S02.3: The avian tongue and larynx: Multiple functions in nutrition and vocalisation
Dominique G. Homberger
Department of Biological Sciences, Louisiana State University, Baton Rouge, Louisiana 70803-1715, USA, fax 225 388 1763, e-mail zodhomb@lsu.edu
Homberger, D.G. 1999. The avian tongue and larynx: Multiple functions in nutrition and vocalisation. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 94-113. Johannesburg: BirdLife South Africa.Two long-standing questions, namely ‘Why did birds lose their teeth?’ and ‘Why can parrots talk?’ were explored by analysing the functional morphology of the lingual apparatus, hyoid suspension, and laryngeal apparatus in birds that differ in their capacity to swallow relatively large food items whole and to generate complex, articulated vocalisations. The conditions in birds were compared to those in mammals and, as a result of this comparison, the first question was reformulated to ‘Why did not all mammals lose their teeth?’ The specific construction of the hyoid suspension in birds allows evolutionary changes that are necessary for swallowing large prey items whole, while the mammalian construction of the hyoid suspension prevents such an evolutionary option. The capacity of parrots to mimic human speech is a byproduct of the unique construction of their larynx which plays a crucial role during swallowing. The kinetic larynx of parrots and the concomitant capacity to generate complex, articulated vocalisations have evolved in convergence to those of songbirds (i.e., oscine Passeriformes).
INTRODUCTION
Avian morphology is one of the oldest biological disciplines dating back to Aristotle and his intellectual ancestors. Recently it has enjoyed an upsurge in widespread interest through the ongoing controversy surrounding the evolutionary origin of birds either from dinosaurs or from archosaurs ancestral to both dinosaurs and birds (see, e.g., Feduccia 1996; Chatterjee 1997; Dingus & Rowe 1997; Shipman 1998). In this controversy, morphological aspects of the locomotory system and integument feature most prominently (e.g., Chatterjee 1997; Shipman 1998; Ackermann 1998; Maderson & Homberger in press). The significance of the morphology of the feeding apparatus for the evolution of birds has been less directly embroiled in this controversy, even though it has been discussed by Zweers et al. (1995), Feduccia (1996), Morrell (1997), Zweers & Gerritsen (1997), and Zweers & Van den Berge (1997 a, b). Nevertheless, factors related to diet and food acquisition are known as powerful agents in the process of natural selection and, therefore, promise to hold at least some of the keys for understanding the evolutionary processes that have contributed to the origin and evolution of birds.
One of the features that are diagnostic of extant birds is their lack of teeth. Reduction or complete loss of teeth is relatively rare among vertebrates, but it does occur whenever teeth are not absolutely needed for a specialized mode of nutrition, such as in turtles, nectarivorous and myrmecophagous mammals, and baleen whales. Toothlessness in birds lends itself less readily to an analysis and understanding of its functional and evolutionary causes than toothlessness in mammals and reptiles, because all extant birds lack teeth and because birds have radiated to exploit the broadest range of foods possible for any vertebrate class. Therefore, toothlessness in birds cannot be correlated directly to diet as readily as it can in mammals. It must also have evolved at an early stage in the evolutionary history of birds (see, e.g., Hou et al. 1995; Zimmer 1996; Sanz et al. 1997). Until now, the loss of teeth in birds has been interpreted as a result of selective pressures towards the optimisation of the flight capacity of birds by reducing the weight of the head and preventing a top-heavy load of the body (e.g., Böker 1937: 88f; Portmann 1950: 29; Gill 1990: 85; Brooke & Birkhead 1991: 28; Bühler 1992, 1996; Feduccia 1996: 3 & 5; Zimmer 1996; Ackermann 1998). This interpretation may appear plausible, but is not compelling. Furthermore, comparative studies do not indicate a strong correlation between bipedalism or a capacity for flight on the one hand and toothlessness on the other hand (see also Bühler 1992).
Another feature that is diagnostic of birds is their capacity for acoustic communication, which in the majority of birds is at least partially vocal. The avian vocal apparatus is usually considered to be a part of the respiratory system, although the larynx and oropharyngeal cavity are shared by both the digestive and respiratory system. This topographical confluence of two seemingly separate functional systems raises the possibility of functional and evolutionary interactions between them (see also Zweers 1985). Until now, an explanation for the propensity of some birds, such as parrots and songbirds, to generate and learn highly complex, articulated vocalisations and even to mimic human articulated speech has been sought mainly in terms of an increased encephalisation and concomitant learning and cognitive abilities or in terms of the morphology of the syrinx and suprasyringeal air passageways (e.g., Thorpe 1958; Tielcke 1970; Kroodsma & Miller 1982, 1996; Ball 1994; Striedter 1994; Catchpole & Slater 1995; Snowdon & Hausberger 1997; Dubbeldam 1998; Fee et al. 1998; Goller 1998).
In the following, I will address two of the oldest, still unanswered questions that concern features that are quintessential to birds, namely: 'Why did birds lose their teeth?' and 'Why can parrots talk?'. By exploring these seemingly independent questions, I will call attention to the potential that integrative, multispective approaches, innovative conceptualisations, and discoveries of new structural conditions may hold for avian anatomy.
WHY DID BIRDS LOSE THEIR TEETH?
To get to an answer to this question, it is necessary to approach it indirectly and to provide some background information.
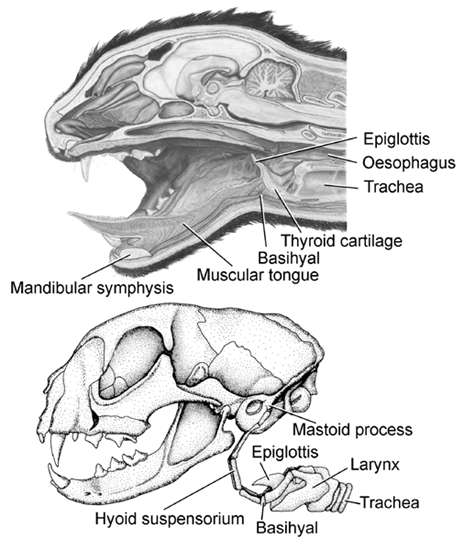
In mammals, the body of the tongue consists mainly of musculature, is devoid of an internal bony skeleton, and functions as a muscular hydrostat (e.g., Kier & Smith 1985). The tongue is anchored rostrally to the symphysis of the mandible and caudally to the basihyal, a relatively small element of the mammalian hyoid skeleton (Fig. 1). The basihyal itself is anchored directly to the otic region of the skull by paired chains of hyoid ossicles, as in dogs and cats, or by paired ligaments, as in human beings, rabbits, and rodents. This suspensory apparatus of the hyoid, or hyoid suspensorium, underprops the narrow, fleshy arch that separates the oral cavity from the pharyngeal cavity and is known as the Isthmus faucium (Schummer & Nickel 1967; Dyce et al. 1987; Walker & Homberger 1992, 1993). Thus, the hyoid skeleton and its suspensory apparatus limit the expansibility of the passage into the oesophagus and effectively determine the maximal size of a food bolus that can be swallowed (Homberger 1994). The muscular tongue is responsible for manipulating food within, and transporting food through, the oral cavity and for finally pushing the food bolus into the oesophagus (e.g. Hiiemae & Crompton 1985). The basihyal is also directly connected to the laryngeal skeleton by articulations. The larynx with its epiglottis, which folds back over the closed glottis during deglutition, guides the traffic between the food and air passageways. The larynx also contains the vocal chords with which mammals generate sounds.
