S02.5: Neuromuscular correlates to the functional reorganisation for flight of the M. Supracoracoideus.
George. E. Goslow, Jr., Donald S. Wilson & Samuel O. Poore
Department of Ecology and Evolution, Brown University, Providence, Rhode Island, 02912, USA, fax:401 863 7544 e-mail ted_goslow@brown.edu
Goslow, G.E., Jr., Wilson, D.S., & Poore, S.O. 1999. Neuromuscular correlates to the functional reorganisation for flight of the M. Supracoracoideus. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
124-136. Johannesburg: BirdLife South Africa.The techniques of electromyography, anatomical tracers, and histochemistry in the hands of vertebrate morphologists have provided a new opportuntity for the investigation of some long-standing questions relating to the evolution of Avian musculoskeletal systems. The neuromotor pattern (i.e., the onset/offset of muscle contractions within the locomotor cycle) is conserved for some homologous muscles of the tetrapod shoulder but not for others, in the transition from terrestrial locomotion to flight. We selected the M. supracoracoideus in the European Starling Sturnus vulgaris, a passerine bird capable of powered flight, to study the non-conserved pattern. During the evolution of birds the tendon of insertion of the M. supracoracoideus migrated from a ventral to a dorsal attachment on the humerus, and when compared to its homologous muscle(s) in reptiles and mammals, its neuromotor pattern has shifted. At what morphological level(s) of neuromuscular organisation does such a change occur? To address this question for the M. supracoracoideus, we examined the position of its motoneuron pool in the cervical spinal cord and its muscle fibre morphology. Our data reveal the organisation of the motoneurons within the spinal cord has remained relatively conserved for this muscle, but muscle fibre composition has undergone extensive change when compared to non-avian tetrapods. These data suggest that synaptic changes of the motoneurons controlling the M. supracoracoideus are responsible for this muscle’s functional reorganisation in Aves.
INTRODUCTION
We are engaged in studies of the musculoskeletal and neuromuscular organisation of the wings of two model species of birds with contrasting wing morphologies and flight styles, the Common Pigeon Columba livia and European Starling Sturnus vulgaris, in order to study the evolution of powered flight. Beginning with the Late Jurassic form Archaeopteryx, a number of advanced skeletal reconfigurations of the shoulder can be seen which accompany the evolution of this extreme form of locomotion in birds (see for review, Chiappe l995; Padian and Chiappe l998). Recent discoveries of a series of Late Jurassic and Early Cretaceous fossil birds, for example, reveal several features of reorganisation which include elongation of the coracoids, scapulae aligned with the vertebral column which join the coracoid at an acute angle, and a keeled sternum.
Coincident with these alterations, some muscles changed only slightly in their morphology, whereas others underwent extensive reorganisation to accomplish the wing beat cycle. A keeled sternum in modern birds, for example, enables the M. pectoralis (pars thoracicus) to produce most of the power during the downstroke required for lift and thrust (Biewener et al. l992; Dial & Biewener l993). The keel also provides for the origin of the M. supracoracoideus, a muscle with a highly derived morphology familiar to most avian biologists. The marked anatomical reconfiguration from its primitive anteroventral insertion on the humerus in ancestral forms enables the supracoracoideus to impart a high-velocity rotation of the humerus about its longitudinal axis thought to be important for execution of the upstroke (Poore et al. l997a,b).
In order to more fully understand the structural and functional changes of the M. supracoracoideus and to determine the basis for its evolutionary transformations from a generalised tetrapod limb to a wing, we must understand the structural and functional properties of the locomotor system at several levels. For a series of muscles surrounding the shoulder we have documented the spatial relationships of their controlling motoneurons within the spinal cord and investigated the histochemical properties of their constituent muscle fibres. The patterns we have discovered for the M. supracoracoideus have particular relevance to our understanding of the evolution of locomotor diversity in tetrapods generally and to bird flight more specifically.
