S01.5: Nutritional constraints on breeding in birds.
David C. Houston
Ornithology Group, Institute of Biomedical and Life Sciences, Graham Kerr Building, Glasgow University, Glasgow G12 8QQ, Scotland, U.K., fax 141 330 5971, e-mail D.Houston@bio.gla.ac.uk
Houston, D.C. 1998. Nutritional constraints on breeding in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
52-66. Johannesburg: BirdLife South Africa.There is some evidence from studies on gulls and tits that egg production can be constrained in birds by the quality of diet that is available to birds at the time they are forming eggs. For tits, protein quality influences egg production. Many bird species undergo loss of female muscle mass at the time they are forming eggs, and muscle proteins may contribute to egg production. This would enable birds to store nutrients which may be limiting in their diet at the time of egg formation, and so make a greater investment in reproduction than their food supply would otherwise permit. Studies on Zebra Finches suggest that the quality of muscle protein a bird is able to store prior to breeding may be a factor influencing egg production and other aspects of breeding performance.
INTRODUCTION
The food supply available to birds during the breeding season is generally recognised to be an important factor in determining the timing of breeding and the size of the investment made in eggs and young. A number of field studies have shown good correlations between estimates of natural food availability and various breeding parameters such as laying date and clutch size; for example, in Great Tits Parus major Perrins (1991) has shown that the abundance of caterpillars in any year is correlated with the mean number of eggs laid. Many studies have investigated whether this is a causal relationship by experimentally manipulating the size of the food supply available to some birds by the provision of supplementary feeding. The results of these studies have, however, been rather equivocal. In general, enhancing the food supply often results in an advancement of laying date, but few studies have shown any effect on the size or number of eggs laid (reviews in Martin 1987; Arcese & Smith 1988; Boutin 1990; Aparicio 1994).
The underlying assumption behind most of these supplementary feeding studies has been that the energy content of the food may limit breeding. Most studies use only a single form of supplementary feeding, and the type of food provided varies considerably between studies, as does the time over which it is provided in relation to the period when egg growth is occurring. It is possible that the nutritional composition of food may also have an influence on breeding in birds, and variations in the nutrients provided by different foods may explain some of the disparity in the results obtained from supplementary feeding studies.
This review considers whether there is any evidence that the nutritional quality of the diet may be a factor influencing breeding. Firstly it considers why nutritional factors might be important, and whether there is evidence for nutritional limitation on egg production in wild birds, particularly for protein quality. It then considers whether birds may be able to use nutrient stores to contribute to egg production. Finally, it considers the aspects of reproduction that may be influenced by nutrient reserve quality in birds.
WHY MIGHT NUTRITIONAL FACTORS BE IMPORTANT FOR EGG PRODUCTION?
Egg production can make extreme demands on a female bird. In some small passerines the mass of eggs laid weighs more than the body weight of the female (Perrins 1979). The scale of this investment varies between species, depending on the number and size of eggs laid and the body mass of the female. It demands not only substantial investment of energy, but also protein. Robbins (1981) estimated the daily energy cost of egg production varied between bird species from 37% to 216% of normal daily energy metabolism, and from 86 to 230% of daily protein requirements. Furthermore, the quality of protein required for egg production is rather specialised. Proteins are built from amino acids, some of which can be synthesised by the bird and are termed non-essential, and others of which are essential and must be obtained from the diet. The balance of amino acids required for egg proteins is rather unusual, having for example rather high requirements for the sulphur amino acids, methionine and cysteine (Paul & Southgate 1978; Paul et al. 1978; Murphy 1994; Houston et al. 1995a). It is, therefore, possible that the quality of protein, as well as the absolute quantity of protein, in the diet could constrain egg production. This is known to occur in domestic poultry, where egg production is enhanced if certain essential amino acids are added to commercial feed (Scott et al. 1982).
