S02.4: Avian respiration: isolation of ventilation and flight?
Richard E. Brown
Respiratory Biology Program, School of Public Health, Harvard University, 665 Huntington Ave. Boston, Massachusetts 02115, USA, fax 617 732 6927, e-mail
Rbrown@zeus.bwh.harvard.eduBrown, R.E., 1999. Avian respiration: isolation of ventilation and flight. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
114-123. Johannesburg: BirdLife South Africa.Airsacs fill >30% of a bird's body cavity, extend from tail to mid-neck, and contribute equally to inspiration and expiration. Thus, lung ventilation requires co-ordinated movements of all bones and muscles forming the body wall (excluding extremities and cranial half of neck). Cineradiographic imaging of awake ducks, finite element modelling of the intact chest-wall and dissection were used to analyse the body-wall motions associated with breathing. The sternum, spine, and coracoids are maintained in a constant angular alignment during breathing. Because the ribs are isolated from the flight musculature and are the only mechanical connection between sternum and spine, any wingbeat induced motion of the sternum results in an identical spinal motion with no change in thoracic volume. The intraclavicular airsac, lying mostly outside the thorax, is ventilated by neck motions and by medio-lateral coracoid motions that occur independently of wingbeat. Abdominal airsacs are ventilated with dorso-ventral motions of the caudal sternum relative to the synsacrum and by hinging of the synsacrum relative to thoracic spine. Although birds do not possess a mammalian-like diaphragm, the abdominal viscera atop the caudal sternum move cranially and caudally with breathing similar to the diaphragm-driven visceral motion seen in mammals.
STUDIES OF AVIAN VENTILATION - HISTORICAL PERSPECTIVES
Nearly all of our knowledge concerning the mechanisms driving air into and out of the bird’s respiratory system, i.e. ventilation, is based on results and conclusions generated prior to 1935 (see Bert 1870; Baer 1896; Soum 1896; Zimmer 1935; King 1966). However, the data gathered in those earlier investigations were limited almost entirely to gross deformations of the thorax without regard to the motions of the bird’s jointed ribs, soft tissue body walls, viscera, body cavity cranial to the sternum and the synsacrum. Importantly, there exist disagreements among the earlier investigators of avian ventilation (see Baer 1896; Soum 1896; Zimmer 1935).
The questions that elicited these earlier investigations of avian ventilation are not different from those that stimulated the present investigation and can be roughly categorised as: (1) questions of comparative respiratory biology (see Bert 1970); (2) specific questions concerning the mechanisms driving flow through the bird’s unique lung-airsac respiratory system (see Baer 1896; Soum 1896); and, (3) questions concerning interactions between breathing and flight (see Zimmer 1935). Many of the ‘wonders’ of avian respiration are beginning to give up their secrets following considerable work of numerous investigators and investigations, for example, existence of- and mechanisms responsible for controlling the unidirectional airflow through the bird’s lung. However, not only does much remain unanswered but new investigators and new approaches will continually redefine what are considered to be the ‘true’ answers to ‘old problems’.
WHAT ARE THE BODY-WALL MOTIONS DRIVING VENTILATION IN BIRDS?
Regardless of the accuracy of the efforts of Baer (1896), Soum (1896), Bert (1970) and Zimmer (1935), because their investigations were centred almost exclusively on sternal motions their results are less than adequate for us to fully appreciate the ventilation of the birds’ lung-airsac respiratory system. Functioning in the manner of bellows and deformed by overlying body walls the airsacs drive airflow into and out of the bird’s body and through its nearly constant volume lung. The several airsacs fill greater than 30% of the body cavity, extend from neck to caudalmost abdomen and contribute about equally to both inspiration and expiration. Because of the widespread distribution of the bird’s several airsacs, ventilation in birds demands co-ordinated motions/control of nearly all body surfaces except for the limbs and cranial neck. Important questions that remain if we are to understand avian ventilation include the following.
The body-wall overlying the airsacs is, in broad areas, composed of highly compliant soft-tissue structures, e.g.: broad thoracic inlet over interclavicular airsac with a membrane composed of skin plus underlying airsac wall; or, abdominal wall, which is passive during inspiration, over the abdominal and caudal thoracic airsacs. These soft tissue body-walls are susceptible to paradoxical respiratory motions, e.g., abdominal wall moving inward during inspiration. If such paradoxical motions were to occur they would reduce the efficiency of the respiratory muscles in that no flow would be produced for the work of paradoxically deforming a soft tissue body surface. And if such paradoxical motions do not occur what prevents them?