Background Studies
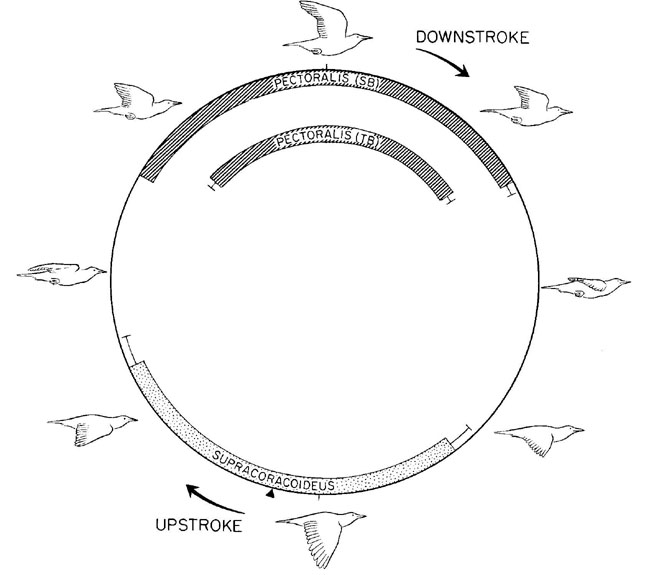
An initial study of European Starlings provides a framework for the present study (Dial et al. l991). The purpose of the study was to document the excursions of the wing skeletal elements by cineradiography and record the electrical activity patterns of eleven shoulder muscles of birds flying in a wind tunnel at a variety of speeds. Patterns of electrical activity, which reflect the neuronal activation of a muscle, primarily coincide with the transitional phases of the wingbeat cycle. The sternobrachialis (SB) and thoracobrachialis (TB) regions of the M. pectoralis, for example, are activated in late upstroke and remain active during the upstroke/downstroke transition to decelerate and reaccelerate the wing for the subsequent downstroke. In contrast, the M. supracoracoideus is activated in late downstroke, remains active during the downstroke/upstroke transition, and continues into early upstroke, a pattern necessary to decelerate and then reaccelerate the wing into the upstroke (Fig. 1). Of particular significance to this report is that intense activation of the M. supracoracoideus at this particular phase of the limb’s movement cycle is distinctly different from that of its homologous muscle(s) in all other terrestrial vertebrates for which there is comparable data (below). Thus this pattern in European Starlings reflects a change in the ordering or selection of motoneurons at the central nervous system level.
At what level of anatomical organisation do we find correlates to this evolutionary change in muscle action across species and within species? Here we report on the distribution within the spinal cord of the motoneurons innervating the M. pectoralis (SB and TB), and the M. supracoracoideus in European Starlings and provide a histochemical profile of each muscle. In this species capable of powered flight, the organisation of motoneurons has remained relatively conserved in the course of a fundamental change in the action of the M. supracoracoideus and its muscle fibre composition has undergone extensive inter-specific specialisation.
METHODS
Tracer Application for Motor Pool Identification
For study we selected ten adult European Starlings (70-90g) which we captured from a wild population near Fall River, Massachusetts. All experiments followed a protocol approved by the Brown University Animal Care and Use Committee. This Committee follows the rules for animal welfare mandated by the US National Institutes of Health. We anesthetised each bird (25 mg/kg ketamine; 2 mg/kg xylazine, I.M.) and administered an antibiotic (Amikacin sulfate, 0.015 mg/g, I.M.) daily for five days postoperatively. We surgically exposed the brachial plexus under aseptic conditions by a dorsal approach between the axial skeleton and the scapula and transected the M. latissimus dorsi and M. rhomboideus muscles. In all experiments, we labeled the motor pools of the entire M. pectoralis (pars thoracicus) or one of its two parts, the sternobrachialis (SB) and thoracobrachialis (TB), by dipping the muscle nerve into one of two tracers. The motoneurons constituting the ‘motor pool’ for the pectoralis served as a reference for the M. supracoracoideus pool. For the M. supracoracoideus we either followed the same procedure, or we exposed the muscle to the axonal tracer fast blue via multiple, small intramuscular injections.