Is there any evidence for this in wild birds? It is known for Zebra Finches Taeniopygia guttata breeding in captivity on a seed diet that those females given access to diets with higher protein content will lay larger clutches (Williams 1997, Houston 1997). However, this is a rather specialised species, adapted for desert conditions and for breeding on poor quality diets where we might expect some nutrient limitation. It might be thought less likely to occur in species feeding on more normal, animal protein diets. Bolton et al. (1992) carried out a supplementary feeding study on Lesser Black-backed Gulls Larus fuscus during the period prior to and during egg formation. Some birds were provided with a supplement of animal fat during the period while they were forming eggs, while another group of nests were given a supplement of fish, both foods being provided in quantities of equivalent calorific value. If energy content of the diet was limiting, then both diets should result in enhanced egg production, but if protein or some other nutrient was limiting then only the fish-fed birds should lay larger eggs. Fig. 1 shows that only the fish-fed birds produced larger eggs than control, unfed, birds.
Bolton et al. (1992) conducted a second trial to consider if nutrient quality might be a factor, by comparing two supplementary feeds (provided in quantities of equivalent calorific and total protein content), one group receiving fish and another group cooked hens eggs. If nutrient quality was a factor, we would predict birds fed the egg supplement would produce larger eggs because presumably all nutrients required for egg formation will be present in eggs themselves in suitable proportions, whereas fish protein does not have such an optimal balance of, for example, amino acids. The results (Fig. 2) showed that egg-fed birds produced heavier clutches than control or fish fed birds.
Ramsay & Houston (1997) carried out a similar study with Blue Tits Parus caeruleus in which birds were given supplementary diets of either fat or a diet containing eggs. This showed (Fig. 3) that while the date of laying was influenced by dietary energy supply and both groups laid earlier than control groups, only those females with access to egg diets laid significantly larger eggs.
In these studies on gulls and tits we suspect that protein quality is the limiting factor, but we do not know this because the supplementary diets contained entire cooked hen’s eggs, and it is possible that other nutrients such as essential fatty acids, vitamin or minerals may have been involved. To confirm that protein quality is a factor limiting egg production we carried out another experimental trial with Blue Tits in which birds were provisioned with two diets which were of identical calorific, total protein and non-protein nutrient balance, and differed only in that one was supplemented with five essential amino acids (selected because they were known to be in higher concentration in egg proteins) and the other was supplemented with sufficient glutamic acid to make the two diets isonitrogenous. Fig. 4 shows that only those females receiving the ‘high quality’ protein diet laid larger clutches than control birds, containing about 18% more eggs.
We might conclude therefore that there is some evidence that quality of diet can influence egg production. Many nutrients might be involved, but amino acid balance is likely to be among the most important factors and there is evidence that this can constrain even a species such as a tit feeding largely on an animal protein diet.
NUTRIENT STORAGE IN BIRDS
Lack (1967) first suggested that the breeding season for birds was determined by food supply, such that the period when the parents had to obtain the maximum amount of food for the young would coincide with the season when food was most abundant. As a consequence, birds may need to lay eggs many weeks before the peak of food supply, when food may be scarse. In this situation there would be strong selective pressures on birds to use endogenous sources of nutrients to contribute to egg production. Females that had good body stores might then be able to lay earlier, to lay larger eggs or clutches than their food supply would otherwise permit.
There has been extensive research on egg production in domestic poultry, and these birds rely totally on food intake for egg formation. However, chickens are not a typical model for wild birds, for the obvious reason that they have been selectively bred by man to produce, in some breeds, one egg every day for their entire productive life. This astonishing rate of egg production can only be sustained if the total daily requirements for egg synthesis are met from the diet, and not from body stores, although some tissues may act as a temporary storage pool over the 24-hour cycle, as is known to occur for the calcium required for shell formation (Scott et al. 1982). For wild birds, however, the production of eggs is one of the most critical periods in their annual cycle and the investment made in the clutch is a major determinant of the fitness of the individual.
Changes in female muscle mass at the time of egg formation.
