inter-laboratory variation, not different learning conditions (Slater 1991). I thus began a series of studies to determine what aspects of input enabled Grey Parrots to learn an allospecific vocal code.
Aspects of input to be tested
To demonstrate the relative importance of various aspects of input for learning, one must first identify the relevant aspects. A psychological paradigm called social modeling theory (Bandura 1971, 1977), characterises input by three main aspects (see Pepperberg 1985, 1992a, 1993, 1994b, 1997, 1998): whether it (1) correlates with a specific aspect of an individual’s environment (i.e. has ‘referentiality’, Smith 1991), (2) has functional relevance for an individual’s environment (what psychologists call ‘contextual applicability’), and (3) is socially interactive.
Reference
Reference is, in part, what signals ‘are about’ (Smith 1991). Reference concerns the direct relationship between a signal and an object or action. Reference is not always easily determined; e.g. ‘key’ generally implies a specific metal object (what Smith [1991] labels an ‘external’ referent), but may also mean an action, as to ‘key’ in data. Similarly, a bird emitting an alarm call may refer to both predator and action it is about to take. Thus not all information contained in a signal involves a single referent, and determining the referent often requires the receiver to engage in cognitive processing. The receiver cannot simply process signal A, remember and interpret it as associated with situation X, but must decide, based on additional information, among the possibilities X, Y, and Z. The more explicit the referent of a signal, the more easily the signal appears to be learned.
Functionality
Functionality involves pragmatics: when a signal is to be used and the effects of using information in the signal. Because use and effect of a signal may depend upon environmental context, functionality helps define reference - in the above example, defines ‘key’ as a noun or verb. Cognitive processes are again important for extracting the function of a signal from what may be many possibilities in a given situation. The more explicit a signal’s functionality, however, the more readily the signal appears to be learned.
Social interaction
Social interaction can highlight which components of the environment should be noted, emphasise common attributes - and thus possible underlying rules - of diverse actions, and allow input to be continuously adjusted to match the level of the receiver. Interaction may also provide a contextual explanation of the reasons for an action and demonstrate its consequences (see Pepperberg 1993, 1994b, 1997, 1998). Interactive input may thus facilitate learning. And, again, cognitive processing is likely involved in sorting out the different facets of a given interaction.
Thus reference and functionality involve real world use of input, and social interaction provides emphasis. Researchers can design input that varies with respect to these aspects and then evaluate the relative effects of such variation on learning. To obtain such information on Grey Parrots, I used different conditions to train one adult and two juveniles to produce English labels to identify various common objects. Much of the material in the following sections, which describes this research, has been reported elsewhere (Pepperberg 1994b, 1997, 1998; Pepperberg & McLaughlin 1996; Pepperberg et al. 1998), but is included for the reader’s convenience.
THE SUBJECTS, FORMS OF INPUT TESTED, AND RESULTS
The juvenile Grey Parrots, Alo and Kyaaro, were 10 and 6.5 months, respectively, at the beginning of the experiments; the adult subject, Alex, was approximately 12 yrs old. I completed Alex’s study before I obtained the juveniles, and birds were physically isolated from one another during the experiments with the juveniles. Alo and Kyaaro had received no formal training prior to these experiments and had acquired no human vocalisations. Alex, however, had had extensive training and had learned referential use of English labels for objects, shapes, colors, and numbers up to 6 to identify, request, refuse, and quantify objects (Pepperberg 1981, 1987a, 1994b). He had been tested on concepts such as the presence or absence of sameness and difference, on his ability to categorise objects with respect to colour, shape, or material (Pepperberg 1983, 1987b, 1988), and his label comprehension (Pepperberg 1990b, 1992b). He also had functional use of several phrases (e.g. ‘Come here,’ ‘You tickle,’ ‘What’s that?’, ‘I’m sorry,’ ‘You tell me,’ ‘Wanna go X’ and ‘Want Y’ where X and Y are location and object labels).
Until the present experiments, Alex’s training system maximised reference, functionality, and social interaction. This system, called the model/rival (M/R) procedure, was adapted from the work of Todt (1975). M/R training involves three-way interactions between two humans and the avian student. M/R training primarily introduces new labels and concepts, but also aids in correcting pronunciation. Because the experiments described here are an in-depth comparisons of training protocols, I provide details of the M/R procedure although the material is available elsewhere (Pepperberg 1981, 1983, 1990a,b,c, 1994b, 1997, 1998).
The M/R technique (Todt 1975) uses human social interaction to demonstrate to a bird the targeted vocal behaviour. Sessions begin with a bird observing two humans handling an object in which the bird has already demonstrated some interest. One human trains the second human (the model/rival), i.e., presents and asks questions about the item (‘What’s here?’, ‘What toy ?’, etc.). The trainer rewards correct identifications with the item to which the label refers, thus demonstrating referential and functional use of labels, respectively, by providing a 1:1 correspondence between label and object, and modeling use of the label as a means of obtaining the object (Pepperberg 1981). A trainer responds to incorrect responses by scolding the trainee and temporarily removing the object from sight. Thus the second human not only is a model for the bird’s responses and a rival for the trainer’s attention, but also illustrates aversive consequences of errors. The model/rival is told to speak more clearly or try again when responses are garbled or incorrect, thereby allowing a bird to observe ‘corrective feedback’ (see Goldstein 1984; Vanayan et al. 1985). The parrot is also included in these interactions. It is rewarded for successive approximations to a correct response; the protocol thus adjusts the level of training to that of the bird. Trainer and model/rival also reverse roles to demonstrate the reciprocity of the communicative process: how it is used by either party to request information or effect environmental change. Without role reversal, birds exhibit two behaviour patterns inconsistent with interactive, referential communication: They do not transfer responses to anyone other than the human who posed the questions during training, and they do not learn both parts of the interaction (Todt 1975).