Each of the bird’s complex ribs is formed of two individual bones (Fig. 2). Each vertebral rib, articulating with the vertebrae, and each sternal rib, articulating with the sternum, are themselves joined via their intercostal articulation. The construction of the bird’s rib-cage confers additional degrees of freedom in motion not found in other homeotherms. Examples of these additional degrees of freedom observed in cadavers include: with minimal force, sternal-spinal angular orientation can be artificially varied over a range of 90o; the sternum’s dorso-ventral motion can be uncoupled from lateral thoracic expansion. What are the specific motions or rotations of the rib’s individual components and articulations relative to each other and to the spine and sternum? What is the specific contribution from each individual component of the ribs to overall sternal motion? How much control does the bird have over its intercostal and paracostal muscles and the resulting rib-rib and sternal-spinal alignment?
Because of the origin of the bird’s large flight muscles upon the sternum we need to thoroughly understand thoracic anatomical design, sternal motion relative to the spine and how that sternal motion is controlled if we are to understand mechanical linkages between respiration and flight. Importantly, we do not know if the bird can control its sternal-spinal angular orientation or is sternal-spinal angular alignment a passive response to applied forces. It is these latter questions related to control of sternal-spinal alignment and the influences of the flight muscles on that alignment that are the focus of this manuscript.
LOCOMOTOR-RESPIRATORY COUPLING
A perennially attractive notion among those interested in animal locomotion is that mechanical linkages might exist between ventilation and locomotion such that the work of breathing is somehow reduced during locomotion when respiratory needs are highest. A century ago Marey (1890) suggested that the anatomical arrangement in birds, whereby the massive flight muscles are attached upon the sternum, would permit the muscle forces driving ‘wingstoke’ to assist in ventilating the bird’s respiratory system, i.e., compress the thorax during the down-stroke. As flight is the most energetically expensive form of locomotion (O2 consumption per unit time; see Tucker 1972; Chai and Dudley 1995), it is rather appealing to think that the ventilation-wingbeat linkage suggested by Marey (1890) does indeed reduce the work of moving the large quantities of air (ventilation) necessary to meet the bird’s respiratory demands. Further, we now have definitive evidence that there is a small, 4 to 11% of tidal volume, expiratory-like flow-event temporally coordinated with the downstroke (Banzett et al. 1992; Boggs et al. 1997). However, we do not understand how the flight mechanism affects respiratory flow or thoracic volume.
Before we consider below the possible linkages between breathing and flight in birds and their mechanism of action we must consider the implication of any such possible mechanical interaction(s). Is the wingstroke-coordinated airflow an unavoidable and energetically costly interference between breathing and flight? (Here airflow refers to respiratory system airflows measured at the mouth.) Under this scenario breathing might be temporally co-ordinated in some fashion with wingbeat so as to reduce interference between ventilation and locomotion. In sharp contrast to the interference scenario, the wingstroke-coordinated airflow might be the product of a beneficial interaction between respiratory and locomotor apparatus. Under this ‘beneficial’ scenario there is an overall reduction in the combined work of breathing plus flight relative to that of both breathing and flight alone? The ‘beneficial’ scenario is only possible where locomotor muscles are more efficient in producing respiratory airflows than are the respiratory muscles themselves; or, the respiratory muscles are more efficient in driving the locomotor apparatus than are the flight muscles themselves. (Efficiency here is taken as either the muscle work done to move a unit volume of air at a given flow rate, or the work to support and propel the bird by its wings at a given velocity). That is, without such an increased efficiency resulting from using flight muscles for breathing or breathing muscles for flight it is simply not possible for a single muscle to do two jobs for the price of one (or any cost less than that required for both jobs)!
It is rather difficult for this investigator to entertain the notion that natural selection would produce a design in which locomotor muscles are more efficient in effecting ventilation than are the respiratory muscles per se. Even when and where inertial effects are assumed to contribute to ventilation, e.g. piston effect of abdominal contents in running quadrupeds, it cannot be ignored that the locomotor muscles must perform work to accelerate that visceral piston and then more work is needed to decelerate the animal surrounding the piston so that the piston can move forward and do work on the respiratory system. I would suggest that the ideal situation physiologically is one in which respiratory and locomotor demands can be accommodated individually without interference between them.