The resources for egg formation could come from three routes: from the diet, from endogenous sources or by reallocation of physiological processes within the bird to permit resources to be diverted from body maintenance to egg formation. The relative importance of these various routes has still not been established for any species of wild bird (Walsberg 1983; Houston et al 1995a). However, it is well known that the muscle system of birds could contribute protein for egg synthesis. Jones and Ward (1976) first described a marked decline in the mass of pectoral muscle of Red-billed Quelea Quelea quelea at the time of egg production, and suggested that protein obtained from muscle stores may play a major role in egg production. The muscle system is the largest source of protein in the body, and so is the most likely source of endogenous protein for egg production. Kendall et al.(1973) suggested that there may be labile sarcoplasm proteins in bird muscle which could be mobilised without impairing the contractile function of the muscle, and reported marked changes in sarcoplasm volume, but not in myofibril volume, from the muscle of birds in good and poor condition. Since those studies the body composition of females of about 29 species of birds have been examined during the period of egg formation. In 22 of these species significant muscle tissue is lost at the time eggs are forming (Houston et al.1995b).
The use of labelled amino acids in Zebra Finches has shown that muscle proteins are broken down at this time and the amino acids are then incorporated into egg proteins (Houston et al. 1995c). This is not a surprising finding, because protein turnover is always taking place in the body and any amino acids liberated into the body pool during muscle protein turnover would be available for egg protein synthesis. But there is an enhanced loss of amino acids from the muscle system of breeding females compared to non-breeding control birds (Fig. 5), which shows that more amino acid is released from muscle stores in females when they are forming eggs than would be expected from routine protein turnover (Houston et al. 1995c).
Changes in muscle proteins during egg formation
The muscle tissue of Zebra Finches undergoes marked changes during the period of egg formation. There is a significant loss of both sarcoplasm and myofibrillar proteins at this time, and analysis of the sarcoplasm proteins suggests that not all proteins are lost: Fig. 6 shows that there is a high molecular weight component of the sarcoplasm, which declines markedly over the few days while eggs are forming, and which may function in the manner proposed by Kendal et al. (1967). This suggests that general muscle proteins might not be used as the only source of protein, because this could weaken flight performance, but that there may be special proteins in bird muscle- perhaps rich in those essential amino acids that are limiting in the diet - and that these proteins can readily be mobilised to contribute to the amino acid pool available for egg formation.
If this is the case, then we might expect that it is the quality of muscle stores a bird lays down that is important rather than the absolute amount of muscle tissue. This does seem to be true. Selman & Houston (1998) compared the breeding performance of two groups of Zebra Finches that differed only in the quality of diet that the females had experienced before the start of the breeding season. One group received a normal seed diet, while the other group was given the same diet but with a supplement containing cooked egg. The underlying assumption was that if storage of specific amino acids or other nutrients, which are limiting in the diet, was an important factor in later egg production, the females on the egg-supplemented diet would have greater opportunities to incorporate these into body tissues because of their higher concentration in the diet. After two weeks on these diets the females were paired with their male and placed into individual breeding cages. At the time of pairing the two groups of females did not differ in body weight or in their muscle volume (determined by a non-invasive method of moulding the shape of the live bird; Selman and Houston 1996). After pairing, both groups of birds were kept on an identical ad libitum diet of seed. During the period between pairing and the laying of the clutch the two groups of females did not differ in their food intake. However, Fig. 7 shows that birds which had previous experience of high quality diets before breeding laid larger eggs and larger clutches, producing almost twice the mass of eggs, and reared three times as many young as the control group. Both groups were taking identical diets during the whole period of egg formation, so the difference between the groups must have been mediated by differences in the female condition established before the start of breeding. Those females that laid more eggs had not lost more muscle tissue. In fact, the low quality diet female group lost more muscle tissue while forming eggs, despite laying much smaller clutches and smaller eggs. Fig. 8 shows that females with a previous experience of high quality feed lost very significantly less muscle tissue per gram of egg laid than did females with a poor quality dietary experience prior to breeding. These results show that the quality of diet available to a female bird well before the start of the breeding season can have a profound influence on breeding success, irrespective of the food supply available during the period when eggs are being formed, presumably because it induces different levels of body stores in the females. In addition, these differences in female condition are not detectable by normal measures of fat and muscle condition in birds, because it is not only the absolute amount of protein or other nutrients that are stored, but the quality of those stores that are important. There are some field studies which suggest support for these findings. Cave (1968) showed that experimentally providing European Kestrels Falco tinnunculus with additional food in the winter could influence the size of the clutch they would lay in the spring. It suggests that the quality of diets available to birds, perhaps even before they arrive at their breeding sites, could have implications for their breeding performance.