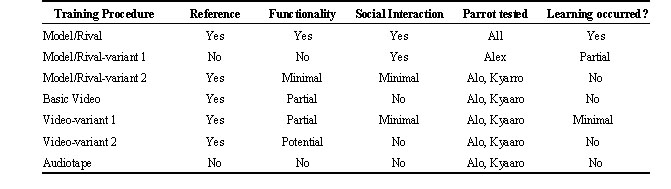
I designed experiments to examine how various levels of reference, social interaction, and functionality affect learning with respect not only to sound reproduction, but also comprehension and appropriate use (i.e. actions that require cognitive processing; see Pepperberg 1998). To provide input that varied with respect to these factors, I contrasted sessions of M/R, videotape, and audiotape tutoring. M/R and videotape training sessions could have varying components of input (Table 1).
To eliminate as much reference and functionality as possible and still retain the two-trainer method, I designed ‘M/R-variant 1’ (Pepperberg 1994b). Two humans enacted the same roles as in basic M/R training, but did not emphasize connections between labels and specific objects or collections. In the bird’s presence, one human posed a question on a specific topic in the absence of any objects and, in response, the other human produced a string of labels. We maintained role reversal and included the parrot in interactions. Correct responses garnered vocal praise and the opportunity to request anything desired (Pepperberg 1987a); errors elicited scolding and time-outs.
To eliminate some functionality and as much social interaction as possible, and to examine in Grey Parrots the effect of what child language researchers call ‘joint attention’ (e.g. Baldwin 1991), I designed ‘MR-variant 2’ (Pepperberg & McLaughlin 1996). The only aspects remaining of the basic M/R procedure were use of a live trainer and referential reward. A single trainer, rather than a pair, sat with her back to a bird, who was seated on a perch so as to be within reach of a suspended object The trainer produced relevant phrases and sentences in which object labels were stressed and in the final position (Pepperberg 1981), e.g. ‘You have a shiny key!’, ‘You gonna get the key?’, etc. so as to replicate what is often heard during language-learning in young children (see de Villiers & de Villiers 1978). Conceivably parrots, like humans, most readily remember ends of word strings (Lenneberg 1967; Silverstone 1989). The trainer never made eye contact with the bird, never presented the object to the bird, and never focused her attention on the object while interacting with the bird. She would reward any attempt at the targeted label with vocal praise.
To provide training that closely followed the M/R procedure but eliminated social interaction and minimised contextual applicability, I used a ‘basic video’ procedure (Pepperberg 1994b). I videotaped M/R sessions of the adult trained parrot, Alex, and exposed the juvenile birds to those tapes. During taping of these training videos, Alex occasionally erred or interrupted with requests for other objects and changes of location (Pepperberg 1983, 1987a, 1994b), which provided the opportunity to engage him in ‘corrective feedback’. Thus, although Alex already knew the targeted labels (Pepperberg 1990a,b), tapes did not present the targeted material as a review but rather recreated training sessions. Trainers also reversed roles and occasionally erred. Tapes retained breaks for nonvocal exchanges (e.g. when trainers preened Alex) and trainers’ departures by using, respectively, scenes of such nonvocal interactions or a blank screen. A zoom lens enabled us to include life-size images of Alex and the targeted objects in addition to the somewhat smaller images of the entire training scenario (the object, Alex, and two humans; Pepperberg et al. 1998). While watching these tapes, juveniles perched in front of a TV monitor in the absence of direct social interaction with trainers; no humans were present after a bird was situated on its perch. By watching a tape of a human or Alex produce a particular sound and either receive an object or be scolded, juveniles saw but did not experience directly the effect of a vocalisation. Videos, therefore, demonstrated reference but lacked clear functionality. A previous study (Rutledge & Pepperberg 1988) showed that Alex could respond to objects presented via a live video link; Grey Parrots thus can recognise two-dimensional video representations.
Because children may learn more from television programs viewed along with an adult (Lemish & Rice 1986; see St. Peters et al. 1989), I designed ‘video-variant 1’ for the parrots. This protocol involved ‘co-viewers’: trainers who merely ensured that birds attended to the monitor (Pepperberg et al. 1998). Trainers provided social approbation for viewing and pointed to the screen, making comments like ‘Look what Alex has!’ To separate the effect of having a human present who interacted with the bird from the effect of having a human present who interacted with both the bird and actions on the tape (see Pepperberg & Gardiner, unpubl. ms), here the co-viewer did not repeat new labels, ask questions, or relate the content to other training sessions. A bird’s attempt at a label would be rewarded with vocal praise, not the object. Thus social interaction was limited, and the amount of functional meaning matched that of basic videotape sessions. For all video presentations, we analysed (Kay 5500 DSP Sona-Graph) the audio portion of the video to ensure that sound was not degraded compared to that of Alex ‘live’ (Pepperberg et al. 1998).
Although neither parrot used labels from basic video sessions in the kind of vocal ‘practice’ we usually heard following M/R training (Pepperberg et al. 1991), possibly they attempted to produce the labels during video sessions and lack of reward extinguished the behaviour (Pepperberg 1994b). To test this possibility, I subsequently designed ‘video-variant 2’ (Pepperberg et al. 1998). Students and I repeated the basic videotape protocol, but included an ‘automatic’ reward system (a pulley) so a parrot could, in the absence of social interaction, receive the item if it attempted to produce the label. The pulley was controlled by a student in another room who monitored a parrot’s utterances through headphones. We taped sessions to test (inter)observer reliability.
To test the effects of total absence of reference, context, and social interaction, I exposed the juveniles to audiotapes (Pepperberg 1994b). Audiotapes consisted of the audio portion of a basic videotape presentation, and thus paralleled M/R and video procedures but eliminated all reference and context. Juveniles received ‘basic audiotape’ sessions in social isolation, and no objects or actions were associated with the sounds presented over the speaker. Note that this procedure replicated the early studies of song learning in birds in social isolation (e.g. Marler 1970).