In birds, the entire tongue is underpinned by the hyoid skeleton (Fig. 2). A central axis is formed by the unpaired paraglossale, which is located in the very lingual tip and articulates caudally with the basihyale, which, in turn, extends caudally into the urohyale (e.g., Homberger 1986; Homberger & Meyers 1989; and references therein). The paired ceratobranchialia articulate with each side of this central axis at the point where the basihyale and urohyale meet. Caudally, the ceratobranchialia articulate with the epibranchialia, with which they form the paired hyoid horns. The distal ends of the hyoid horns are encased in powderhorn-shaped sheaths of connective tissue that are anchored to the surface of the connective tissue that envelopes the jaw muscles as epimysia (Fig. 3; Homberger 1986; Homberger & Meyers 1989). Thus, the hyoid skeleton of birds, unlike that of mammals, is not directly attached to the skull (Fig. 2 & Fig. 3).
The laryngeal skeleton, which caps the upper end of the trachea, lies on the dorsal side of the urohyale and caudal end of the basihyale; it is embraced by the paired hyoid horns (Fig. 2 & Fig. 4). The laryngeal skeleton forms the skeletal framework of the prominent fleshy laryngeal mound, which can be protracted or retracted relative to the hyoid skeleton by the extrinsic laryngeal muscles. Thus, the larynx in birds, unlike that in mammals, does not articulate directly with the hyoid skeleton.
A hyoid sheath consists of two layers of connective tissue that are separated by a synovial cavity containing lubricating fluid and are interconnected by a mesentery-like membrane (Fig. 5; Homberger & Meyers 1989). The internal, or visceral, layer is fused to the connective tissue envelope (i.e., epimysium) of the M. branchiomandibularis, which winds around the hyoid horn and originates from the mandible (Fig. 3 & Fig. 5). When the M. branchiomandibularis contracts, it protracts the hyoid horn together with the visceral layer of the hyoid sheath and, thereby, pulls it out of the parietal layer of the hyoid sheath, which remains anchored to the epimysium of the jaw muscles. The protracted hyoid horn is pushed back into the hyoid sheath when the M. serpihyoideus and M. stylohyoideus contract. Both muscles originate from the caudal ends of the mandibular ramus (Fig. 3) and insert directly or indirectly on the ventral surface of the rostral ends of the ceratobranchialia and the adjoining areas of the basihyale and (Homberger 1986; Homberger & Meyers 1989).
The back-and-forth movements of the hyoid skeleton and tongue play a crucial role for the transport of food through the mouth cavity and for pushing food items into the oesophagus during deglutition in birds that swallow relatively small food items. Zweers (1982 a, b, 1985) showed for the Domestic Pigeon (Columba livia) how this lingual, or 'slide-and-glue', feeding mechanism works for ingesting relatively small grains. A food item is grasped between the tips of the mandible, glued to the moist tip of the tongue, and lodged in the pharyngeal cavity by the retracting tongue. The tongue then slides forward under the food item until the laryngeal mound is situated in front of the food item (see also Fig. 4 for topographical relationships between the hyoid and laryngeal skeletons). The food item is held in place by the caudally pointing palatine papillae on the palate during the protraction of the tongue and is finally pushed into the oesophagus by the laryngeal mound with its caudal row of caudally pointing laryngeal papillae [i.e., ‘dermal flap’, ‘laryngeal spines’, or ‘scrapers’ of Zweers (1982a, b, 1985)] when the tongue is retracted again. Such choreographed movements of the tongue and larynx are typical for birds, such as parrots, pigeons, and chickens, that exclusively, or at least commonly, swallow relatively small food items.
However, many birds, such as herons, many piscivorous and frugivorous birds, and owls, are able to, and usually do, swallow relatively enormous prey items whole. For such birds it is necessary to expand the entrance to the pharyngeal cavity and oesophagus as much as possible. Many of these birds have evolved intraramal joints, or flexion zones, in their mandibles to widen their gape (e.g., Bühler 1981, 1992; Zusi & Warheit 1992). This structural modification, however, only expands the entrance to the oesophagus at the level of the bill gape, but not at the level of the pharyngeal cavity where a food item needs to pass between the palate and the hyoid skeleton. Furthermore, intraramal joints are not an option for birds, such as herons and egrets (Ardeidae) that depend on a rigid, lance-like bill to capture prey.
In herons and egrets, such as the Snowy Egret Egretta thula as an example of birds that swallow relatively large prey items whole, the hyoid horns within their hyoid sheaths are located well below the mandible, which creates a greater space between the palate and the hyoid skeleton (Fig. 6). The hyoid sheaths, therefore, are not anchored to the surface of jaw muscles, but to the fascial wall of the throat. Furthermore, the antagonistic pair of muscles that protract or pull down the hyoid skeleton are bipartite with widely separate insertions, in contrast to the corresponding muscles that insert only on the hyoid horn in birds that swallow smaller food items and have a tight hyoid suspension (Cummins 1986, Cummins & Homberger 1986). The M. branchiomandibularis originates from the medial surface of the mandibular ramus. Its cranial slip inserts on the distal end of the hyoid horn after winding around it, but its caudal slip inserts along the dorsal side of the parietal layer of the hyoid sheath. The M. tracheohyoideus originates from the epimysium of the pectoral muscle at the base of the neck. Its cranial slip inserts on the medio-ventral surface of the proximal half of the ceratobranchiale, while its caudal slip inserts on the lateral surface of the parietal layer of the hyoid sheath. In this manner, the M. branchiomandibularis and M. tracheohyoideus do not move the hyoid horns relative to their hyoid sheaths, as in birds that swallow relatively small food items, but move the hyoid horns together with their hyoid sheaths and the fascial wall of the throat to which they are anchored. The ensuing loss of manoeuverability for the hyoid skeleton in this construction may be tolerable for birds, such as herons and egrets, that do not use their tongue to manipulate and transport prey items during deglutition. This type of loose hyoid suspension allows a maximal expansion of the pharyngeal cavity and entrance to the oesophagus. This expansion is limited only by the extensibility of the oesophagus and wall of the throat because the hyoid skeleton with a loose suspension is built into the wall of the throat and, thus, moves with it as it expands and contracts. Hence, the expansion of the oesophageal entrance is not limited by the dimensions of the hyoid suspensory apparatus as it does in mammals.