These results suggest that there are subtle changes in the quality of muscle proteins. The Zebra Finch is probably unusually dependent on endogenous sources of protein for egg production, because it is adapted for breeding on a low protein diet. During egg formation the amount of protein lost from body tissues is roughly equivalent to the protein requirements of the entire clutch (Houston et al. 1995a), and rather perversely the food intake of these birds actually declines during the period they are forming eggs (Houston et al. 1995a). We should not assume that other bird species rely so extensively on endogenous stores, for as many authors have pointed out (Drent & Daan 1980; Ankney & Alisauskas 1991), the extent to which a species depends on endogenous resources for egg production will vary with many factors, especially the quality of diet available to the bird at the time.
HOW NUTRIENT RESERVE QUALITY MIGHT INFLUENCE BREEDING
It has already been shown that the nutritional quality of food available to a female bird can influence the size and number of eggs that she will lay. Other factors are also likely to influence the fitness of the female. Male birds are able to discriminate between females who, because of their past dietary experience, will lay large or small clutches: Monaghan et al. (1996) showed that male Zebra Finches will selectively choose females that will lay large clutches. The two female groups in this trial differed only in the quality of diet they received prior to pairing, but at the time of pairing they did not differ in body mass or in muscle mass. In a monogamous species like the Zebra Finch the securing of a mate with high reproductive potential is important to the male, and in this species male birds have clearly evolved sophisticated discriminatory abilities. Females which had previously been feeding on high quality diets in this trial, and which therefore subsequently laid larger clutches, were three times more likely to obtain a mate.
There are also likely to be fitness consequences for the female if endogenous resources are depleted at the time of egg formation. Muscle tissue mobilised and used to form egg proteins may result in a deterioration in a female bird’s flying performance and an increase in her risk of predation. The extent to which flight performance varies may depend on the quality of reserve protein the female has available, as well as the absolute mass of muscle present on the bird. A loss of body protein may also have implications for the ability of the female to repair damaged tissues or fight infection. Fig. 8 showed that the amount of muscle tissue a female bird needed to mobilise at the time of breeding was influenced by the nutritional quality of her diet before the start of the breeding season, rather than by the number of eggs she had laid. Females from poor nutritional backgrounds may therefore be in worse protein condition at the end of laying than females that had access to better quality diets.
ACKNOWLEDGEMENTS
I am extremely grateful to Pat Monaghan, Neil Metcalfe, Ruedi Nager, David Donnan, Peter Jones, Dan Osborne, Richard Selman, Mat Cottam and Jake Veasey who have all collaborated on the studies reported here.
REFERENCES
Ankney, C.D. & Alisauskas, R.T. 1991. The use of nutrient reserves by breeding waterfowl. Proceedings of the International Ornithological Congress 20:2170-2176.
Aparicio, J.M. 1994. The effect of variation in the laying interval on proximate determination of clutch size in the European Kestrel. Journal of Avian Biology 25: 275-280.
Arcese, P. & Smith, J.N.M. 1988. Effects of population density and supplemental food on reproduction in song sparrows. Journal of Animal Ecology 57: 119-136.
Betts, M.M. 1955. The food of titmice in oak woodland. Journal of Animal Ecology. 24: 282-323.
Bolton, M., Houston, D.C. & Monaghan, P. 1992. Nutritional constraints on egg formation in the lesser black-backed gull: an experimental study. Journal of Animal Ecology 61: 521-532.
Boutin, S. 1990. Food supplementation experiments with terrestrial vertebrates: patterns, problems, and the future. Canadian Journal of Zoology 68: 203-220.
Cave, A.J. 1968. The breeding of the kestrel Falco tinnunculus in the reclaimed area Oosterlijk Flevoland. Netherlands Journal of Zoology. 18:313-407.