Experiments with Alo and Kyaaro
Each bird was trained on English labels under several conditions (Pepperberg 1994b, Pepperberg & McLaughlin 1996; Pepperberg et al. 1998). I chose labels that Alex could produce (Pepperberg 1981, 1990a) to ensure that the vocalisations were within the capacity of the species. I also chose objects in which the birds had previously demonstrated interest so that differential motivation to obtain an item would not affect label acquisition (see Pepperberg et al. 1991). Each bird received labels in M/R, M/R-variant 2, basic video, video-variant 1, video-variant 2, and audiotape sessions. Neither juvenile received M/R-variant 1 training.
I counterbalanced labels, so that, with some exceptions, labels used for one bird with one technique were used for the other bird with another technique. Alo, for example, was trained on ‘cork’ in M/R sessions, whereas Kyaaro initially had ‘cork’ in basic video. Both birds, however, were trained on ‘paper’ via live tutors, on ‘rock’ via audiotape, and on ‘key’ and ‘block’ in M/R-variant 2 to compare their speeds of learning. For each bird, I repeated a label from the basic video in video-variant 1 to test for co-viewers effects, and repeated some labels (e.g. ‘cork’) not learned from non-basic M/R procedures in subsequent M/R sessions (Pepperberg et al. 1998).
Alo's results
Alo never clearly produced, in the presence of trainers, any labels trained via M/R-variant 2 (key, block), basic or video-variant 2 (wood, nail [initial training], chalk, chain) or audio (key, rock) training (Pepperberg 1994b, Pepperberg & McLaughlin 1996; Pepperberg et al. 1998). Tapes of solitary sound productions also revealed a total lack of ‘practice’ (see Pepperberg et al. 1991) of these labels, in contrast to the frequent practice of labels trained in M/R sessions (Pepperberg 1994b; Pepperberg & McLaughlin 1996). Alo did attempt to produce ‘nail’, trained during video-variant 1, but failed to identify the object or produce an approximation of the correct label on formal tests for labels trained in this and all other non-basic M/R procedures. In contrast, for labels taught via the basic M/R procedure, first trial test scores were 34/40 for both cork and paper, 35/40 for truck, and 38/40 for wool (Pepperberg 1994b; Pepperberg & McLaughlin 1996; Pepperberg et al. 1998).
Kyaaro's results
Kyaaro also did not produce, either in the presence of trainers or in private practice, labels he experienced via audio (wood, rock) or any form of video training (cork [initial training], truck [initial training], key, block, chalk, bear) except video-variant 1 (truck). He attempted to produce labels taught via the M/R technique (paper, nail), but, at the end of his first 11 months, ran them together (‘ail-er’) in a manner too difficult to distinguish by trainers for testing (Pepperberg 1994b). He did, however, produce clearly differentiated versions of nail and paper during private practice. After additional training, his labels were at criterion for testing (see Pepperberg 1981 for a discussion of such criteria). On identification tests for items trained under non-basic M/R procedures and video-variant 1, Kyaaro scored 0 on every trial. For labels taught via the basic M/R procedure, first trial test scores were 34/40 for paper and 35/40 for nail, wood, wool, and cork (Pepperberg 1994b; Pepperberg & McLaughlin 1996; Pepperberg et al. 1998).
Experiments with Alex
Results with the juveniles can be compared with previous work with Alex. As noted earlier, Alex learned to produce and comprehend many labels and concepts via basic M/R training (e.g. Pepperberg 1990a,b, 1992b, 1994b). Would a bird that had previously succeeded under M/R training fail under conditions that lacked certain aspects of input?
Alex thus had M/R-variant 1 training. He was given a sequence of eight number labels that were trained without reference either to specific objects in the laboratory or to his previously acquired English number labels (Pepperberg 1994b, 1997). These labels were part of a study on ordinality, counting, and serial learning (Pepperberg & Silverstone, unpubl. ms). The set, il ee bam ba oo yuk chil gal, was derived from Korean count labels both to permit comparisons with children (Fuson 1988) and to be maximally different from English. Bam (pronounced ‘baem’) and ba were substituted for the Korean sam and sa because of Alex’s occasional difficulty in producing ‘ss’.
Although we attempted to train Alex in the total absence of reference, he would not attend to sessions until we included a minimal point of reference: a sheet of paper with the symbols 1-8 traced along the diagonal (NB: He did not know that his English number labels corresponded to these symbols; Pepperberg 1994b). In a typical session, the human acting as trainer held the paper up to the model and stated, ‘Say number!’; all previous quantity-related queries were ‘How many?’ The model produced the altered Korean labels and was allowed to request a toy or food, or erred and was scolded. As in basic M/R sessions, we reversed roles of model and trainer; Alex was also given a turn. Although we usually reward Alex either with the object that he has labelled or the opportunity to request a favoured item (‘I want X’, Pepperberg 1987a), here we used only the latter reward. Training, therefore, lacked the usual functional meaning and all but minimal referentiality. The procedure did, however, maintain joint attention between bird, humans, and the pictured numbers.
Alex eventually learned the modelled string of vocal labels, although he insisted on producing nuk instead of yuk. His results, however, differed from those of previous studies in two important ways. First, acquisition took an unusually long 9 months (Pepperberg 1981, 1994b; Pepperberg & Silverstone, unpubl. ms). Second, and most striking, was that he could not immediately use, nor subsequently learn to use, these labels referentially, i.e., for either serial labelling or quantity. Even after we modelled 1:1 correspondences between 8 objects and the string of labels, he could not use elements in the string to refer to smaller quantities, e.g. to say ‘il ee bam ba’ when shown 4 items and asked to ‘Say number’. He had learned to produce, but not comprehend the use of, these human vocalisations (Pepperberg 1994b). Given his previous successful production and comprehension of human labels after M/R training (e.g. Pepperberg 1990b, 1992b, 1994b), his failure was likely a consequence of the training and not a general lack of cognitive capacity (Pepperberg 1994a,b).