After post-surgical survival times of 5-9 days, we anaesthetised the animal and immediately perfused through the left ventricle with 0.25 ml of 0.1% sodium nitrite followed by 100 ml of 0.9% heparinized saline. A fixative containing 2.5% paraformaldehyde and 0.5% glutaraldehyde in 200 ml of 0.1M phosphate buffer (pH 7.4) was introduced. We removed the spinal cord from spinal nerve levels 8-13 and placed it in 25% sucrose (at 4°C) in 0.1M phosphate buffer solution for 24 hrs. The cord was embedded in 5% agarose (37 – 38°C) and cut in serial cross sections of 100µm on a vibratome. We collected tissue sections in a cold (4°C) 0.1M phosphate buffer solution. We processed the spinal cord tissue with a modified version of Mesulam’s (l978) tetramethylbenzidine method, counterstained in neutral red, coverslipped with DPX mountent, and viewed with brightfield and fluorescent microscopy. We documented neurons and their corresponding spinal cord level with photographs.
Histochemistry
Following euthanasia of three birds, we removed the supracoracoideus in its entirety for histochemical analysis. We traced and measured the muscle and cut it into 10 cm blocks representative of its proximal, middle and distal regions, both anterior and posterior to its central tendon. Care was taken to preserve the tissue’s orientation relative to the muscle’s in vivo position. Blocks were frozen in isopentane at -160°C and stored at - 70°C. We cut cryostat cross-sections of 12µm thickness at -20°C and mounted them directly onto coverslips. We processed sections simultaneously for acid-stable myosin ATP-ase activity (preincubated at pH 4.4, 4.5, and 4.6), alkaline-stable myosin ATP-ase activity (preincubated at pH 10.4), NADH-diaphorase activity, and alpha-glycerophosphate dehydrogenase (alpha-GPD) activity. These markers provide an indirect assessment of contraction speed (Guth & Samaha l970), oxidative (aerobic) capacity (Novikoff et al. l961), and glycolytic (anaerobic) capacity (Wattenberg & Leong l960), respectively. We dehydrated each coverslip, covered it with permount, and mounted it on a slide.
We identified muscle fibres from photographic prints of the four stains. All fibres from the supracoracoideus possess alkaline-stable myosin ATP-ase activity and all fibres possess relatively high oxidative capacity. We categorised fibres by their intensity of reaction to alpha-GPD; those fibres in a sample field with the darkest reaction to this enzyme we identified as FG fibres regardless of cross-sectional area. In the majority of cases, when compared to FG fibres, the FOG fibres had the strongest reaction for NADH-D but a relatively less intense reaction to alpha-GPD. A small percentage of fibres in each muscle illustrate relatively strong reactions to both the oxidative and glycolytic stains; we identified these as FG fibres. For the most part, the fibres identified here as FG and FOG correspond to the ‘intermediate’ and ‘red’ fibres of Rosser & George (l986).
RESULTS
General Anatomy
A detailed myology of the shoulder of the European Starling is described by Dial et al. (l991) and the general anatomy of the Avian spinal cord and configuration of the brachial plexus are provided by Breazile & Yasuda (1979). The bipinnate supracoracoideus, which lies deep to the pectoralis, arises primarily from the lateral plate of the sternum and courses through the foramen triosseum before inserting onto the proximal humerus. A lateral view of the supracoracoideus provides the location of the histochemical samples discussed in this paper (Fig. 2)
Motor Pool Organisation
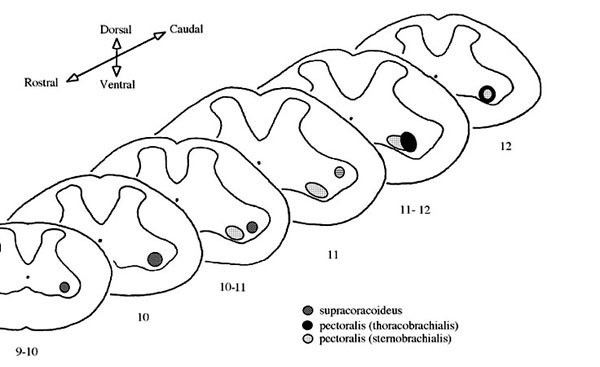
Along the rostro-caudal axis, the diameter of the spinal cord begins to increase at levels corresponding to spinal segments 10 to 12 . The increase corresponds to the brachial plexus which is derived from spinal nerves 10 - 12. As has been reported for the Domestic Fowl Gallus domesticus, the gray matter in this region segregates into a medial motor column and a lateral motor column (Huber l936; Leonard & Cohen l975). Ipsilateral nerve soaks of the SB and TB nerves labelled motoneuron cells in the ipsilateral ventral horn only; there was no labelling on the contralateral side. For each muscle tested, the extent of the labelled cells differs by as much as one spinal segment in the rostral and caudal direction.