Drent, R.H. & Daan, S. 1980. The prudent parent: energetic adjustment to avian breeding. Ardea 68:225-331.
Houston, D.C. , Donnan, D. & Jones, P.J. 1995a. The source of nutrients required for egg production in zebra finches. Journal of Zoology 235: 469-844.
Houston, D.C. , Donnan, D. , Jones, P.J., Hamilton, I.D. & Osborne, D. 1995b. Changes in the muscle condition of female zebra finches during egg laying and the role of protein storage in bird skeletal muscle. Ibis 137: 322-329.
Houston, D.C. , Donnan, D. & Jones, P.J. 1995c. Use of labelled methionine to investigate a protein storage function of avian muscle to contribute to egg production. Journal of Comparative Physiology B 165, 161-164.
Houston, D.C. 1997. Nutritional constraints on egg production in birds. Proceedings of the Nutrition Society 56:1057-1065.
Jones, P.J. & Ward, P. 1976. The level of reserve protein as the proximate factor controlling the timing of breeding and clutch-size in the Red-billed Quelea Quelea quelea. Ibis 118: 547-574.
Kendall, M.P., Ward, P & Bacchus, S. 1973. A protein reserve in the pectoralis major flight muscle of Quelea quelea. Ibis 118:547-574.
Lack, D. 1954. The natural regulation of animal numbers. Oxford University Press.
Martin, T.E. 1987. Food as a limit on breeding birds: a life-history perspective. Annual Review of Ecology and Systematics 18: 453-487.
Monaghan, P. Metcalfe, N.B. & Houston, D.C. 1996. Male finches selectively pair with fecund females. Proceedings of the Royal Society B. 263:1183-1186.
Murphy, M.E. 1994. Animo acid compositions of avian eggs and tissues: nutritional implications. Journal of Avian Biology 25: 27-38.
Paul, A.A. & Southgate, D.A.T. 1978. McCance and Widdowson’s ‘The Composition of Foods’, 4th revised edition. London H.M. Stationary Office.
Paul, A.A., Southgate, D.A.T. & Russell, J. 1978. First supplement to McCance and Widdowson’s ‘The Composition of Foods’, 4th revised edition. London H.M. Stationary Office.
Perrins, C.M. 1979. British Tits. Collins, England; London.
Perrins, C.M. 1991. Tits and their caterpillar food supply. Ibis 133: 49-54.
Ramsay, S.L. & Houston, D.C. 1997. Nutritional constraints on egg production in the blue tit: a supplementary feeding study. Journal of Animal Ecology 66: 649-657.
Ramsay, S.L. & Houston, D.C. 1998. The effect of dietary amino acid composition on egg production in Blue Tits. Proceedings of the Royal Society B. 265:1-5.
Robbins, C.T. 1981. Estimation of the relative protein cost of reproduction in birds. Condor, 83: 177-179.
Scott, M.L., Nesheim, M.C. & Young, R.J. 1982. Nutrition of the Chicken, Third Edition. Ithaca, New York : M.L. Scott & Associates.
Selman, R.G. & Houston, D.C. 1996. A technique for measuring lean pectoral muscle mass in live small birds. Ibis 138:348-350.
Selman, R.G. & Houston, D.C. 1996. The effect of prebreeding diet on reproductive output in zebra finches. Proceedings of the Royal Society B. 263: 1585-1588.
Walsberg, G.E. 1983. Avian Ecological Energetics. In: Avian Biology, Vol. 7, Farner, D.S., King, J.R. & Parkes, K.C. Academic Press, London. 161-220.
Williams, T.D. 1997. Variation in reproductive effort in female Zebra Finches (Taeniopygia guttata) in relation to nutrient specific dietary supplements during egg laying. Physiological Zoology 69: 1255-1275.