An Explanation of Our Findings
Clearly, social interaction, reference, and full functional meaning are all important factors in learning to produce and comprehend an allospecific code, even for a mimetic parrot (Pepperberg 1994b, Pepperberg & McLaughlin 1996, Pepperberg et al. 1998). Absence of some of these factors affects whether and what type of acquisition occurs, and one of two outcomes is likely: (a) birds fail to learn because they lack adequate information to be processed (i.e. to direct the learning process), or (b) the impoverished input corrupts the birds’ processing abilities, causing them learn in ways that would be aberrant in the wild (e.g. they learn to reproduce sounds but not in a meaningful manner). How the absence of various factors specifically affects a bird’s behaviour can be described in detail.
Birds failed to learn anything from audiotapes, which lacked reference, functionality, and social interaction (Pepperberg 1994b, 1997). The juveniles did not attempt to reproduce the human sounds while they listened to the tapes, nor subsequently in private practice, in the presence of trainers, nor on identification tests. Possibly they treated the taped human sounds as background noise, maybe corresponding to what would be environmental sounds in the wild. Although environmental noise might have some referential meaning relevant to their lives, audiotape sessions gave birds no opportunity to deduce explicit meanings for the sounds they heard nor demonstrated any purpose for which the sounds could be used: Little information existed to process. Too, their response to the sounds had no effect on what they subsequently heard or received, either vocally or physically. Did the birds engaged in a cognitive evaluation of the situation and ‘tune out’ the sounds because they lacked information to process, or fail to master the labels because no detectable outcome existed between a stimulus and their response? In either case they had no reason to acquire the sounds. Their total lack of vocal learning suggests the former rather than the latter mechanism. If the latter mechanism had occurred, birds would likely have attempted to learn the sounds, possibly because the sounds simply reminded them of other types of interactions with humans (Mowrer 1954) or because an arbitrary connection could initially have been made with some irrelevant cue; the subsequent lack of effect of their vocalisations on their situation would have then extinguished the behaviour. The overall effect would be no net learning, but we would have detected practice. Had the birds engaged in other types of cognitive processing, they might have transferred skills from M/R sessions to audio sessions: Remembering that other human vocalisations were presented in conjunction with objects in M/R sessions, birds might have attended to the tapes without overt learning but then made some association between the novel sound and the novel object subsequently presented to them in testing. They might then have attempted to produce the targeted label. They did not, however, behave in any of these ways (Pepperberg 1994b, 1997, 1998).
Birds attended to, but also failed to learn from, videos (Pepperberg 1994b, 1997). Failure to learn in the basic video condition, which included reference and limited meaning in the absence of interaction, may have occurred for at least three reasons: (1) Birds failed to realise that what they saw could be transferred to their own situation; (2) birds could not determine what aspect of Alex’s behaviour caused transfer of the desired objects; or (3) birds stopped responding because they received no encouragement for what could have been their first attempts at the targeted vocalisation. The first two reasons suggest that inadequate information was available for processing; the third reason suggests that a potential association either did not occur or was extinguished. That the birds also failed in video-variant 2, where reward was possible, shows that the third factor was irrelevant: No attempts occurred that could have been rewarded (Pepperberg et al. 1998). Clearly, watching another individual receive objects for producing particular sounds provided insufficient information for acquisition. Even video-variant 1, where a trainer directed birds’ attention to onscreen actions, was unsuccessful: Although each bird did attempt to produce the labels, neither learned what these labels represented or how these labels could be used outside of training (Pepperberg et al. 1998).
The contrast between Alex’s M/R-variant 1 versus basic M/R training data shows that social interaction along with severely limited functionality and meaning engender, at best, allospecific vocal production without comprehension (Pepperberg 1994b). In M/R-variant 1, Alex was rewarded merely for making certain sounds to a specific cue. He had no reason to work towards understanding what he was saying or information about the meaning of his utterances. Subsequently, he could not transfer his learned behaviour to related situations (Pepperberg 1994b, 1997, 1998). Such results suggest his learning involved only the simplest form of processing, i.e., associating a situation and reward, and possibly recognising that no additional processing was needed. Such training represents that of most pet birds, and explains why parrots were once thought capable of only mindless mimicry (e.g. Lenneberg 1973). Pet birds did what was necessary for reward (be it the attention of their owners or food), but no more. In the wild, however, reward for such specific associations is unlikely and such learning improbable; otherwise many instances of random psittacine mimicry would have been recorded. Learning under M/R-variant 1 thus was plausibly an artifact of captivity.
I have not studied the effect of social interaction and functional input lacking reference, but such training will likely engender production without comprehension. This condition is the case for pet birds that, for example, appropriately produce greeting or farewell routines (‘Good night dear,’ ‘Goodbye, and thank you’; Amsler 1947). These birds have a more general sense of situations in which to use their vocalisations than do birds taught without functionality, but do not comprehend individual words in their routines (Pepperberg 1997). They connect a situation and utterance with reward (attention) from their owners, but have experienced a condition that does not occur in nature.
Given the M/R-variant 2 data, I suggest that Grey Parrots are also unlikely to acquire or comprehend elements of an allospecific code from input that is referential, fully functionally meaningful, but noninteractive (see Pepperberg 1997). Thus, the presence or absence of a reward item is less important than the presence or absence of social interaction. In M/R training, for example, reward is not likely a critical factor for acquisition because a bird is rewarded only after it attempts the targeted label, i.e., after some acquisition has occurred (Pepperberg 1981, 1994b). Rewards primarily reinforce referentiality.
Overall, I have sought to determine conditions that enable Grey Parrots to acquire a referential, allospecific communication code. Although mimetic birds are characterised by their extensive capacities to acquire allospecific vocalisations, Grey Parrots (at least) seem to acquire a meaningful allospecific code most readily under certain conditions. Various conditions remain to be tested (e.g. reference and full functionality in the absence of social interaction; reference and limited functionality with either full or limited interaction), but input that is fully referential, functional, and socially interactive ensures that Greys will both produce and eventually comprehend allospecific vocalisations (Pepperberg 1987a,b, 1990b, 1992b, 1994b, 1997, 1998). Lack of some or all of these aspects appears to prevent a Grey Parrot from acquiring meaningful allospecific communication.