HOW MIGHT BODY-WALL DEFORMATIONS DRIVING VENTILATION BE INFLUENCED BY WINGSTROKE?
It appears that two features of avian ventilation have reinforced the belief that wingstroke and breathing are linked in birds: (1) the notion that sternal hinging relative to the spine’s axis is the major respiratory motion of the thorax; and, (2) the obvious attachment of the large flight muscles, M. pectoralis, to the sternum. Here I argue that (1) sternal-spinal hinging does not occur and if it did it could not affect avian respiration and (2) that the design and construction of the avian sternum, attached pectoral girdle and attachments of the flight muscles do not allow for any volume changes of the body cavity to be produced by the flight muscles per se. Comparisons will be made between the conclusions of previous investigations and the results from recently completed fluoroscopic and anatomic studies of the bird’s respiratory mechanism (unpublished observations). The body-wall motions associated with breathing were quantified in spontaneously breathing wild-type Mallards Anas platyrhynchos from sequential fluoroscopic images analysed digitally (Fig. 1). During these examinations the ducks rested in sternal recumbency in a cloth sling, a support position that mimics, at least statically, the forces upon the sternum encountered in flight (Zimmer 1935). Anatomical observations were completed in a diverse range of taxa with different body sizes and different flight styles.
RESPIRATORY MOTIONS OF THE STERNUM AND RIBS
Sternal-Spinal Hinging
It has been assumed that the primary breathing-related volumetric change within the thorax is mediated via a rotation of the sternum relative to the spine’s axis, with a hinge point located somewhere near the cranial sternum (Bert 1870; Baer 1896; Soum 1896; Zimmer 1935; King 1966). The attachments of the large flight muscles (M. pectoralis) originating from the caudal sternum and inserting at the shoulder joint (mechanically via the proximal humerus), when combined with the notion of a prominent sternal-spinal hinging, led many investigators to the conclusion that a linkage between wingbeat and breathing must exist.
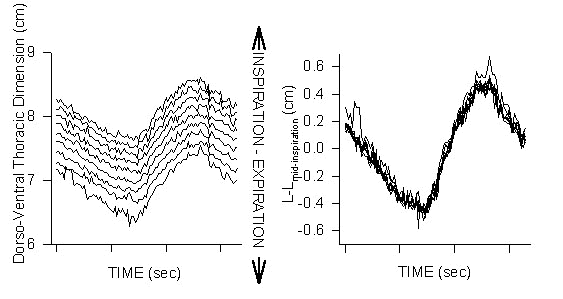
When the literature is closely examined it is found that Baer (1896), Soum (1896), and Zimmer (1935) came to somewhat conflicting conclusions regarding the ventilatory motions of the sternum relative to the spine. All these investigators indicate that during leg support the bird’s sternum not only moves towards (expiration) and away (inspiration) from the spine but that the sternum’s primary motion is that of a rotation or hinging (pivot point of hinge at sternum’s or coracoid’s cranial end) relative to the longitudinal axis of the spine. In sharp contrast, Zimmer (1935) indicates that during sternal recumbency the angular orientation between sternum and spine is constant during respiration. Interestingly, this result is ignored in later reports concerning avian ventilation. Regardless of this contrast in conclusions, spinal-sternal hinging has been considered to be the predominant if not the only mode of respiratory-related sternal motion (e.g. see Fig. 12, p.243, King 1966; Fig. 8, p. 37, Duncker 1971.
Analysed fluoroscopically where sternal position and orientation were referenced to the spine’s position and orientation, there is no sternal-spinal (or sternal-coracoidial) hinging during breathing while ducks rested in sternal recumbency (Fig. 1), similar to the results described by Zimmer (1935). Additionally, no sternal-spinal hinging was found fluoroscopically in birds in dorsal recumbency in contrast to the results of Baer (1896) and Soum (1896).
Anatomical design of sternum, pectoral girdle, ribs and spine
It is in fact quite striking seeing the bird’s large flight muscles (³ 20% of body mass) attach to its respiratory apparatus, i.e., the bird’s broad sternum. In strong contrast to the interpretations of Marey (1890) and later investigators, I suggest that the anatomical design, connections and relationships between the bird’s sternum, pectoral girdle, ribs, spine and pectoral musculature provides for no anatomical mechanisms by which the flight musculature could directly alter the volume of the bird’s body cavity. That is, except for an acclerative-inertial coupling between sternum and spine (see below), the muscles driving the bird’s wingstroke cannot produce any significant respiratory flows.