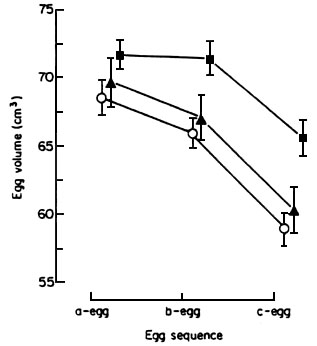
Fig.1. Mean volume (cm3 ±SE) of eggs of Lesser Black-backed Gulls in three egg clutches laid by controls (o ), and birds given supplementary feeding of fish (g ) and fat (D ) during the period of egg formation and laying. Repeat measures ANOVA comparing control vs. fat-fed: effect of egg sequence: Wilks F = 64.50, df = 1.28, P<0.001, effect of feeding treatment: F = 0.57, df = 1,29, P>0.05, interaction not significant. From Bolton et al. (1992).

Fig. 2. Mean volume (cm3 ± SE) of eggs of Lesser Black-backed Gulls in three egg clutches laid by controls (o ), and birds given supplementary feeding of fish (g ) and fat (n ) during the period of egg formation and laying. Repeated measures ANOVA: effect of laying sequence Wilks F = 111.61 df = 2.59, P<0.001, effect of feeding treatment F= 10.86, df = 2.60, P < 0.001, interaction not significant. From Bolton et al. (1992).
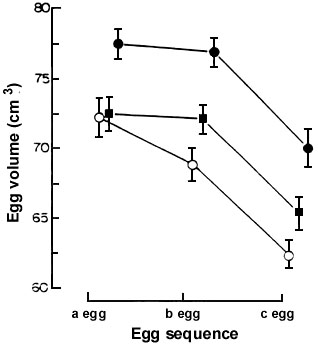
Fig. 3. Mean values (± SE) of intercepts, for intraclutch regressions of egg volume through the laying sequence for Blue Tits which were provisioned with a supplementary diet of animal fat or a diet containing cooked eggs. There was a significant difference in volume between treatments ( F=3.570, df=2,64. P<0.05), however only in the birds fed the egg supplement were the eggs significantly larger than control, Scheffe test(*). From Ramsay & Houston 1997.
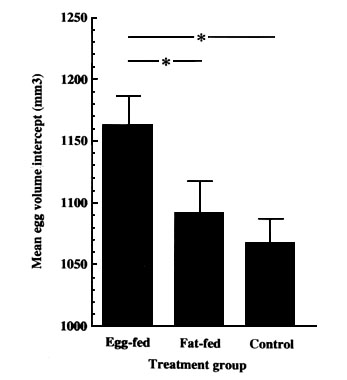
Fig. 4. Number of eggs laid by Blue Tits which had been provided with supplementary feeding which differed only in the balance of five amino acids such that one was of higher quality (HQ protein) than the other (LQ protein) in respect to the balance of amino acids required to form egg proteins. Clutch size declines significantly with season in Blue Tits and to control for this we used analysis of covariance with laying date as a covariate. After controlling for laying date mean clutch size did not differ between LQ protein group and control birds, which had received no supplementary feeding, but the HQ protein group showed significantly greater mean clutch size than either control or LQ protein group (effect of laying date, F1,57 =26.36, p<0.0005, effect of treatment, F2,55 = 4.15, p<.025; clutch size data transformed by squaring to normalise), producing on average about 1.6 additional eggs per clutch compared to controls. From Ramsay & Houston 1998.
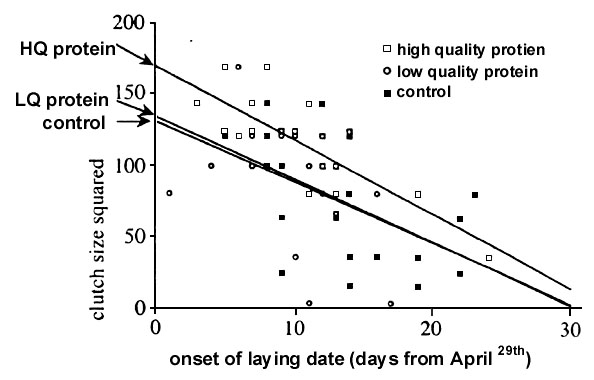
Fig. 5. Two groups of female Zebra Finches were given daily doses of 35S-methionine. One group was then introduced to a male and laid a clutch of eggs, the other group did not breed and was used as controls. In the breeding group the 35S-methionine was incorporated into egg proteins. Eleven days after the last administration of 35S-methionine the mean (± SD) level of 35S-methionine found in pectoral muscle samples showed that breeding (n=14) birds had lost significantly more labelled amino acid than had the control non-breeding (n=14) birds (t test P<0.01). From Houston, Donnan & Jones 1995.