CONCLUSION
All my experiments involve laboratory subjects. Can my work be related to conditions in the real world, particularly with respect to referentiality and constraints on acquisition? Furthermore, does a cognitive approach help us understand the data?
We do not yet know if Grey Parrots use referential vocalisations in the wild. Limited data for other psittacids on duetting, sentinel behaviour, and individual recognition (review in Pepperberg 1994b), however, suggest that referentiality may indeed exist. Too, I believe that a Grey Parrot’s acquisition of referential communication in the laboratory (e.g. Pepperberg 1990a, 1992b, 1994b) is likely only if based on a pre-existent cognitive architecture (Premack 1983; Rice 1980) involving perception, memory, and communicative intent (Pepperberg 1997, 1998; Pepperberg et al. 1998).
If constraints found in the laboratory (i.e., that input be referential, functional, and socially interactive) exist in the wild, an intriguing scenario emerges (Pepperberg 1997, 1998; Pepperberg & McLaughlin 1996). Unconstrained mimicry would be maladaptive. Mimicry must thus be flexible to allow for producing a range of sounds and constrained enough to be useful. What evolutionary pathway opted for widespread mimicry in parrots yet constrained it to appropriate situations? Do specific mechanisms exist that constrain mimicry to meaningful vocalisations? Something like an innate sound detector or filter could be too limiting and lack flexibility to change with varying environmental conditions. What if, instead, psittacine allospecific vocal learning occurred only in conjunction with cognitive choice or processing that relied upon meaning, function, and interaction?
Such a cognitive mechanism could not be based only on meaning and function, for it would lack selectivity; i.e., too many opportunities exist for acquisition based on associating a sound and an object or action. Of course, sound-action-object associations are not irrelevant to allospecific vocal learning. Researchers on human language acquisition argue that to learn language, children must first perceive the incoming signals and represent both the structural and informational content of these signals (Chomsky 1965; Morgan & Demuth 1996). A similar argument can be made for avian song acquisition (Pepperberg 1998). Such a prescription for acquisition, however, defines what is necessary but not sufficient. It is not difficult to imagine how environmental sounds - e.g. rustling of leaves - might be processed for their structure, their arrangement, and their information content - the approach of a predator - and one might further hypothesise a functional use for mimicking such sounds: deceiving a sympatric bird into taking cover and missing out on a food source (Munn 1986; Pepperberg 1997, 1998; Pepperberg et al. 1998). Similarly, water is vital to survival, and parrots could easily associate the sound of a stream with the act of searching for or reporting the presence of water. Yet, to my knowledge, mimicry of such sounds such has not been recorded in wild parrots.
But birds, like children, appear to use social interaction to learn how to link vocalisations they hear with the correct objects, events, and properties in the world and, most importantly, need social interaction to direct their attention to what is worthy of learning (Bruner 1977; Dore 1980; Baldwin 1995; Pepperberg & McLaughlin 1996). Children develop ‘denotive symbols’ (intentional communication involving specific referents) from ‘indexical signs’ (intentional communication without such referents) through social, interactive dialogs that explicitly demonstrate both reference and function (Dore 1980). If a subject cognitively processes the social interactions to sort out the different facets that have been observed, a mechanism involving sound/object/action and social interaction could be maximally adaptive. In the above example, lack of social interaction would signal reproduction of the rustling of leaves as maladaptive compared to, for example, mimicry of actual alarm calls (i.e., rustling may have many implications whereas an alarm call has far fewer; Munn 1986). For children, mere temporal contiguity of a label and object is insufficient for label acquisition (e.g. Baldwin 1991); children apparently use lack of social interaction as a specific nonvocal cue to inhibit mapping of label to object (Baldwin 1993). For children such a strategy is adaptive: 30-50% of mothers’ labels do not correspond to the object a child is in the process of viewing (Collis 1977; Harris et al. 1983); thus using temporal contiguity of label and object without social interaction to establish reference could cause numerous mapping errors. Given our results for M/R-variant 2 training (Pepperberg & McLaughlin 1996), I suggest a similar case is likely for Grey Parrots, particularly given the often dense foliage in which they live (Pepperberg 1998).
And, although devising a scenario is possible in which sound/object/action/interaction engenders vocal learning in the absence of cognitive processing, such is unlikely for Grey Parrots. Arguably, a combination of cues (e.g. hormonal levels, amount of daylight, a live tutor) might enable allospecific vocal learning (e.g. Baptista & Petrinovich 1994, 1996) without invoking a need for cognitive processing. Here student birds needn’t sort out different facets of their interaction with the live tutor, nor determine the referent or the functionality of the tutor’s vocalisations; students are simply primed to learn and, because a live tutor focuses their attention, they use the information presented by the tutor (its song) as a model; how ‘well’ they then use their learned vocalisations has not been carefully examined. Previously, I stressed that allospecific vocal learning requires input that includes reference, functionality, and social interaction (e.g. Pepperberg 1985, 1986, 1990a, 1994b; Pepperberg & McLaughlin 1996; Pepperberg et al. 1998). I did not, however, examine underlying mechanisms that may be involved for mimetic parrots. These mechanisms become apparent only if I determine when cognitive processing, rather than simpler information processing or associative learning, is required. I now suggest that mimetic parrots must cognitively process all the elements of the input, including social interaction, to acquire allospecific utterances and use the acquired vocalisations appropriately.
Although social interaction is likely critical for determining what is appropriate to learn (even for parrot-parrot interactions), such interaction requires cognitive processing to assess not only the nature of the interaction, but also whether the input is worthy of processing. Humans assess whether input comes from a dominant individual, and decide if status or acceptance may be gained through specific behavioural reproduction (Bandura 1977); some positive correlation exists between high model status and rate and amount of acquisition of modeled behaviour (Mischel & Liebert 1967). Thus social interaction in humans may facilitate or modify the course of development, and passerine birds may also use such a strategy (e.g. Payne & Groschupf 1984); parrots could respond similarly. (Note that social interaction might facilitate development of dialects, which have been observed in Amazon parrots [Nottebohm 1972; Nottebohm & Nottebohm 1969; Schindlinger 1995; Wright 1996] and which might help maintain flock identity. One wonders if the dialects include allospecific vocalisations of particular, limited-range sympatric species to identify different dialect groups.)