Neurons for the SB portion of the pectoralis extend from spinal nerves 10 to 12 whereas the neurons for the TB extend from middle 10 to middle 12 (Fig. 3). In general, the SB motoneuron pool extends one-half to one spinal segment rostral to the TB pool and the TB pool extends caudal to the SB pool an equal distance. We also observed a dorsolateral separation between SB and TB neurons; TB neurons are located in the dorsal half of the lateral motor column (Fig. 4).
Nerve soaks for the supracoracoideus labelled cells from middle 9 to rostral 11 (Fig. 3). Supracoracoideus neurons were located dorsolaterally to the pectoralis motor pool at middle 10 and laterally to the pectoralis motor pool at rostral 11 (Fig. 4).
Muscle Histochemistry
The histochemical profile of the M. supracoracoideus reveals a ‘mixed’ population of FG and FOG fibers. As in the M. pectoralis of many species of birds (Rosser and George, l986) and some muscles of reptiles (Laidlaw et al. l995) and mammals (Windhorst et al. l989), we observed significant variation of fibre type within the belly of this muscle as well. All fibres identified in the M. supracoracoideus possess relatively high oxidative capacity and had a dark reaction to NADH-D (Fig. 2). In the majority of cases, when compared to FG fibres, the FOG fibres have the strongest reaction for NADH-D but a relatively less intense reaction to alpha-GPD (Fig. 2, A,B, FOG). A small percentage of fibres in each muscle illustrate relatively strong reactions to both the oxidative and glycolytic stains and were identified as FG fibres (Fig. 2, FG). The FG fibres account for close to 46% of the total population of the supracoracoideus. Mean (±S.D.) sizes of the FG fibers is 1384±354 µm2 and for the FOG fibers is 990±246 µm2. We also observed a range of cross-sectional areas of the FG and FOG fibers within the supracoracoideus as well which accounts for the relatively large standard deviations
.DISCUSSION
In pursuit of an understanding of the evolution of the shoulder in vertebrates, Jenkins and Goslow (1983) compared the limb kinematics and patterns of electrical activity of the shoulder muscles in the Savannah Monitor lizard, Varanus exanthematicus, and the Virginia Opossum, Didelphis virginiana. Some muscles, thought to be homologous and designated as ‘functionally equivalent’, illustrated patterns of activity which were similarly timed within the phases of the step cycle of these two forms. Comparable kinematic and electromyographic data from pigeons Columba livia flying short distances enabled extension of this idea to birds, but focused the need for a broader comparative data base (Goslow et al. l989). We generated additional data for multiple wing beats of starlings flying in a wind tunnel, incorporated Hermanson & Altenbach’s (l983) data for a flying mammal, (the Pallid bat, Antozous pallidus), and extended the discussion (Dial et al. l991). Two basic evolutionary patterns of homologous muscles emerged when these flying forms were compared to terrestrial forms: (1) Some muscles maintain a similar elecromyographic burst within either the propulsion (=downstroke) or swing (=upstroke) phase of the limb’s movement. In the Savannah Monitor lizard, Virginia Opossum, Pallid bat and European Starling, for example, the pectoralis is consistently a propulsive-phase muscle that initiates activity in the latter part of swing or upstroke, and (2) Some muscles do not exhibit similar activity patterns across species, an observation of particular interest here. The supracoracoideus, for example, seems to ‘shift its phase of primary activity’ during evolution. In terrestrial forms, this muscle in the Savannah Monitor lizard and its putative mammalian homologues, the infra- and supraspinatus, are biphasic with discrete bursts evident in both the propulsive and swing phases. The primary burst of the homologous muscles in the Pallid bat and European Starling, in contrast, occurs at the downstroke/upstroke transition.