Importantly, the pectoral muscles driving the wing’s downstroke are attached between sternum and humerus, and do not have any connections with the bird’s ribs or spine. Thus the pectoral muscles cannot influence spinal-sternal separation (ventro-dorsal motion of the sternum relative to the spine), or hinging of the sternum or coracoids relative to the spine or directly effect changes in thoracic volume. The coracoids are strongly attached to the cranialmost sternum such that there is very little to no possible dorso-ventral flexion of the coracoids relative to the sternal axis (Fig. 2). In a cadaver with its the sternum wired to a support along its internal surface and the coracoids disposed horizontally, a load greater than 7 times the birds body mass could be suspended from the distalmost coracoids without producing more than 3o of coracoid ventroflexion (unpublished observation). The strongly united sternum-coracoids-furcula is a mechanically independent and nearly inflexible anatomic subassembly that supports the flight apparatus composed of wings and flight muscles. The proximalmost humerus, to which is attached the large flight muscles (M. pectoralis), is supported at the shoulder joint which is primarily supported by the coracoids. The flight muscles are attached between sternum and humerus can and must affect humeral alignment, the wingbeat, however they cannot deform the nearly rigid sternal- or flight subassembly. That is, M. pectoralis cannot produce any hinging of the coracoids relative to the sternum.
The longitudinal axis of the coracoids is approximately aligned with the lateral edge of the sternum to which the ribs are articulated. The great majority if not all of M. pectoralis lies below the longitudinal axis of the coracoids. Thus if the flight muscles could effect coracoid-sternal alignment the muscles would produce a ventro-flexion of either sternum or coracoids relative to the other which would have an inspiratory effect. Importantly, downstroke driven by the pectorals has an associated expiratory flow-event (Banzett et al. 1992; Boggs, et al. 1997) and thus cannot be produced by sternal or coraoid ventro-flexion. Remember however, the sternal-coracoidial joints are constructed and supported by very tough connective tissue sheets such that any ventro-flexion is strongly constrained.
The ribs along with their attached intercostal and paracostal musculature are, with the exception of the scapulae, the only structures mechanically attaching the sternum and bones of the pectoral to the remainder of the bird’s body (spine) (Fig. 2). The scapulae are attached between shoulder and ribs, however all of their connections (ribs to scapula and scapula to coracoid-furcula) are highly deformable as they must be to allow normal ‘at rest’ respiratory motions to occur. That is, the loose attachments of the scapulae to the dorsal ribs must be easily deformed as the sternum and attached coracoids and furcula move dorso-ventrally towards and away from the scapula’s during breathing. Further, if the ribs served as an origin of the pectoral muscles then any shortening of the flight muscles would rotate the ribs cranially which is an inspiratory rib motion and not the expiratory flow-event coordinated with downstoke described by Banzett et al. (1992) and Boggs et al. (1997).
Importantly, even if the pectoral muscles could by some unknown mechanism directly influence sternal alignment relative to the coracoids such changes would be merely reflected in identical changes in the spine’s orientation (here we ignore accelerations). That is, the spine and remainder of the body are suspended atop the sternum via the ribs such that any change in sternal position that did not concomitantly produce motion of the ribs could not influence thoracic volume or ventilation. As an analogy consider a large spider standing atop a floating leaf where the leaf=bird’s sternum, spinder’s body=birds spine and suprasternal body, and the spider’s legs=bird’s ribs. As the leaf moves up and down with passing waves (i.e., any influence of pectoral muscles on sternal alignment), the body of the spider supported above the leaf via its legs smoothly moves up and down identical to the motion of the leaf/sternum upon which it stands.
Airsac diverticulae lying between the layers of the pectoral muscle would be deformed during contractions of the pectoralis and those diverticulae surrounding the shoulder joints would be deformed with the wingstoke. However since muscle shortening and joint motion are isovolume events it is difficult to image anything but a miniscule flow effect secondary to shape changes of these relatively small airsac diverticulae. Pennycuick (1986) suggested that ventilation of these intra-pectoralis airsac diverticulae would function to remove heat from the flight musculature. The very small, if any, tidal volume of these diverticulae eliminates any effective convective heat transport and, importantly, that the air in these deep diverticulae is saturated (water vapour) there can be no evaporative cooling.