Although flexibility in use of allospecific vocalisations has not been documented in Grey Parrots in the wild, evidence, although limited, exists in the laboratory for Alex. Alex was trained to use the term ‘none’ to report if nothing was same or different with respect to two exemplars, i.e., to report if objects were identical or completely different with respect to colour, shape, and material (Pepperberg 1988). In a subsequent study involving questions concerning relative size (Pepperberg & Brezinsky 1991), Alex was, without prior training, given two objects of identical size and asked to report which was bigger or smaller. He initially asked ‘What’s same?’, and then responded to our repeated question with ‘none’. Such flexibility suggests the use of cognitive processing to determine elements of correspondence in the trained and untrained situations.
Interestingly, cues that I propose that wild parrots use for choosing what they are to learn are almost always subverted in captivity, i.e., adapted in highly peculiar ways. The pet parrot, for example, hears the microwave ‘beep’, sees its owners rush to the source of the signal, and processes the possibility that by reproducing this beeping noise it, too, can attract the members of its new ‘flock’. Alternatively, vocal input given a pet bird often lacks explicit, consistent reference, and thus the bird likely acquires some rote ability to emit vocalisations it has heard, but is unable to use the code productively and lacks true comprehension. The cognitive process cannot work appropriately because the relevant information is lacking, and the bird falls back on an associative mechanism. And if input lacks functionality, the subject is unaware of the pragmatics of the vocalisations it has acquired, i.e., will lack knowledge of when and how to use the utterances meaningfully, particularly in novel situations (see above; Pepperberg 1997, 1998). The pet parrot’s cognitive process are subverted to enable the bird to make some form of contact with its allospecific owners, who likely respond to any human vocalisation the bird uses.
Finally, if cognitive processing and social interaction are both needed for appropriate allospecific learning in wild parrots, another intriguing correlate appears for the observed behaviour. Remember, Rozin (1976) defined intelligence as flexibility in transferring skills acquired in one domain to another. Humphrey (1976) proposed that intelligence (and presumably the need for cognitive processing) was a correlate of having a complicated social system and a long life; i.e., that intelligence was the outcome of a selection process favouring animals that could remember and act upon knowledge of detailed social relations among group members. How these two patterns might drive parrot vocal behaviour seems obvious; i.e., long-lived birds in complex social systems use abilities honed for social gains to direct vocalisations. I suggest, however, a somewhat different scenerio: That intelligence was an evolutionary outcome of the need not only for memory and flexibility, but also for choosing what to ignore as well as what to process. Social primates also likely actively engage in such choice; an animal that could not form a hierarchy of information would be unable to act. Only in parrots, however, can we clearly see, by what is vocally reproduced and what is not, the outcome of such choices.
In conclusion, learning how a particular species comes to acquire and use allospecific sounds engenders more questions than answers. My students and I have studied Grey Parrots; how do they compare to other psittacids? Certainly an increased or decreased capacity for accurate sound reproduction would influence how vocal learning is used. Might additional data weaken the hypothesis that cognitive processing and social interaction are critical factors in vocal learning? Clearly, no shortage exists with respect to topics for further study, and for the possibility of extending these studies to other mimetic species.
ACKNOWLEDGEMENTS
The research reported here has been supported by NSF grants BNS 91-96066 and IBN 92-21941, REU supplements, the University of Arizona Undergraduate Biology Research Program, and The Alex Foundation.
REFERENCES
Adret, P. 1993. Vocal learning induced with operant techniques: An overview. Netherlands Journal of Zoology 43: 125-142.
Amsler, M. 1947. An almost human Grey Parrot. Aviculture Magazine 53: 68-69.
Baldwin, D.A. 1991. Infants’ contributions to the achievement of joint reference. Child Development 62: 875-890.
Baldwin, D.A. 1993. Early referential understanding: Infants’ ability to recognise referential acts for what they are. Developmental Psychology 29: 832-843.
Baldwin, D.A. 1995. Understanding the link between joint attention and language. In: Moore, C.& Dunham, P.J. (eds) Joint attention: its origin and role in development. Hillsdale, NJ; Erlbaum: 131-158.
Bandura, A. 1971. Analysis of modeling processes. In: Bandura, A. (ed.) Psychological modeling. Chicago; Aldine-Atherton: 1-62.
Bandura, A. 1977. Social modeling theory.Chicago; Aldine-Atherton.
Banta, P.A. 1998. Neurobiology of the Budgerigar vocal control system: Effects of lesions on natural vocalisations and acquired English utterances. PhD Thesis, University of Arizona, Tucson, Arizona.
Baptista. L.F. & Gaunt, S.L.L. 1994. Advances in studies of avian sound communication. Condor 96: 817-830.
Baptista, L.F. & Petrinovich, L. 1984. Social interaction, sensitive phases, and the song template hypothesis in the White-crowned Sparrow. Animal Behaviour 32: 172-181.
Baptista, L.F. & Petrinovich, L. 1986. Song development in the White-crowned Sparrow: social factors and sex differences. Animal Behaviour 34: 1359-1371.
Bruner, J.S. 1977. Early social interaction and language acquisition. In Schaffer, H.R. (ed.) Studies in mother-infant interaction. London; Academic Press: 271-289.
Chomsky, N. 1965. Aspects of the theory of syntax. Cambridge, MA; MIT Press.
Collis, G.M. 1977. Visual co-orientation and maternal speech. In Schaffer, H.R. (ed) Studies in mother-infant interaction. London; Academic Press: 355-375.