These evolutionary shifts in the activation pattern of the motor units within a muscle might occur at a number of anatomical or physiological levels. There may be change in the order of nerve cell selection at the level of the brain or synaptic changes in the spinal cord which affect neuron recruitment. There is some evidence that the similar activation patterns of homologous muscles, such as seen for the supracoracoideus and its homologues, for example, might represent a pattern inherited from early tetrapods and hence reflect a phylogenetic ‘conservatism’. In other words, during the anatomical reconfiguration of a muscle in derived forms, the timing of the motor outflow pattern within the intra-limb cycle of movement (reflected by neuronal impulse traffic) is retained (Jenkins & Goslow l983). Smith (l994) in a critical review of this idea of ‘neuromotor conservatism’ as it relates to tetrapod feeding and locomotor systems emphasises two problems in testing such a hypothesis; (1) the uncertainty of determining muscle homologies across taxa, and (2) a general lack of detailed information about the underlying musculoskeletal and neuroanatomical features of the neuromotor program. This paper is a response to the second criticism and provides relevant musculoskeletal and neuroanatomical data for an Avian flight muscle that illustrates neuromotor patterns divergent from its homologues in other major taxa.
Motor Pool Distribution
The distribution patterns of motoneuron motor pools within the spinal cord for groups of muscles, individual muscles, or even portions of muscles have provided insight into the organisational basis of the nervous system for motor control across tetrapods (Burke et al. l977; Brichta et al. l987; Windhorst et al. l989; Landmesser l978). In light of the shifted and disconnected motor patterns seen in the European Starling when compared to other non-flying and flying tetrapods, one intent of this study is to determine if these altered neuromotor patterns are reflected in motor pool organisation.
In a recent series of studies designed to determine the variability of motor pool location within the spinal cord for homologous muscles across tetrapods, Ryan et al. (l997, l998) investigated the motor pools of four shoulder muscles (including the pectoralis and supracoracoideus) of the Iguana lizard Iguana iguana and their homologues in a terrestrial mammal, the Mouse Mus musculus in addition to two species of bats, the Big Brown bat Eptesicus fuscus and the Little Brown bat Myotis lucifugus. Ryan and colleagues compared their findings to the distribution pattern of motor pools in the only bird for which the data are available, the Domestic Fowl, Gallus domesticus. They concluded the intra-pool relationships within the spinal cord have been conserved ‘during the evolution of a wide variety of locomotor strategies because of developmental constraints.’
The aim of the present study is to investigate and elucidate the structural correlates to the evolutionary change in peripheral muscle function. To accomplish this, we examine the relationships and general morphology of the motor pools associated with the wings of the European Starling, a passerine capable of powered flapping flight. Our motor pool distributions for the supracoracoideus compare favorably to the retrograde degeneration study of Ohmori et al. (l982), and the tracer studies of Straznicky & Tay (l983) and Hollyday & Jacobson (l990). This muscle’s motor pool extends three segments in the Domestic Fowl, two in the Common Pigeon (Sokoloff et al. (l989) and European Starling, and remains closely associated with the ventrally positioned pectoralis pool. In all cases, the supracoracoideus pool extends one to two segments more rostral than the pectoralis pool and its relative position is retained when compared to the distribution of the motor pools for shoulder muscles in reptiles and terrestrial and flying mammals. We agree with Ryan et al. (l998) that the motor pool distribution of the supracoracoideus is conserved and does not reflect the muscle’s new function in birds.
Muscle Histochemistry
Presumably, motor units of different contractile parameters possess characteristic biochemical properties, which must relate to function. When compared to the homologous muscles of terrestrial tetrapods, there exists marked variation in the overall fibre type of the supracoracoideus and pectoralis across species in addition to regional variation within muscles (Table 1). In the pectoralis and supracoracoideus of the Savannah Monitor lizard, for example, both muscles are comprised of different percentages of the FOG and FG fibre types and in addition, possess a third type not present in the European Starling, the slow twitch, oxidative (S/O) fibre (Young et al. l990). Armstrong et al. (l982) surveyed these muscles in the Domestic dog Canis famaliaris and found no FG fibres but a substantial number (25 - 46%) of slow twitch, oxidative (Type I = SO) fibres.