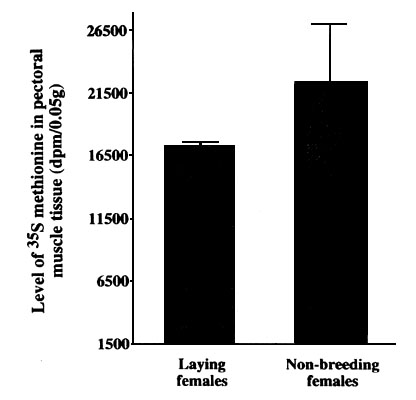
Fig. 6. Results of gel filtration of water-soluble proteins from the pectoral muscles of female Zebra Finches. (a) shows a typical trace from a bird three days before the first egg is laid and (b) a typical trace from a bird after the laying of the last egg of the clutch. The two traces show the elution volume for material of decreasing molecular weight from left to right, and show that protein loss mainly occurs among heavy molecular weight proteins, which we refer to as Peak 1. (c) shows that there is a significant decline in the protein content of the peak 1 fraction from females at different stages of the laying cycle, with the first egg laid on day 1, the second on day 2 etc. and with days prior to the start of laying during which ova growth is occurring marked -1, -2 etc. (r31 = -0.82, P<0.0001). From Houston et al. 1995b.
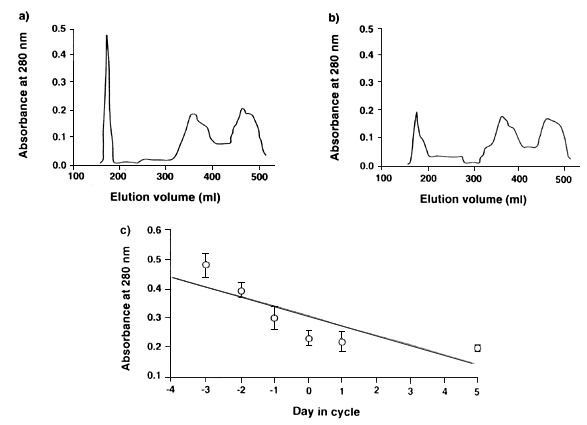
Fig. 7. Comparison of the clutch masses (± SE) laid by female Zebra Finches that have previously experienced a diet of high quality (HQD) and low quality (LQD) before the start of egg production (t47 =4.67, p<0.001). From Selman & Houston 1996.
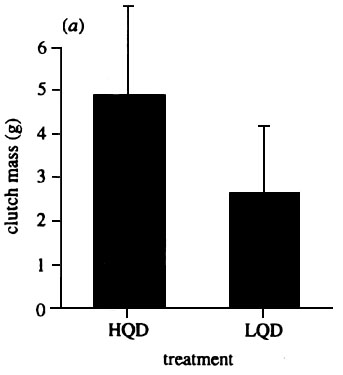
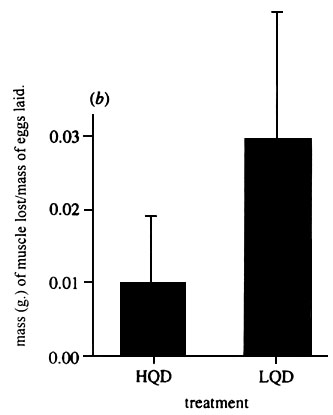
Fig. 8. Estimated mean (± SE) loss of lean, dry pectoral muscle tissue per gram of egg laid for females with past experience of high quality (HQD) and low quality (LQD) diets before the start of egg production (t50 = 4.12, p < 0.001). From Selman & Houston 1996.