Furcular-coricoidial motion
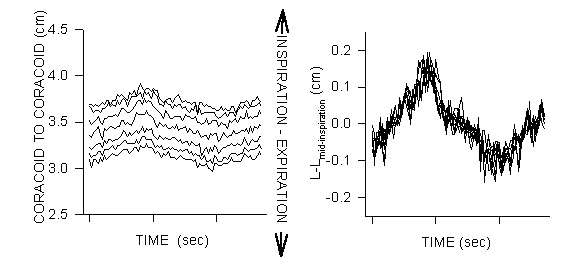
While any possible dorso-ventral motion of the coracoids relative to the sternum is strongly constrained, the coracoids do move medio-laterally (expiration-inspiration) with respiration (Fig. 3). The construction of the coracoido-sternal joints is such that the coracoids maintain a nearly constant orientation relative to one another during their respiratory-related medio-lateral motion. The dorsal extremes of the furcular bars are connected to the distal coracoids such that the furcular bars must also flex with respiration. Jenkins et al. (1988) show in flying birds that lateral furcular bending was coordinated with the wing’s downstroke; and if the dorsalmost furcular bars were displaced laterally then the attached coracoids must have also been displaced laterally. Thus there is a possible common respiratory mode between breathing-related furcular-coracoid motion and that seen during flight. In consideration of the volume of the interclavicular recess, of which the coracoids and furcular bars form a part of the lateral walls, and the small range of medio-lateral motion of the coracoids (Fig. 3) any flow associated with wingstroke driven medio-lateral coracoid motion plus furcular bending would be small. Importantly, if the lateral coracoid motion plus lateral furcular bending coordinated with the downstroke had any significant flow consequence, such flow would be inspiratory-like during the downstroke. This is not consistent with the results of Banzett et al. (1992) and Boggs et al. (1997).
What is the mechanism by which wingstroke drives a respiratory flow?
The airflow measurements reported by Banzett et al. (1992) and Boggs et al. (1997) have established the fact that there is indeed an expiratory-like flow event temporally coordinated with the wing’s downstroke. Above I describe how the pectoral muscles per se cannot directly effect changes in the volume of the thorax and thus cannot directly generate a respiratory flow. However, I suggest there is an accelerative-inertial coupling between sternal subassembly and spine that would produce the downstroke related expiratory flows seen by Banzett et al. (1992) and Boggs et al. (1997) and the downstroke-related dorso-ventral dimensional change deduced by Boggs et al. (1997).
During the downstroke the wing produces an aerodynamic force, the upward component of which is lift. This upward lift force varies during the course of a single wingbeat relative to a taxon’s specific flight styles. In gliding flight where the wings are held in constant extension and position the lift force is constant across the wingbeat, e.g., Laridae. In the taxa that nearly completely fold their wings during the upstroke the lift force varies from zero during the upstroke to a maximum during some portion of the downstroke, e.g. Starlings Sternus vulgaris examined by Banzett et al. (1992) and Magpies (Pica pica) examined by Boggs et al. (1997). Lift would vary much less in the slow flapping flight of gulls or large herons in which the wings are held in extension and generate lift throughout the wingbeat.
For the purpose of this description consider a bird in straight and level flight and belonging to a taxon that folds their wings during the upstroke, i.e., birds that have strongly cyclic lift production. Without gravity and drag, inertial forces would tend to maintain a bird on a constant path even during the folded-wing, non-lift-producing upstroke. However gravity and drag cannot be ignored and thus during straight and level flight these birds’ bodies are falling and decelerating during the upstroke. During the downstroke the wings generate an aerodynamic force that reverses the trends found during the upstroke, accelerating the bird upward (lift) and forward (thrust). The wing and its driving muscles transfer the lift and thrust forces they generate to the sternal subassembly such that it is accelerated upward and forward.