In addition, fibres may vary in their frequency throughout a muscle and be compartmentalised in different regions of the parent muscle. In the pectoralis, for example, a superficial to deep gradient of FG to FOG fibres exists and there is variation in the cross-sectional areas of fibres within this muscle and the humeral triceps. Whereas in most instances the cross-sectional area of the FG fibres is greater than the FOG fibres in a given sample, in the ventral head and ventral midbelly region of the humeral triceps, the opposite is seen.
It has long been recognised that the locomotory muscles of vertebrates are composed of fibres with different contractile and metabolic properties that are organised to meet the specific functional requirements of the muscle (Close l972; Talesara & Goldspink, l978; Windhorst et al. l989). Thus it is not surprising that there has been a concomitant specialisation of fibre types in the supracoracoideus to reflect its evolutionary plasticity. It remains to be determined whether the fibre distributions found within the supracoracoideus (which has undergone an evolutionary change in the ordering or selection of its motoneurons) relates specifically to the evolution of powered flight or if they are simply correlative but not essential.
ACKNOWLEDGEMENTS
We thank Chris Kovacs for his assistance in preparing the histochemistry figures, Kena Fox-Dobbs for assistance with the histochemistry, and Suncana Kuljis for assistance with the data analysis. Supported by National Science Foundation; Contract grant number: IBN 9220097.
REFERENCES
Armstrong, R.B., Saubert, C.W., IV, & Taylor, C.R. 1982. Distribution of fiber types in locomotory muscles of dogs. American Journal of Anatomy 163: 87-98.
Breazile, J.E. & Yasuda, M. 1979.
Systema nervosum peripherale. In: Baumel, J.J., King, A.S., Lucas, A.M., Breazile, J.E. & Evans, H.E. (eds) Nomina Anatomica Avium. London, Academic Press: 473-503.Biewener, A. A., Dial, K. P. & Goslow, G. E., Jr. 1992. Pectoralis muscle force and power output during flight in the starling. Journal of Experimental Biology 164: 1-18.
Brichta, A.M., Callister, R.J. & Petersen, E.H. 1987. Quantitative analysis of cervical musculature in rats: Histochemical composition and motor pool organisation. I. Muscles of the spinal accessory complex. Comparative Neurology 255: 351-368.
Burke, R.E., Strick, P.L., Kanda, K., Kim, C.C., & Walmsley, B. l977. Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. Journal of Neurophysiology 174: 709-712.
Chiappe, L. M. 1995. The first 85 million years of avian evolution. Nature 378: 349-355.
Close, R.I. l972. Dynamic properties of mammalian skeletal muscles. Physiological Reviews 52: 129-197.
Dial, K. P. & Biewener, A. A. 1993. Pectoralis muscle force and power output during different modes of flight in pigeons. Journal of Experimental Biology 176: 31-54.
Dial, K. P., Goslow, G. E., Jr. & Jenkins, F. A., Jr. 1991. The functional anatomy of the shoulder in the European Starling (Sturnus vulgaris). Journal of Morphology 207: 327-344.
Goslow, G.E., Jr., Dial, K.P. & Jenkins, F.A., Jr. 1989. The avian shoulder: an experimental approach. American Zoologist 29: 287-301.
Guth, L. & Samaha, F.J. 1969. Qualitative differences between actomyosin ATP-ase of slow and fast mammalian muscle. Experimental Neurology 25: 138-152.
Hermanson, J.W. & Altenbach, J.S. l983. The functional anatomy of the shoulder of the Pallid bat, Antrozous pallidus. Journal of Mammalogy 64: 62-75.
Hollyday, M. l980. Organisation of motor pools in the chick lumbar lateral motor column. Journal of Comparative Neurology 194: 143-170.
Huber, J.F. l936. Nerve roots and nuclear groups in the spinal cord of the pigeon. Journal of Comparative Neurology 65: 43-91.
Hollyday, M. & Jacobson, R.D. 1990. Location of motor pools innervating chick wing. Journal of Comparative Neurology. 302: 575-588.