Cruickshank, A. J., Gautier, J.-P. & Chappuis, C. 1993. Vocal mimicry in wild African Grey Parrots Psittacus erithacus. Ibis 135: 293-299.
de Villiers, J.G. & de Villiers, P.A. 1978. Language acquisition. Cambridge, MA; Harvard University Press.
Dore, J. 1980. Holophrases revisited: their logical development from dialog. In Barrett, M. (ed) Children’s single word speech. New York; John Wiley & Sons: 23-58.
Fuson, K. 1988. Children’s counting and concepts of number. New York; Springer-Verlag.
Goldstein, H. 1984. The effects of modeling and corrected practice on generative language and learning of preschool children. Journal of Speech and Hearing Disorders 49: 389-398.
Gramza, A.F. 1970. Vocal mimicry in captive budgerigars (Melopsittacus undulatus). Zeitschrift für Tierpsychologie 27: 971-983.
Harris, M., Jones, D. & Grant, J. 1983. The nonverbal context of mothers’ speech to infants. First Language 4: 21-30.
Hensley, G. 1980. Encounters with a hookbill-II. American Cage Bird Magazine 52: 11-12, 59.
Humphrey, N.K. 1976. The social function of intellect. In Bateson, P.P.G. & Hinde, R.A. (eds) Growing points in ethology. Cambridge, UK; Cambridge University Press: 303-317.
Kamil, A.C. 1988. A synthetic approach to the study of animal intelligence. In Leger, D (ed) Nebraska symposium on motivation: comparative perspectives in modern psychology. Vol. 7; Lincoln, NE; University of Nebraska Press: 257-308.
King, A.S., Freeberg, T.M. & West, M.J. 1996. Social experience affects the process and outcome of vocal ontogeny in two populations of cowbirds (Molothrus ater). Journal of Comparative Psychology 110: 276-285.
Lemish, D. & Rice, M.L. 1986. Television as a talking picture book: a prop for language acquisition. Journal of Child Language 13: 251-274.
Lenneberg, E.H. 1967. Biological foundations of language. New York; John Wiley & Sons.
Lenneberg, E.H. 1973. Biological aspects of language. In Miller, G.A. (ed) Communication, language, and meaning. New York: Basic Books: 49-60.
Lieberman, P. 1984. The biology and evolution of language. Cambridge, MA; Harvard University Press.
Marler, P. 1970. A comparative approach to vocal learning: Song development in white-crowned sparrows. Journal of Comparative and Physiological Psychology 71: 1-25.
Mischel, W. & Liebert, R.M. 1967. The role of power in the adoption of self-reward patterns. Child Development 38: 673-683.
Morgan, J.L. & Demuth, K. 1996. Signal to syntax: an overview. In Morgan, J.L. & Demuth, K. (eds) Signal to syntax: bootstrapping from speech to grammar in early acquisition. Mahwah, NJ; Erlbaum: 1-22.
Mowrer, O.H. 1954. A psychologist looks at language. American Psychologist 9: 660-694.
Mowrer, O.H. 1958. Hearing and speaking: An analysis of language learning. Journal of Speech and Hearing Disorders 23: 143-152.
Munn, C.A. 1986. The deceptive use of alarm calls by sentinel species in mixed-species flocks of neotropical birds. In Mitchell, R.W. & Thompson, N.S. (eds) Deception: Perspectives on human and nonhuman deceit., Albany, NY; SUNY Press: 169-175.
Nottebohm, F. 1972. The origins of vocal learning. American Naturalist 106: 116-140.
Nottebohm, F. & Nottebohm, M. 1969. The parrots of Bush-Bush. Animal Kingdom: 19-23.
Patterson, D.K. & Pepperberg, I.M. 1994. A comparative study of human and parrot phonation: Acoustic and articulatory correlates of vowels. Journal of the Acoustical Society of America 96: 634-648.
Payne, R.B. & Groschupf, K.D. 1984. Sexual selection and interspecific competition: a field experiment on territorial behavior of nonparental finches (Vidua spp.). Auk 101: 140-145.
Pepperberg, I.M. 1981. Functional vocalisations by an African Grey Parrot (Psittacus erithacus). Zeitschrift für Tierpsychologie 55: 139-160.
Pepperberg, I.M. 1983. Cognition in the African Grey Parrot: Preliminary evidence for auditory/vocal comprehension of the class concept. Animal Learning & Behavior 11: 179-185.
Pepperberg, I.M. 1985. Social modeling theory: A possible framework for understanding avian vocal learning. Auk 102: 854-864.
Pepperberg, I.M. 1986a. Acquisition of anomalous communicatory systems: Implications for studies on interspecies communication. In Schusterman, R., Thomas, J. &Wood, F. (eds) Dolphin behavior and cognition: comparative and ecological aspects. Hillsdale, NJ; Erlbaum: 289-302.
Pepperberg, I.M. 1986b. Sensitive periods, social interaction, and song acquisition: the dialectics of dialects? Behavioral and. Brain Sciences 9: 756-757.
Pepperberg, I.M. 1987a. Evidence for conceptual quantitative abilities in the African Grey Parrot: Labeling of cardinal sets. Ethology 75: 37-61.
Pepperberg, I.M. 1987b. Acquisition of the same/different concept by an African Grey Parrot (Psittacus erithacus): Learning with respect to color, shape, and material. Animal Learning & Behavior 15: 423-432.
Pepperberg, I.M. 1988. Comprehension of ‘absence’ by an African Grey Parrot: Learning with respect to questions of same/different. Journal of the Experimental Analysis of Behavior 50: 553-564.
Pepperberg, I.M. 1990a. Some cognitive capacities of an African Grey Parrot (Psittacus erithacus). In Slater, P.J.B., Rosenblatt, J.S. & Beer, C. (eds) Advances in the study of behavior. Vol. 19; New York; Academic Press: 357-409.
Pepperberg, I.M. 1990b. Cognition in an African Grey Parrot (Psittacus erithacus): Further evidence for comprehension of categories and labels. Journal of Comparative Psychology 104: 41-52.