If we return to the original question 'Why did birds lose their teeth?', we now realise that the proper question should be 'Why did not all mammals lose their teeth'. The loss of teeth economises energy and materials and is prevented by natural selection only if the presence of teeth plays a crucial role in the nutrition of an organism. In most mammals, teeth are critical not only to grab prey or acquire food, but also to break it down physically so that it can pass the Isthmus faucium during deglutition. The expansion of the Isthmus faucium beyond a certain limit that would be necessary to let pass relatively large prey items whole is not an evolutionary option for mammals because the hyoid skeleton serves as an anchoring place for the muscular tongue and cannot lose its firm moorings to the base of the skull. That a masticatory dentition in mammals is indeed selectively maintained in this manner is corroborated by the numerous cases of edentulous mammals who feed exclusively on very small food items, such as ants, pollen, or plankton. Birds, unlike mammals, do not necessarily need to break down their food before swallowing it, even if they feed on large prey items, though some birds, such as raptors and vultures, do. Because the avian hyoid skeleton is not, and does not need to be, attached directly to the skull, it can be moved away from the skull in response to selective pressures for a larger entrance to the oesophagus to allow the swift ingestion of large prey items (see also Homberger 1994).
WHY CAN PARROTS TALK?
Parrots, like songbirds and hummingbirds, can generate and learn complex vocalisations for intraspecific communication (e.g., Pepperberg 1994, 1997; Farabaugh & Dooling 1996; Pepperberg & McLaughlin 1996; Dent et al. 1997; Homberger 1997, in press a; Pepperberg, Naughton & Banta 1998). Parrots, like many songbirds, are also very adept at imitating human speech, that is at generating articulated vocalisations with vowel-like and consonant-like qualities in addition to frequency-modulated and amplitude-modulated complex vocalisations, even though their imitation is not perfect (e.g., Nottebohm 1976; Patterson & Pepperberg 1994, 1998; Warren et al. 1996). This imperfect imitation is most probably due to the completely different anatomy of the sound producing and sound modulating and resonating structures and organs in birds and human beings, even though the basic physical principles of voice production are remarkably similar in both vertebrate classes.
In human beings, sound of variable frequencies and amplitudes is generated in the larynx by the vocal chords. This sound is then modulated while it passes through the supralaryngeal cavities, such as the narrow perilaryngeal tube and the pharyngeal and oral cavities, which selectively resonate and, thereby, alter the various frequencies generated by the larynx. The mobile tongue and lips can change the configurations and resonating qualities of the supralaryngeal cavities and, thereby, generate articulated speech with a variety of vowels and consonants (Denes & Pinson 1973; du Brul 1976; Titze & Story 1997).
In birds, sound is generated in the syrinx at the base of the trachea and resonates in the suprasyringeal cavities, such as the trachea and the oropharyngeal cavities, whose configurations can be changed by movements of the neck, trachea, glottis, tongue, and beaks (e.g., Rüppell 1933; Thorpe 1959; Hinsch 1972; Nottebohm 1976; Nowicki 1987; Nowicki & Marler 1988; Hausberger et al. 1991; Westneat et al. 1993; Patterson & Pepperberg 1994, 1998; Goller & Suthers 1996a, b; Warren et al. 1996; Goller & Larsen 1977a, b; Patterson et al. 1997; Fee et al 1998; Goller 1998; Pepperberg, Howell et al. 1998). However, these structures and resonating cavities, in contrast to the laryngeal chamber, do not differ in a fundamental or regular manner between birds that generate and learn complex, articulated vocalisations, such as parrots and songbirds, and birds that produce only relatively simple stereotyped vocalisations, such as chickens, herons, geese, and pigeons (Homberger 1997). What has not been considered is the role of the laryngeal chamber being an entity separate from the trachea and serving a crucial role as a modifiable resonating cavity for vocalizing birds, although Rüppel (1933) noticed that the removal of the larynx modified the sound quality, and Zweers (1985) analogized the laryngeal muscles and ligaments of songbirds with the cheeks and lips of human beings, and the laryngeal dorsal cricoids of songbirds with the human tongue, when analyzing the structural basis of whistling and glissando song in songbirds.
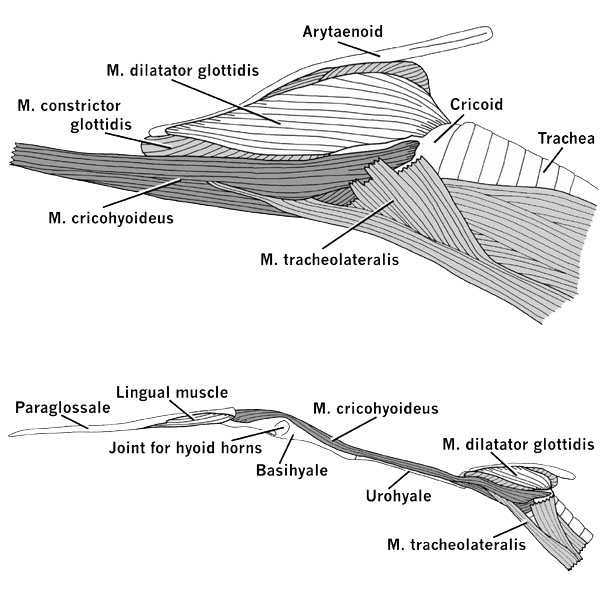
The laryngeal skeleton of a bird that produces simple vocalisations, such as the Snowy Egret Egretta garzetta among the Ardeidae (see, e.g., Bayer 1984), consists of a large cricoid that encloses a laryngeal chamber at the upper end of the trachea. The paired arytaenoids, which open and close the glottis when the intrinsic laryngeal muscles contract, are attached caudally to the cricoid through the unpaired procricoid (Fig. 7). The larynx can be moved back and forth relative to the hyoid skeleton (Fig. 8). The M. tracheolateralis is the laryngeal retractor; it originates from the Membrana sternocoracoclavicularis at the base of the neck and inserts on the ventral and ventrolateral surfaces of the cricoid. The M. cricohyoideus is a laryngeal protractor; it originates on the basihyale and inserts on the ventral surface of the cricoid. A box-like cricoid and undivided extrinsic laryngeal muscles that act as simple protractors and retractors are also characteristic of other birds with simple vocalisations (Homberger 1997), such as geese, chickens, and pigeons (Göppert 1937; White 1975; Zweers et al. 1981).
The laryngeal skeleton of a parrot, such as the Grey Parrot Psittacus erithacus, however, differs in several aspects that turn out to be critical for the generation of complex, articulated speech (Fig. 4). The cricoid is reduced to a flat ring that sits atop the upper end of the trachea. The paired arytaenoids and the unpaired procricoid are connected to the cricoid in essentially the same manner as in other birds. But the laryngeal chamber is formed mainly by half-rings of the trachea.
The extrinsic laryngeal muscles that move the larynx relative to the hyoid skeleton are much more complex than in other birds (Fig. 4). The M. tracheolateralis originates from the base of the neck and inserts with an rostral slip on the ventral surface of the rostral end of the cricoid and with a caudal slip to the dorsal surface of the caudal end of the cricoid. The M. cricohyoideus consists of two main parts with different attachments. The dorsal part originates with two heads, one from the caudal end of the paraglossale, the other from the basihyale, but inserts by a common slip on the dorsal surface of the caudal end of the cricoid. The ventral part of the M. cricohyoideus originates from the basihyale and inserts on the ventral surface of the caudal end of the cricoid.