Jenkins, F.A., Jr. & Goslow, G.E., Jr. 1983. The functional anatomy of the shoulder of the Savannah monitor lizard (Varanus exanthematicus). Journal of Morphology 175: 195-216.
Laidlaw, D.H., Callister, R.J. & Stuart, D.G. l995. Fiber-type composition of hindlimb muscles in the turtle, Pseudemys (Trachemys) scripta elegans. Journal of Morphology 225: 193-211.
Leonard, R.B. & Cohen, D.H. l975. A cytoarchitectonic analysis of the spinal cord of the pigeon (Columba livia). Journal of Comparative Neurology 163: 159-180.
Landmesser, L. 1978. The distribution of motoneurons supplying chick hindlimb muscles. Journal of Physiology 284: 371-389.
Mesulam, M. M. l978. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. Journal of Histochemical Cytochemistry 26: 106-117.
Novikoff, A.B., Shin, W. & Drucker, J. 1961. Mitochondrial localization of oxidative enzymes: Staining results with two tetrazolium salts. Journal of Biophysical Biochemistry and Cytology 9: 47-61.
Ohmori, Y., Wantanabe, T., & Fujioks, T. 1982. Localization of the motoneurons innervating the forelimb muscles in the spinal cord of the domestic fowl. Zbl. Vet. Med. C. Anat. Histol. Embryol. 11:124-137.
Padian, K., & Chiappe, L.M. 1998. The origin and early evolution of birds. Biological Reviews 73: 1-42.
Poore, S.0., Ashcroft, A., Sanchez-Haiman, A., & G.E. Goslow Jr. 1997. The contractile properties of the M. supracoracoideus in the pigeon and starling: a case for long-axis rotation of the humerus. Journal of Experimental Biology 200: 2987-3002.
Poore, S.O., Sanchez-Haiman A., & G.E. Goslow Jr. 1997. Wing upstroke and the evolution of flapping flight. Nature 387: 799-802.
Rosser, B.W.C. & George, J.C. 1986. The avian pectoralis: histochemical characterization and distribution of muscle fibre types. Canadian Journal of Zoology 64: 1174-1185.
Ryan, J.M., Cushman, J. & Baier, C. l997. Organisation of forelimb motoneuron pools in two bat species (Eptisicus fuscus and Myotis lucifugus). Acta Anatomica 158: 121-129.
Ryan J.M., Cushman, J., Jordan, B., Samuels, A., Frazer, H. & Baier, C. l998. Topographic position of forelimb motoneuron pools is conserved in vertebrate evolution. Brain Behavior & Evolution 51: 90-99.
Smith, K. 1994. Are neuromotor systems conserved in evolution? Brain Behavior & Evolution 43: 293-305
Sokoloff A., Deacon, T., & G.E. Goslow Jr. 1989. Musculotopic innervation of the primary flight muscles, the pectoralis (pars thoracicus) and supracoracoideus, of the pigeon (Columba livia): a WGA-HRP study. Anatomical Record 225: 35-40.
Straznicky, C. & Tay, D. 1983. The localization of the motoneuron pools innervating wing muscles in the chick. Anatomical Embryology 100: 209-218.
Talesarus, G.L. & Goldspink, G. l978. A combined histochemical and biochemical study of myofibrillar histochemical and biochemical study of myofibrillar ATP-ase in pectoral, leg and cardiac muscle of several species of bird. Histochemistry Journal 10: 695-709.
Wattenburg, L.W. & Leong, J.L. 1960. Effects of coenzyme Q (subscript) 10 and menadione on succinate dehydrogenase activity as measured by tetrazolium salt reaction. Journal of Histochemical Cytochemistry 8: 296.
Windhorst, U., Hamm, T.M. & Stuart, D.G. 1989. On the function of muscle and reflex partitioning. Behavioral and Brain Science 12: 629-681.
Young, B.W., Magon, D.K., and Goslow, G.E., Jr. 1990. Length-tension and histochemical properties of select shoulder muscles of the Savannah monitor lizard (Varanus exanthematicus): Implications for function and evoution. Journal of Experimental Zoology 256: 63-74.