The remainder of the bird’s body, i.e., everything that is not attached to or resting on the sternal subassembly or wings, must of course follow the motions/accelerations of the sternal subassembly. However the inertial forces acting on the remainder of the bird’s body tending to keep the body falling at the end of the wing’s upstroke somewhat resist the lift and thrust accelerations applied by the sternal subassembly through the ribs to the spine. The inertial resistance of the suprasternal body mass to being accelerated upward and forward results in a deformation of the bird’s elastic rib cage constructed of jointed ribs and attached intercostal and paracostal muscles. This deformation of the bird’s rib cage between the upward acceleration of the sternal subassembly and the inertial forces acting on the remainder of the body result in the downstroke-related reduction of the dorso-ventral dimensions of the thorax (as deduced by Boggs et al. 1997) and an expiratory flow excursion (as measured by Banzett et al. 1992 and Boggs et al. 1997). There is of course an inertial force operating on the sternal subassembly resisting the lift force generated by the wing’s downstroke. Overcoming that inertial force is an obvious cost of flight. It would be expected that in slow flapping flight the aerodynamic force varies little during the wingbeat cycle such that respiration would be much less affected, if at all, by wingstroke.
Why is there no inspiratory flow-event coordinated with the upstroke? During the upstroke the folded wings do not generate an inertial force. To the extent that the wing’s generation of the aerodynamic force over the course of a downstroke is best described by a sine-wave (not a square wave) lift and thrust slowly (relatively) abate during the last half of the downstroke such that there would be no discontinuity in force between end-dowstroke and any time during the folded wing uptrokes. That is, there is no sudden downward acceleration generated during the upstroke but only a reduction of (later half of downstroke) and then an absence of any opposition to the otherwise wingbeat invariant action of gravity and drag.
Consequences of inertial coupling between respiratory and flight apparatus
The accelerative-inertial coupling between sternum and spine driven by the aerodynamic force generated by the wings is interference between breathing and locomotion (flight). That is, there is a mechanical cost of deforming the costal mechanism, i.e., ribs and attached musculature. The work performed by the flight muscles in stretching respiratory muscles is then not available for driving the downstroke. Additionally, there is a flow related cost whenever wingstroke produces a flow opposite in sign to that occurring in the respiratory system, e.g., expiratory wingbeat-related flow occurring during the inspiratory cycle of the respiratory system. If the rib mechanism was stiff enough to resist chest-wall deformation driven by the accelerative-inertial wingbeat-driven coupling of sternum and spine then it would require more work by the breathing muscles for respiration at all times. Breathing and wingbeat in birds appears to be temporally coordinated so as to reduce interference between locomotion and ventilation; that is, there is an inescapable expiratory flow coupled with the wing’s downstroke that if expiration is coordinated with downstroke there is, to some degree, an amelioration of the cost of this respiratory-locomotor interference.
Even with a bird in which every downstroke related expiratory flow-event was coordinated with an active expiration by the bird’s respiratory system, I suggest there would be no net energy savings in the cost of wingbeat plus breathing. It is not conceivable to this investigator that the locomotor muscles through their inertially driven deformation of the rib cage can produce flow as efficiently as the direct action of the respiratory muscles themselves. However, the inertially driven deformation of the rib cage may in fact provide a positive benefit to the bird. It may be that the wingbeat driven deformation of the costal mechanism acts to reduce stress-strain on the components of the flight apparatus as such a deformation may reduce peak forces, absorbing some and somewhat spreading the remainder out over time. Thus the cost of the ventilatory-locomotor interference may be in reality the cost of protecting musculo-skeletal structures.
ACKNOWLEDGEMENTS
This work would not have been possible without extensive intellectual interactions with Dr. Robert Banzett, School of Public Health, Harvard University and the financial support of the Scandinavian-American Foundation and Dr. Jeff Fredberg of the School of Public Health, Harvard University. I appreciate the invitation extended by Drs. Dominique Homberger and Gart Zweers to participate in their symposium. Additionally, Dominique Homberger contributed to the preparation of this manuscript.
REFERENCES
Baer, M. 1896. Beiträge zur Kenntnis der Anatomie und Physiologie der Atemwerkzeuge bei den Vögeln. Zeitschrift für Wissenschaften Zoologie 61: 420-498.
Banzett, R.B., Nations, C.S., Wang, N., Butler, J.P., & Lehr, J.L. 1992. Mechanical independence of wingbeat and breathing in starlings. Respiration. Physiology 89: 27-36.
Bert, P. 1870. Leçons sur la physiologie comparée de la respiration. Paris:Baillière.
Boggs, D.F., Jenkins, F.A. & Dial, K.P. 1997. The effects of the wingbeat cycle on respiration in the black-billed magpie (Pica pica). Journal of Experimental Biology 200: 1403-1412.