This complex arrangement of the extrinsic laryngeal muscles permits a multitude of laryngeal movements depending on which muscle parts contract synchronously with others. If all the parts of the M. cricohyoideus contract simultaneously, the larynx is protracted relative to the hyoid skeleton, and if the various slips of the M. tracheolateralis contract simultaneously, the larynx is retracted relative to the hyoid skeleton. If, however, the rostral slip of the M. tracheolateralis contracts at the same time as the dorsal part of the M. cricohyoideus, or if the caudal slip of the M. tracheolateralis contracts at the same time as the ventral part of the M. cricohyoideus, then the cricoid pivots on top of the laryngeal chamber and, thereby, changes the configuration of this resonating chamber considerably. For example, the pivoting of the cricoid can determine not only the size and the shape of the laryngeal chamber but also whether, and to what extent, the ventrally projecting caudal end of the cricoid and procricoid protrudes into the laryngeal cavity (see Fig. 4). Even slight contractions of individual muscle parts have the potential to modify the configuration of the laryngeal cavity and, thereby, to contribute to the articulation of sounds. In addition, the extremely complex intrinsic laryngeal musculature (Homberger 1979), which is independent of the extrinsic laryngeal musculature, is capable of modifying the laryngeal chamber by moving the arytaenoids and, thereby, modifying the size and shape of the glottal opening, as well as by moving the procricoid-arytaenoid complex relative to the cricoid and, thereby, modifying the configuration of the roof of the laryngeal chamber. At the same time, any movement of the laryngeal mound, be it through a pivoting of the underlying cricoid or through a translational movement of the entire larynx along the basihyale and urohyale, will also affect the configuration and, thereby, the resonating properties, of the oropharyngeal chamber. Furthermore, movements of the larynx are superimposed on those of the hyoid skeleton (Homberger 1979, 1986), so that the number of possible configurations of the laryngeal and oropharyngeal chambers is multiplied by the combined movements of the hyoid and laryngeal skeletons. Finally, because the hyoid is connected to the mandible by its extrinsic lingual muscles (Homberger 1986), movements of the jaw also affect any movements and positions of the tongue and larynx and, thereby, the configuration of the oropharyngeal cavity.
The present biomechanical model of the role of the larynx in vocalisation is amenable to testing by recording the synchronisation of actual muscle contractions and skeletal motions in vocalising birds by electromyography and other instrumental approaches, with synchronous recordings of the vocalisations and movements of the beak, tongue, and larynx. This model also provides a functional-anatomical basis for the configurations of the tongue, laryngeal mound, oropharyngeal cavity, and trachea that were linked to the production of articulated speech by Warren et al. (1996), Patterson et al. (1997), and Patterson (pers. comm.) in a Grey Parrot.
Thus, the answer to the old question 'Why can parrots talk?' can now be answered. Parrots possess a kinetic larynx, which has a unique structure and is capable of modulating its configuration and resonating properties in subtle and rapid ways. This type of larynx, in combination with the particular mobility of the psittacine tongue, allows for a multitude of motions that modulate not only the configuration of the laryngeal cavity, but also that of the oropharyngeal cavity.
WHY CAN PARROTS AND SONGBIRDS TALK - AND HERONS, PIGEONS, GEESE, AND CHICKENS NOT?
Parrots are not known to mimic human speech under natural condition, and, therefore, the question arises as to the nature of the selective regime that was responsible for the evolution of a kinetic larynx and the concomitant capacity to generate complex articulated vocalisation in parrots. Most parrots swallow only relatively small food items (Homberger 1980, 1990, 1991, 1996) and use their tongue and larynx to push food items into their oesophagus in a similar manner as that described by Zweers (1982 a, b, 1985) for pigeons and crows that swallow small food items. As Zweers (1982 b, 1985) has shown, the piston-like action of the laryngeal mound is made more effective by raising the caudal end of the laryngeal mound above the floor of the oesophagus. Parrots achieve this by pivoting the cricoid above their laryngeal chamber with the help of their extrinsic laryngeal muscles. This pivoting of the cricoid has also a concomitant effect on the configuration of the laryngeal chamber. It must have evolved under a selective regime affecting the feeding mechanism, which had the side-effect of also affecting the ability to modulate vocalisations. This is supported by the fact that various birds that generally swallow relatively small food items have evolved a capacity for raising the caudal end of the laryngeal mound above the floor of the oesophagus, but not all have evolved a capacity for generating complex songs at the same time (see also Zweers 1985).
Pigeons raise the row of laryngeal papillae on the caudal end of the laryngeal mound [i.e., the 'ventral pharyngeal valve' of Zweers et al. (1981) and Zweers (1982 a, b); 'scrapers' of Zweers (1985)] above the oesophageal floor with the help of a special muscle. However, because the large cricoid of the pigeon encloses the laryngeal chamber rigidly in a similar manner as that of the Snowy Egret (Fig. 7), this movement does not modify the configuration of the laryngeal chamber (Zweers et al. 1981).
Songbirds, as shown by Bock (1972, 1978), Zweers (1985) and Zweers & Barkhoudt (1988) for various corvid and other oscine passeriform species, possess a special muscle [i.e., 'M. thyreohyoideus superior' of Bock (1972), 'M. cricohyoideus superior' of Bock (1978), 'dermocricoid muscle' of Zweers (1985), and 'M. cricodermoideus' of Zweers & Berkhoudt (1988)], which raises the laryngeal papillae at the caudal end of the laryngeal mound [i.e., 'posterior flap' of Bock (1972), 'posterior laryngeal flap' of Bock (1978), 'scrapers' of Zweers (1985), and 'pharyngeal scrapers' of Zweers & Berkhoudt (1988)]. Bock (1972) conjectured that this mechanism serves to prevent live prey that is being swallowed from escaping the gullet. The cricoid of the larynx of songbirds consists of two parts, namely a ventrally located actual cricoid and a pair of dorsal cricoids, which movably articulate with the cricoid (Bock 1978, Zweers 1985, Zweers & Berkhoudt 1988). These dorsal cricoids are rotated upwards, when the special muscle that raises the laryngeal papillae contracts, and are likely to have evolved as integral mechanical elements of the mechanism responsible for raising the caudal end of the laryngeal mound to push food items into the oesophagus. Because of the involvement of laryngeal skeletal elements, the mechanism of raising the laryngeal papillae simultaneously also modifies the configuration of the laryngeal chamber (see also Zweers 1985). Therefore, the movements of the kinetic larynx of songbirds, like that of parrots, can play a role in either vocalisations or swallowing movements.
Thus, the question 'Why can parrots and songbirds talk - but herons, geese, pigeons, and chickens, not?' can now also be answered. The raising of the caudal end of the laryngeal mound with its laryngeal papillae is an integral part of the feeding mechanism in birds that swallow relatively small food items and has been evolved several times independently by different avian taxa. If this mechanism involves only the soft tissues of the laryngeal mound, then the configuration of the laryngeal chamber is not affected, and the capacity for generating complex, articulated vocalisations is not given, such as in herons, pigeons, geese, and chickens. If, however, the mechanism of raising the laryngeal papillae is based on movements of skeletal elements of the larynx, so that the configuration of the laryngeal chamber is affected, then the evolution of this mechanism results in a concomitant capacity for complex, articulated vocalisations, as has happened independently in parrots and songbirds. That parrots and songbirds have evolved a capacity for complex articulated vocalisation independently from each other is evidenced not only by the fundamentally different construction of their kinetic larynges, but also by the different neural circuitry in their telencephala (see Ball 1994; Striedter 1994; Dubbeldam 1998) and the fundamentally different role of complex, articulated vocalisations in the maintenance and evolutionary history of their social behaviour.