Table 1. Histochemical properties of selected shoulder muscles
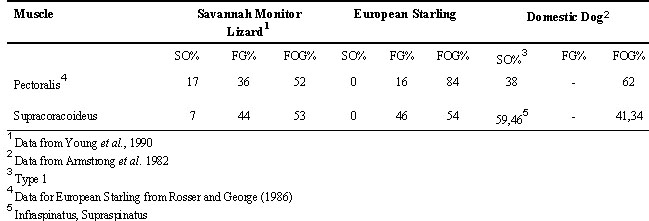
Fig. 1. The cycle of electrical activity in four shoulder muscles of the European Starling. For each muscle, mean onset, offset, and standard errors are represented with reference to wing position. Muscles are associated in three groupings: downstroke (hatched), upstroke (stippled), and transitional (unmarked). The black triangle represents the mean initiation of upstroke at 53.5% of a normalised wing beat cycle (After Dial et al. l991).

Fig. 2. Top:Lateral view of some of the deep muscles of the shoulder in the European Starling; the pectoralis major has been resected, leaving only its origin and insertion (after Dial et al. l991). Bottom: Histochemical profiles of FOG and FG fibres of the M. supracoracoideus. The two histochemical fibre types are shown reacted for (A) NADH-D activity and (B) alpha-GPD activity. All fibres were high in myosin ATP-ase activity (not shown) and relatively high in their NADH-D activity. We separated FOG fibres from FG fibres on the basis of their alpha-GPD (FOG, low; FG, high) and NADH-D (FOG, high; FG relatively low). Scale = 50 µm.
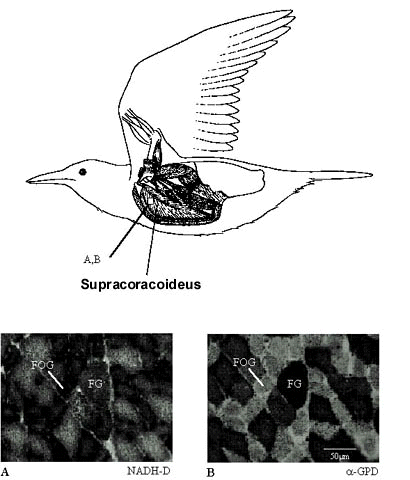
Fig. 3. Distribution of labelled motoneurons along the rostro-caudal axis of the spinal cord for the pectoralis (sternobrachialis, SB; thoracobrachialis, TB) and the supracoracoideus (SC). All cases for each muscle are aligned at the exit of the ventral root of spinal nerve 10; the absolute position of nerves 11 and 12 are shown. Note the relative rostral distribution of the motoneurons of SB when compared to TB.
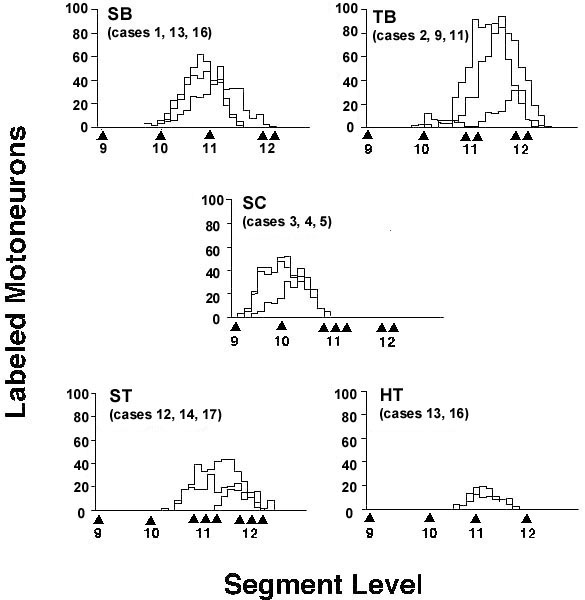
Fig. 4. Summary map illustrating the positions of the motor pools of the pectoralis and the three test muscles. The slices correspond to either the nerve exit position or mid-segment level. Each pool is represented graphically to illustrate its absolute and relative position within the lateral motor column of the spinal gray.