Chai, P. & Dudley, R. 1995. Limits to vertebrate locomotor energetics suggested by hummingbirds hovering in heliox. Nature 377: 722-725.
Jenkins, F.A. Jr., Dial, K.P., & Goslow, G.E. 1988. A cineradiographic analysis of bird filght: The wishbone in starlings is a spring. Science 241: 1495-1498.
King, A.S. 1966. Structural and functional aspects of the avian lungs and airsacs. International Review of General and Expperimental Zoology. 2: 171-267.
Pennycuick, C.J. 1986. Mechanical constraints on the evolution of flight. Memoirs of the California Academy of Science. 8: 83-98.
Soum, J.H. 1896. Recheerches physiologisques sur l'appareil respiratoire des oiseaux. Annales de l'université de Lyon. 28: 1-126.
Tucker, V.A. 1972. Respiration during flight in birds. Respiration Physiology. 14: 75-82.
Zimmer, K. 1935. Beiträge zur Mechanik der Atmung bei den Vögeln in Stand und Flug. Zoologica (Stuttgart) 33: 1-69.
Fig. 1. Sternal motion relative to the spine in awake, spontaneously breathing Mallards. Birds breathing 10% inspired CO2 with 20% O2 to produce ventilatory volumes similar to those seen in exercise. Fluoroscopic (video) images were converted to digital format from which identical measurements were taken from sequential frames. A wire surgically implanted along the dorsal spineous processes of the thoracic vertebrae served as the ‘fixed’ reference for all measurements. From many fixed points along the axis of the reference wire measurements, perpendicular to the spine, were taken to a wire implanted along the sternal keel. Left graph: The mid-inspiration dorso-ventral thoracic dimension increases from cranial to caudal, however note that at any point along the spine the motion of the sternum relative to the spine is nearly identical; i.e., the parallel nature of the measurements from different positions along the spine. Right graph: When each of the measurement sets from the various positions along the spine are reduced to the absolute motion at that point (L-Lmid-insp) about the value at mid-inspiration the different measurements collapse to nearly a single value. Nearly identical dorso-ventral thoracic dimensional changes regardless of position along the spine are only possible if there is no sternal-spinal rotation or hinging.

Fig. 2. Diagram of the bird’s chest-wall anatomy. Note that other than the ribs and scapula (not shown) there are no mechanical connections between the spine and either the sternum or coracoid. The bird’s large pectoral muscles originate from the stippled area of the sternum and the furcula and insert on the proximalmost humerus supported in the shoulder joint formed of distal coracoid, furcula and scapula. Essentially all the pectoral muscle lies ventral to the longitudinal axis of the coracoid, itself aligned with the lateral edge of the sternum. The coracoido-sternal joint, in the parasagittal plane, is constructed such that almost no (<30) dorso-ventral motion of the coracoid is possible. Thus the sternum and attached pectoral girdle form a nearly rigid anatomic ‘flight subassembly’ that is, except for the ribs, mechanically isolated from the remainder of the bird. The birds complex jointed ribs are composed of two bones. Vertebral ribs, articulating with the spine, and the sternal ribs, articulating with the sternum, are joined at the intercostal articulations joining each rib’s two segments
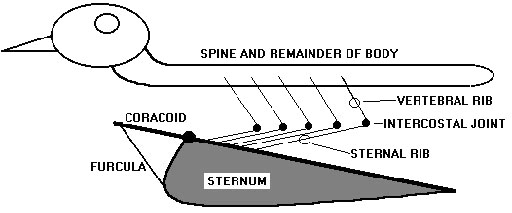
Fig. 3. Motion of the corocoids during breathing in awake Mallards; measurements taken perpendicular to spinal axis. A coracoids moves medio-laterally (expiration-inspiration), towards and away from its pair during breathing. Note that the respiratory-related motion of the coracoids is small (˜10%) relative to that of the thorax’s D-V motion (compare with Fig. 1). Left graph: The inter-coracoid distance increases from the coracoids’ proximal to distal ends. Right graph: When all the various measurement sets are normalised to their mid-inspiration value they collapse to nearly a single value for each temporal stage of the breath demonstrating that the coracoid-coracoid angular alignment is nearly constant. Thus coracoid motion is not a medio-lateral rocking but instead it is constrained to a medio-lateral sliding of the coracoids along their ‘saddle’ articulation with the curved profile of the cranialmost sternum.