CONCLUSION
As long as the questions 'Why did birds lose their teeth?' and 'Why can parrots talk?' were pursued separately and directly, little progress was made in finding conclusive answers. The key for a breakthrough in these evolutionary riddles lay in integrative approaches and conceptual innovations. As I tried to show, the structure and function of the hyoid apparatus, its suspensory apparatus, and the laryngeal apparatus with its extrinsic musculature are indeed highly interdependent, and their specific conditions have fundamental ecological and evolutionary implications.
Even though vertebrate morphology is presently one of the more integrative branches of biology, it tends to analyse the structure of organisms by looking at body parts and organs that are usually defined in terms of their functional contributions to the organism, such as the feeding apparatus or the locomotor apparatus. This approach tends to obscure the fact that most organs and apparatus, such as the head, are highly integrated systems with multiple functions and roles (see also Dubbeldam 1998). Traditionally, the jaw, lingual and laryngeal apparatus have been analysed separately, and the individual apparatus were assigned to particular systems, such as the jaw and lingual apparatus to the digestive system, and the laryngeal apparatus to the respiratory system. This particulate conceptualisation of the head, although heuristically still useful, harbours the danger of hindering a proper understanding of how the various components of the head are interconnected and interacting in multiple and overlapping roles of feeding, drinking, breathing, and vocalisation. It is, therefore, necessary to maintain a holistic view of the organism while dissecting and analysing the structure and function of its components. A holistic view is also required because it is not possible to decide a priori which aspects of a system or apparatus will turn out to have been crucial in determining the direction and extent of evolutionary changes.
Vertebrate morphology relies heavily on the comparative method. For the last century, under the influence of Darwin's theory, the assumption that only phylogenetically related organisms should and can be compared has become widely accepted. However, as the examples presented here demonstrate, the comparison of organisms that are only very distantly related, such as birds and mammals, can yield unique insights, provided that the organisms are selected carefully to create natural experiments (see also Homberger 1988, 1996, in press b). Thus, innovations in concepts and methodology are necessary to open new doors on old questions.
Finally, the examples presented here also point to the fact that the solution to old problems in biology often depends on new anatomical information. The acquisition of such anatomical data may be less dependent on novel techniques than what is usually assumed, but more on an innovative conceptualisation of how organisms are built and constructed. In any case, the examples presented in this review show how a deficit in anatomical data may hold back scientific progress, even if other biological disciplines are flowering and productive.
ACKNOWLEDGMENTS
Rholene Heintze typed the manuscript. Karen Westphal prepared the original illustrations, and Ron Bouchard prepared the electronic files and graphic manipulations of all figures. Drs R. Prakash Dixit and A. Ravi P. Rau, Louisiana State University, provided feedback on the physics of speech production. Dr. Irene M. Pepperberg and Dianne K. Patterson, University of Arizona, and Dr. Gart A. Zweers, Leiden University, read a draft of the manuscript and provided valuable comments. I thank all for their contributions.
REFERENCES
Ackermann, J. 1998. Dinosaurs take wing. National Geographic 194 (1): 74-99.
Ball, G.F. 1994. Comparative studies of the avian vocal control circuit reveal neural specializations associated with vocal learning and production. Journal für Ornithologie 135 (3): 420.
Bayer, R.D. 1984. Vocalisations of Great Blue Herons at Yaquina Estuary, Oregon. Colonial Waterbirds 7: 35-44.
Bock, W.J. 1972. Morphology of the tongue apparatus of Ciridops anna (Drepanididae). Ibis 114: 61-78.
Bock, W.J. 1978. Morphology of the larynx of Corvus brachyrhynchos (Passeriformes: Corvidae). Wilson Bulletin 90 (4): 553-565.
Böker, H. 1937. Einführung in die vergleichende biologische Anatomie der Wirbeltiere. Volume 2; Biologische Anatomie der Ernährung. Jena; Verlag von Gustav Fischer: 258pp.
Brooke, M. & Birkhead, T. 1991. The Cambridge Encyclopedia of Ornithology. Cambridge; Cambridge University Press: 363pp.
Bühler, P. 1981. Functional anatomy of the avian jaw apparatus. In: King, A.S. & McLelland, J. (eds) Form and function in birds, Volume 2. London; Academic Press: 439-468.
Bühler, P. 1992. Light bones in birds. In: Campbell, K.E. (ed) Papers in avian paleontology honoring Pierce Brodhorb. Los Angeles; Natural History Museum of Los Angeles County: 385-393.
Bühler, P. 1996. Die neotropischen Tukane (Rhamphastidae) als Modell einer ökomorphologischen Evolutionsanalyse. Oekologie der Vögel (Ecology of Birds) 18: 127-162.
Catchpole, C.K. & Slater, P.J.B. 1995. Bird song: Biological themes and variations. Cambridge; Cambridge University Press: 248pp.
Chatterjee, S. 1997. The rise of birds: 225 million years of evolution. Baltimore; Johns Hopkins University Press: 312pp.
Cummins, C.L. 1986. The morphology of the hyoid apparatus and gular region of the Snowy Egret, Egretta thula (Molina), (Aves: Ardeidae). M.S. Thesis, Louisiana State University and A&M College, Baton Rouge.
Cummins, C.L. & Homberger, D.G. 1986. The morphology of the gular region of the Snowy Egret, Egretta thula (Molina). American Zoologist 26: 66A.
Denes, P.B. & Pinson, E.N. 1973. The speech chain: The physics and biology of spoken language. Garden City, New York; Anchor Press/Doubleday: 217pp.
Dent, M.L., Brittan-Powell, E.F., Dooling, R.J. & Pierce, A. 1997. Perception of synthetic /ba/-/wa/ speech continuum by budgerigars (Melopsittacus undulatus). Journal of the Acoustical Society of America 102 (3): 1891-1897.
Dingus, L. & Rowe, T. 1997. The mistaken extinction: Dinosaur evolution and the origin of birds. New York; W.H. Freeman & Co.: 332pp.
Dubbeldam, J.L. 1998. Intratelencephalic circuits in birds - what have feeding and vocalisation in common? Netherlands Journal of Zoology 48 (3): 199-212.
Du Brul, E.L. 1976. Biomechanics of speech sounds. In: Harnad, S.R., Steklis, H.D. & Lancaster, J. (eds). Origins and evolution of language and speech. Annals of the New York Academy of Sciences 280: 631-642.
Dyce, K.M., Sack, W.O. & Wensing, C.J.G. 1987. Textbook of veterinary anatomy. Philadelphia; W.B. Saunders Company: 820pp.
Farabaugh, S.M. & Dooling, R.J. 1996. Acoustic communication in parrots: Laboratory and field studies of budgerigars, Melopsittacus undulatus. In: Kroodsma, D.E. & Miller, E.H. (eds) Ecology and evolution of acoustic communication in birds. Ithaca; Cornell University Press: 97-117.
Feduccia, A. 1996. The origin and evolution of birds. New Haven; Yale University Press: 420pp.