Pepperberg, I.M. 1990c. Referential mapping: A technique for attaching functional significance to the innovative utterances of an African Grey Parrot (Psittacus erithacus). Applied Psycholinguistics 11: 23-44.
Pepperberg, I.M. 1992a. What studies on song learning can teach us about playback experiments. In McGregor, P.K. (ed) Playback and animal communication: problems and prospects. New York; Plenum Press: 47-57.
Pepperberg, I.M. 1992b. Proficient performance of a conjunctive, recursive task by an African Grey Parrot (Psittacus erithacus). Journal of Comparative Psychology 106: 295-305.
Pepperberg, I.M. 1993. A review of the effects of social interaction on vocal learning in African Grey Parrots (Psittacus erithacus). Netherlands Journal of Zoology 43: 104-124.
Pepperberg, I.M. 1994a. Evidence for numerical competence in an African Grey parrot (Psittacus erithacus). Journal of Comparative Psychology 108: 36-44.
Pepperberg, I.M. 1994b. Vocal learning in Grey Parrots (Psittacus erithacus): Effects of social interaction, reference, and context. Auk 111: 300-313.
Pepperberg, I.M. 1997. Social influences on the acquisition of human-based codes in parrots and nonhuman primates. In Snowdon, C.T. & Hausberger, M. (eds) Social influences on vocal development. Cambridge; Cambridge University Press: 157-177.
Pepperberg, I.M. 1998. The African Grey parrot: how cognitive processing might affect allospecific vocal learning. In Balda, R., Pepperberg, I.M. & Kamil, A.C. (eds) Animal cognition in nature. London; Academic Press.
Pepperberg, I.M. & Brezinsky M.V. 1991. Acquisition of a relative class concept by an African Grey Parrot (Psittacus erithacus): Discriminations based on relative size. Journal of Comparative Psychology 105: 286-294.
Pepperberg, I.M. & McLaughlin, M.A. 1996. Effect of avian-human joint attention on allospecific vocal learning by Grey Parrots (Psittacus erithacus). Journal of Comparative Psychology 110: 286-297.
Pepperberg, I.M., Brese, K.J. & Harris, B.J. 1991. Solitary sound play during acquisition of English vocalisations by an African Grey Parrot (Psittacus erithacus): Possible parallels with children’s monologue speech. Applied Psycholinguistics 12: 151-178.
Pepperberg, I.M., Naughton, J.R. & Banta, P.A. 1998. Allospecific vocal learning by Grey parrots (Psittacus erithacus): A failure of videotaped instruction under certain conditions Behavioural Processes zz: xx-yy.
Price, P.H. 1979. Developmental determinants of structure in Zebra Finch songs. Journal of Comparative and Physiological Psychology 93: 260-277.
Premack, D. 1983. The codes of man and beast. Behavioral and Brain Sciences 6: 125-167.
Rice, M. 1980. Cognition to language: Categories, word meanings, and training. Baltimore, MD; University Park Press.
Rozin, P. 1976. The evolution of intelligence and access to the cognitive unconscious. In Spragues, J.M & Epstein, A.N. (eds) Progress in psychobiology and physiological psychology. Vol. 6; New York; Academic Press: 245-280.
Rowley, I. 1980. Parent-offspring recognition in a cockatoo, the Galah, Cacatua roseicapilla. Australian Journal of Zoology 28: 445-456.
Rowley, I. & Chapman, G. 1986. Cross-fostering, imprinting and learning in two sympatric species of cockatoo. Behaviour 96: 1-16.
Rutledge, D. & Pepperberg, I.M. 1988. Video studies of same/different. Unpublished data.
Saunders, D.A. 1983. Vocal repertoire and individual vocal recognition in the short-billed white-tailed Black cockatoo, Calyptorhynchus funereus latirostris. Carnaby. Australian Wildlife Research 10: 527-536.
Schindlinger, M.D. August, 1995. The evolution of vocal repertoires in parrots: Evidence from the wild. Paper presented at the XXIVth International Ethological Congress, Honolulu, Hawaii.
Silverstone, J.L. 1989. Numerical abilities in the African Grey Parrot: Sequential numerical tags. Senior Honors Thesis, Northwestern University, Evanston, Illinois.
Slater, P.J.B. 1991. Learned song variations in British storm-petrels? Wilson Bulletin 103:515-517.
Slater, P.J.B., Eales, L.A. & Clayton, N.S. 1988. Song learning in Zebra Finches (Taeniopygia guttata): Progress and prospects. In Rosenblatt, J.S., Beer, C., Busnel, M-C. & Slater, P.J.B. (eds) Advances in the Study of behavior. Vol 18; New York; Academic Press: 1-34.
Smith, W.J. 1991. Animal communication and the study of cognition. In Ristau, C.A. (ed.) Cognitive ethology: the minds of other animals. Hillsdale, NJ; Erlbaum: 209-230.
St. Peters, M., Huston, A.C. &Wright, J.C. April, 1989. Television and families: parental coviewing and young children’s language development, social behavior, and television processing. Paper presented at the Society for Research in Child Development, Kansas City, KS.
Todt, D. 1975. Social learning of vocal patterns and modes of their applications in Grey Parrots. Zeitschrift für Tierpsychologie 39: 178-188.
Vanayan, M., Robertson, H.A. & Biederman, G.B. 1985. Observational learning in pigeons: The effects of model proficiency on observer performance. Journal of General Psychology 112: 349-357.
Wright, T. 1996 Regional dialects in the contact call of a parrot. Proceedings of the Royal Society of London - Series B: Biological Sciences 263: 867-872.
Zentall, T.R. 1993. Animal cognition: An approach to the study of animal behavior. In Zentall, T.R. (ed.) Animal cognition: A tribute to Donald A. Riley. Hillsdale, NJ; Erlbaum: 3-15.
Table 1. Components and Results of Tutoring (after Pepperberg, 1997)