Fee, M.S., Shraiman, B., Pesaran, B. & Mitra, P.P. 1998. The role of non-linear dynamics of the syrinx in the vocalisations of a songbird. Nature 395: 67-71.
Gill, F.B. 1990. Ornithology. New York; W.H. Freeman & Co.: 660pp.
Goller, G. 1998. Vocal gymnastics and the bird brain. Nature 395: 11-12.
Goller, G. & Larsen, O.N. 1997a. A new mechanism of sound production in songbirds. Proceedings of the National Academy of Sciences of the United States of America 94:14787-14971.
Goller, F. & Larsen, O.N. 1997b. In situ biomechanics of the syrinx and sound generation in pigeons. Journal of experimental Biology 200 (16): 2165-2176.
Goller, F. & Suthers, R.A. 1996a. Role of syringeal muscles in gating air flow and sound production in singing brown thrashers. Journal of Neurophysiology 75 (2): 867-876
Goller, F. & Suthers, R.A. 1996b. Role of syringeal muscles in controlling the phonology of bird song. Journal of Neurophysiology 76: 287-300.
Göppert, E. 1937. Kehlkopf und Trachea. In: Bolk, L., Göppert, E., Kallius, E. & Lubosch, W. (eds) Handbuch der vergleichenden Anatomie der Wirbeltiere, Volume 3. Berlin; Urban & Schwarzenberg: 797-866.
Hausberger, M., Black, J.M. & Richard, J.-P. 1991. Bill opening and sound spectrum in barnacle goose loud calls: individuals with wide mouth have higher pitched voices. Animal Behaviour 42: 319-322.
Hiiemae, K.M. & Crompton, A.W. 1985. Mastication, food transport, and swallowing. In: Hildebrand, M., Bramble, D.M., Liem, K.F. & Wake, D.B. (eds), Functional vertebrate morphology. Cambridge, Massachusetts; Belknap Press of Harvard University Press: 262-290.
Hinsch, K. 1972. Akustische Gesangsanalyse beim Fitis (Phylloscopus trochilus) zur Untersuchung der Rolle der Luftröhre bei der Stimmerzeugung der Singvögel. Journal für Ornithologic 113 (3): 315-322.
Homberger, D.G. 1979. Functional morphology of the larynx in the parrot Psittacus erithacus. American Zoologist 19 (3): 988.
Homberger, D.G. 1980. Funktionell-morphologische Untersuchungen zur Radiation der Ernährungs- und Trinkmethoden der Papageien. Bonner Zoologische Monographien No. 13: 192pp.
Homberger, D.G. 1986. The lingual apparatus of the African Grey Parrot, Psittacus erithacus Linné (Aves: Psittacidae): Description and theoretical mechanical analysis. Ornithological Monographs, No. 39: 233pp.
Homberger, D.G. 1988. Models and tests in morphology: The significance of description and integration. American Zoologists 28 (1):217-229.
Homberger, D.G. 1990. Filing ridges and transversal step of the maxillary rhamphotheca in Australian cockatoos (Psittaciformes: Cacatuidae): A homoplastic structural character evolved in adaptation to seed shelling. In: van den Elzen, R., Schuchmann, K.-L. & Schmidt-Koenig, K. (eds) Proceedings of the International 100. Deutsche Ornithologen-Gesellschaft Meeting: Current topics in avian biology. Bonn; Deutsche Ornithologen-Gesellschaft: 43-48.
Homberger, D.G. 1991. The evolutionary history of parrots and cockatoos: A model for evolution in the Australasian avifauna. Acta XX Congressus Internationalis Ornithologici: 398-403.
Homberger, D.G. 1994. The hyoid suspension apparatus as a structural constraint of feeding mechanisms in birds and mammals. Journal of Morphology 220 (3): 355.
Homberger, D.G. 1996. The comparative feeding ecology and functional morphology of Australian cockatoos: A case study in the phylogenetic reconstruction of a complex group, the Psittaciformes. In: Vielliard, J.M.E., da Silva, M.L. & Silva, W.R. (eds) Anais V Congresso Brasileiro de Ornitologia. Campinas, Brasil; UNICAMP: 43-50.
Homberger, D.G. 1997. The role of the larynx in articulated vocalisation of birds. American Zoologist 37 (5): 136A.
Homberger, D.G. In press a. Psittacidae. In: Ross, G.J.B. (ed) Fauna of Australia, Volume 2. Canberra; Australian Government Publishing Service.
Homberger, D.G. In press b. Similarities and differences: The distinct approaches of systematics and comparative anatomy towards homology and analogy. Theory in Bioscience.
Homberger, D.G. & Meyers, R.A. 1989. Morphology of the lingual apparatus of the domestic chicken, Gallus gallus, with special attention to the structure of the fasciae. American Journal of Anatomy 186: 217-257.
Hou, L.-H., Zhou, Z., Martin, L.D. & Feduccia, A. 1995. A beaked bird from the Jurassic of China. Nature 877: 616-618.
Kier, W.M. & Smith, K.K. 1985. Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zoological Journal of the Linnean Society 83: 307-324.
Kroodsma, D.E. & Miller, E.H. 1982. Acoustic communication in birds, Volume 2: Song learning and its consequences. New York; Academic Press: 388pp.
Kroodsma, D.E. & Miller, E.H. 1996. Ecology and evolution of acoustic communication in birds. Ithaca, New York; Cornell University Press: 587pp.
Maderson, P.F.A. & Homberger, D.G. In press. The evolutionary origin of feathers. Introduction to the Symposium at the Annual Meeting of the Society for Integrative and Comparative Biology in Denver, Colorado, January 1999. American Zoologist 39.
Morell, V. 1997. Fossilized hatchling heats up the bird-dinosaur debate. Science 276 (6 June): 1501.
Nottebohm, F. 1976. Phonation in the Orange-winged Amazon Parrot Amazona amazonica. Journal of Comparative Physiology A 108:157-170.
Nowicki, S. 1987. Vocal tract resonances in oscine bird sound production: Evidence from bird songs in a helium atmosphere. Nature 325: 53-55.
Nowicki, S. & Marler, P. 1988. How do birds sing? Music Perception 5 (4): 391-426.
Patterson, D.K. & Pepperberg, I.M. 1994. A comparative study of human and parrot phonation: Acoustic and articulatory correlates of vowels. Journal of the Acoustical Society of American 96 (2): 634-648.
Patterson, D.K. & Pepperberg, I.M. 1998. Acoustic and articulatory correlates of stop consonants in a parrot and a human subject. Journal of the Acoustical Society of America 103 (4): 2197-2215.
Patterson, D.K., Pepperberg, I.M., Story, B.H. & Hoffman, E.A. 1997. How parrots talk: Insights based on Ct scans, image processing and mathematical models. Proceedings of the Society of Photo-Optical Instrumentation Engineers 3033-02: 14-24.
Pepperberg, I.M. 1994. Vocal learning in Grey Parrots (Psittacus erithacus). Effects of social interaction, reference, and context. The Auk 111 (2) 300-313.
Pepperberg, I.M. 1997. Social influences on the acquisition of human-based codes in parrots and non-human primates. In: Snowdon, C.T. & Hausberger, M. (eds) Social influences on vocal development. Cambridge; Cambridge University Press: 157-177.
Pepperberg, I.M. & McLaughlin, M.A. 1996. Effect of avian-human joint attention on allospecific vocal learning by Grey Parrots (Psittacus erithacus). Journal of Comparative Psychology 110 (3): 286-297.
Pepperberg, I.M., Naughton, J.R. & Banta, P.A. 1998. Allospecific vocal learning by Grey Parrots (Psittacus erithacus): A failure of videotaped instruction under certain conditions. Behavioural Processes 42: 139-158.
Pepperberg, I.M., Howell, K.S., Banta, P.A., Patterson, D.K. & Meister, M. 1998. Measurement of Grey Parrot (Psittacus erithacus) trachea via magnetic resonance imaging, dissection, and electron beam computed tomography. Journal of Morphology 238: 81-91.
Portmann, A. 1950. Squelette. In: Grassé, P.-P. (ed.) Traité de zoologie: Anatomie, systématique, biologie. Volume 15: Oiseaux. Paris; Masson et Cie: 78-107.
Rübel, G.A., Isenbügel, E. & Wolvekamp, P. 1991. Atlas of diagnostic radiology of exotic pets. Philadelphia; W.B. Saunders Company: 224pp.
Rüppell, W. 1933. Physiologie und Akustik der Vogelstimme. Journal für Ornithologie 81 (3): 433-542.
Sanz, J.L., Chiappe, L.M., Pérez-Moreno, B.P., Moratalla, J.J., Hernándex-Carrasquilla, F., Buscalioni, A.D., Ortega, F., Poyato-Ariza, F.J., Rasskin-Gutmann, D. & Martínez-Delclõs, X. 1997. A nestling bird from the lower Cretaceous of Spain: Implications for avian skull and neck evolution. Science 276: 1542-1546.
Schummer, A. & Nickel, R. 1967. Eingeweide. In: Nickel, R., Schummer, A. & Seiferle, E. (eds) Lehrbuch der Anatomie der Haustiere, Volume 2. 2nd edition; Hamburg; Paul Parey: 412pp.
Shipman, P. 1998. Taking wing: Archaeopteryx and the evolution of bird flight. New York; Simon & Schuster: 336pp.
Snowdon, C.T. & Hausberger, M. 1997. Social influences on vocal development. Cambridge; Cambridge University Press: 352pp.
Striedter, G.F. 1994. The vocal control pathways in budgerigars differ from those in songbirds. Journal of comparative Neurology 343: 35-56.
Thielcke, G. 1970. Vogelstimmen. Berlin; Springer-Verlag: 156pp.
Thorpe, W.H. 1959. Talking birds and the mode of action of the vocal apparatus in birds. Proceeding of the Zoological Society of London 132 (3): 441-455.
Titze, I.R. & Story, B.H. 1997. Acoustic interactions of the voice source with the lower vocal tract. Journal of the Acoustical Society of America 101 (4): 2234-2243.
Walker, W.F. & Homberger, D.G. 1992. Vertebrate Dissection. 8th edition; Philadelphia; Saunders College Publishing: 459pp.
Walker, W.F. & Homberger, D.G. 1993. A study of the cat with references to human beings. 5th edition; Philadelphia; Saunders College Publishing: 294pp.
Warren, D.K., Patterson, D.K. & Pepperberg, I.M. 1996. Mechanisms of American English vowel production in a Grey Parrot (Psittacus erithacus). The Auk 113 (1): 41-58.
Westneat, M.W., Long, J.H., Hoese, W. & Nowicki, S. 1993. Kinematics of birdsong: Functional correlation of cranial movements and acoustic features in sparrows. Journal of experimental Biology 182: 147-171.
White, S.S. 1975. Larynx. In: Getty, R. (ed) Sisson and Grossmans The anatomy of the domestic animals. Volume 2; 5th edition; Philadelphia; W.B. Saunders: 1891-1897.
Zimmer, C. 1996. From teeth to beak. Discover 17 (1): 44-50.
Zusi, R.L. & Warheit, K.I. 1992. On the evolution of intraramal mandibular joints in pseudodontorns (Aves: Odontopterygia). In: Campbell, K.E. (ed) Papers in avian paleontology honoring Pierce Brodkorb. Los Angeles; Natural History Museum of Los Angeles County: 351-360.
Zweers, G.A. 1982 a. The feeding system of the pigeon (Columba livia L.). Advances in Anatomy, Embryology and Cell Biology 73: 1-108.
Zweers, G.A. 1982 b. Pecking of the pigeon (Columbia livia L.). Behaviour 81: 173-230.
Zweers, G.A. 1985. Generalism and specialism in avian mouth and pharynx. Fortschritte der Zoologie 30: 189-201.
Zweers, G.A. & Berkhoudt, H. 1988. Larynx and pharynx of crows (Corvus corone L. and Corvus monedula L., Passeriformes: Corvidae). Netherlands Journal of Zoology 37 (3-4): 365-393.
Zweers, G.A. & Gerritsen, A.F.C. 1997. Transitions from pecking to probing mechanisms in waders. Netherlands Journal of Zoology 47 (2): 161-208.
Zweers, G.A. & Vanden Berge, J.C. 1997 a. Evolutionary transitions in the trophic system of the wader-waterfowl complex. Netherlands Journal of Zoology 47 (3): 255-287.
Zweers, G.A. & Vanden Berge, J.C. 1997 b. Birds at geological boundaries. Zoology 100 (3): 183-202.
Zweers, G.A., van Pelt, H.C. & Beckers, A. 1981. Morphology and mechanics of the larynx of the pigeon (Columba livia L.): A drill-chuck system (Aves). Zoomorphology 99: 37-69.
Zweers, G.A., de Jong, F., Berkhoudt, H. & Vanden Berge, J.C. 1995. Filter feeding in flamingos (Phoenicopterus ruber). The Condor 97 (2): 297-324.
Fig. 1. Anatomy of the tongue and hyoid suspensory apparatus in a mammal, such as the Domestic Cat Felis catus. Upper figure: Longitudinal section through the head. (Modified from Walker & Homberger 1992: 317). Lower figure: Skull with hyoid suspensory apparatus, larynx, and upper end of trachea. (Modified from Walker & Homberger 1993: 25).

Fig. 2. Lateral views of the position of the hyoid apparatus relative to the skull in a parrot, such as a Blue-and-Yellow Macaw Ara ararauna. (Modified from Rübel, Isenbügel & Wolvekamp 1991: 84). Upper figure: X-ray photograph. Lower figure: Interpretative diagram of the x-ray photograph.
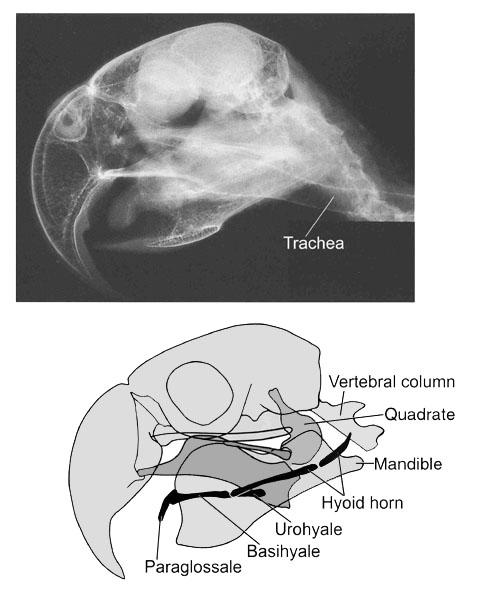
Fig. 3. Lateral view of the superficial muscles of the head in a Grey Parrot Psittacus erithacus with the caudal end of the hyoid horn embedded between the jaw and neck muscles and not connected to the skull. The hyoid sheath, which envelops the hyoid horn, is removed. Most of the hyoid apparatus is hidden from view by the mandible and external jaw muscles. All lingual muscles that are visible in this view are labelled. Abbreviations: EB epibranchiale; Mbm M. branchiomandibularis; Mdm M. depressor mandibulae; Mmh M. mylohyoideus; Msh M. serpihyoideus.
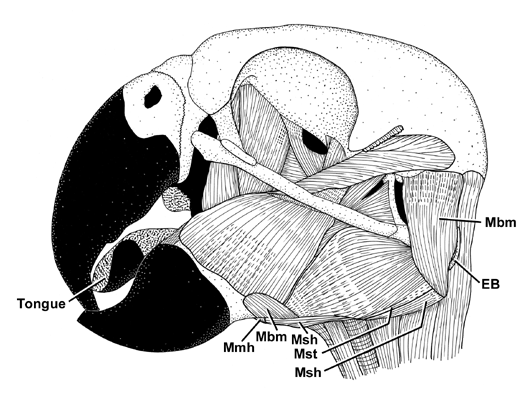
Fig. 4. Model of selected actions of the extrinsic laryngeal muscles in a Grey Parrot Psittacus erithacus. The hyoid skeleton is shown in its protracted position. Upper figure: Depression of the elevated caudal end of the cricoid. Lower figure: Elevation of the depressed caudal end of the cricoid.
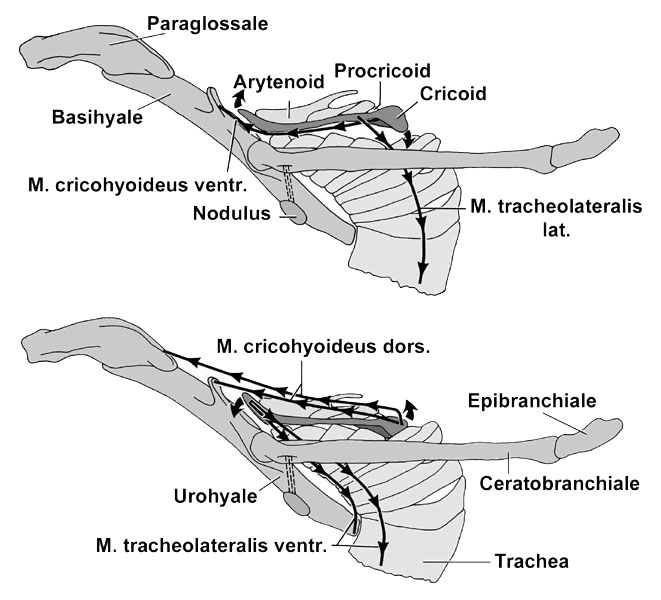
Fig. 5. Diagrammatic longitudinal section through a hyoid horn within its hyoid sheath of a bird, such as the Domestic Chicken Gallus gallus. The mesentery-like membrane connecting the parietal and visceral layers is not seen in these particular sections. Upper figure: Protraction of the retracted hyoid horn. Lower figure: Retraction of the protracted hyoid horn.
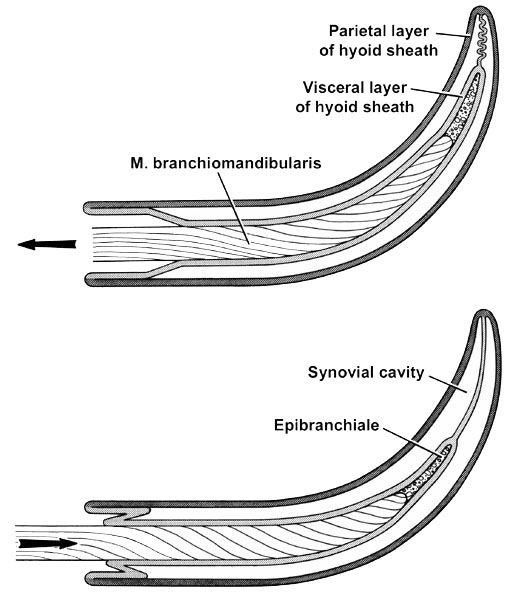
Fig. 6. Head and upper neck of a Snowy Egret Egretta thula. The constrictor and gular muscles are removed. The visceral layer of the hyoid sheath is not shown. Abbbreviations: Mbm M. branchiomandibularis; Mch M. ceratohyoideus; Mst M. stylohyoideus; Mtrh M. tracheohyoideus.
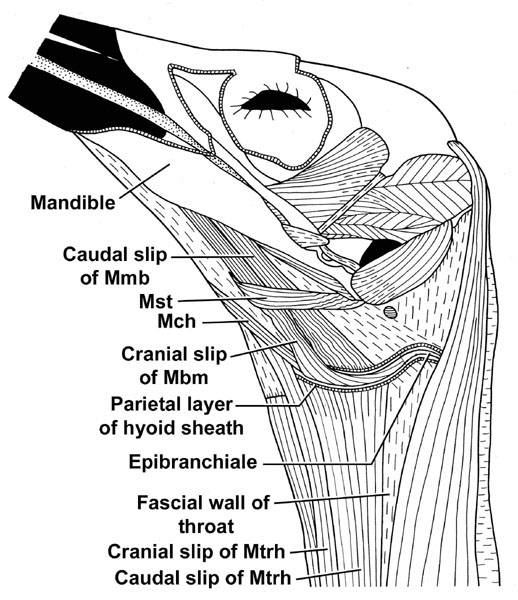
Fig. 7. Lateral view of the skeletal elements of the larynx and upper end of the trachea in a Snowy Egret Egretta thula.
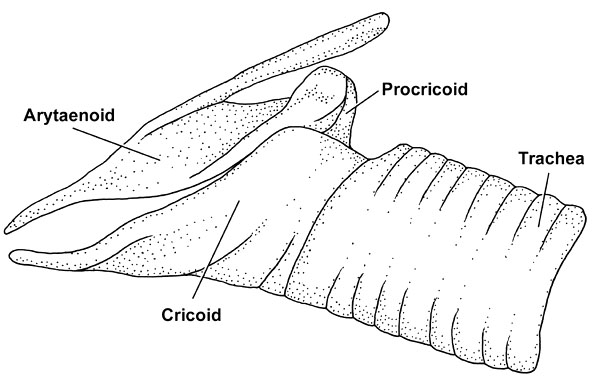
Fig. 8. Lateral views of the laryngeal apparatus of the Snowy Egret Egretta thula. Upper figure: Extrinsic and superficial intrinsic laryngeal muscles. Lower figure: Laryngeal apparatus with its muscular connections to the hyoid apparatus.