S01.4: Methods of studying the functional ecology of protein and organ dynamics in birds
Theunis Piersma1,2 & Marcel Klaassen3
1Netherlands Institute for Sea Research (NIOZ), P.O. Box 59, 1790 AB Den Burg, Texel, The Netherlands, fax 31 222 319674, e-mail theunis@nioz.nl; 2Centre for Ecological and Evolutionary Studies, University of Groningen, P.O. Box 14, 9750 AA Haren, The Netherlands; 3Centre for Limnology, Netherlands Institute of Ecology, P.O. Box 1299, 3600 BG Maarssen, The Netherlands, e-mail klaassen@cl.nioo.knaw.nl
Piersma, T. & Klaassen, M. 1999. Methods of studying the functional ecology of protein and organ dynamics in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
36-51. Johannesburg: BirdLife South Africa.Birds are capable of adaptive responses to ecological challenges involving changes in body composition, including both body stores and functional tissues. These physiological adjustments may affect aspects of the birds’ ecology, such as choice of diet and micro-habitat or susceptibility to aerial predators. Carcass analysis provides accurate data on body composition; however, ethical considerations apart, this method neither enables studies of temporal changes within individuals nor allows compositional analyses to be followed up by studies on the birds’ life history. Various non-terminal methods are available to quantify gross body composition in terms of fat, protein, and water. In addition, energy and mass (nitrogen) balance measurements in caged song birds and shorebirds provide sensitive and robust methods to estimate protein and fat contents of anabolised and catabolised body stores, albeit under laboratory conditions rather than in the field. The potential of new non-terminal methods (i.e. ultrasound, nuclear magnetic resonance imaging and computer tomography), which allow repeated size estimation of various organs, was evaluated in recent studies on shorebirds and swans. These methods probably have the greatest potential in break-through studies in the field of ecophysiological adaptation, because they allow non-invasive, repeated quantification of the size of different organs in individual birds.
INTRODUCTION
Even though birds are organisms that attain fixed size at maturity, many body components vary with time, usually in response to instantaneous or future demands on the body (Piersma & Lindström 1997; Klaassen 1998). Prime examples are provided by the highly variable size of fat deposits carried by birds in anticipation of energy shortfalls due to bad weather or long flights (Blem 1976, 1990), and the enormously, seasonally variable size of the gonads in relation to reproductive events (Murton & Westwood 1977). Here we will discuss methods to study temporal changes in body composition of birds during their adult lives. Rather than treating the estimation of fat stores (Gessaman 1999), we will focus on the nonlipid, lean, proteinaceous parts of the body. These parts--the organs in the body cavity and the various muscle blocks--do all the ‘work’ in an animal. In addition, a partitioning in proteinaceous and fatty components is so important because the energetic equivalents of the two differ almost by an order of magnitude (see below and Table 1). In respect to possible energetic repercussions, it is therefore critical to know the composition of changing bodies.
Birds starting on very long-distance flights carry huge fat deposits, thus requiring large pectoral muscles to get this load into the air (Evans et al. 1992; Driedzic et al. 1993; Jehl 1997; Piersma & Gill 1998). As the fat stores gradually deplete in the course of these flights, muscle size necessary to keep the birds aloft is expected to also decrease (Pennycuick 1978, 1998). An economically built avian machine may thus dispense with pectoral muscle mass in the course of such migratory flights (in spite of intense activity). The sparse available evidence suggests that this is indeed what happens (Lindström & Piersma 1993). [Note that there may be multiple, non-mutually exclusive explanations to additionally account for muscle mass loss during flight. For example, protein converted to glucose through gluconeogenesis may be used as fuel for the brain, protein may provide citric acid cycle intermediates, and may provide free water for evaporation.]
Any bird that needs to eat a lot in order to store fuel for ongoing flights, or to cover high daily energy expenditures, or a bird that eats a difficult food-type, may all need a voluminous gut to process the food (Karasov 1996). However, such guts are costly to carry and maintain, so an economical bird would dispense with such machinery when this option is open. Again, the sparse evidence suggests that this is what happens (Piersma et al. 1993; Hume & Biebach 1996; Piersma & Lindström 1997).
During investigations of the ecophysiological aspects of avian performance, whether it be flying or foraging or other activities, the size of key organs and energy stores help indicate adaptations and constraints on the working systems (Diamond 1993; Piersma 1998). The trouble is that measurements of organ sizes and energy stores cannot be repeated within individuals with the otherwise suitable method of carcass analysis (Brown 1996; and see Kerr et al. 1982; Dobush et al. 1985). However, there exist a few non-terminal methods that enable measurements of sizes of active avian machinery--the lean component of birds--in various grades of detail, and these will be reviewed below. For the heuristically very useful distinction between the terms ‘stores’ (the ‘strategic’ nutrient deposit) and ‘reserves’ (the ‘last-resort’ nutrient deposit), see King & Murphy (1985), Lindström & Piersma (1993), and Van der Meer & Piersma (1994).
THE METHODS
1. Total body density
It would be extremely useful in studies of the body compositional flexibility in birds if we were able to distinguish between the amount and development of functional tissue and of body stores. Body stores largely consist of fat, whereas functional tissue mainly consists of tissue other than fat. The fat and non-fat compartments in an animal’s body have distinct specific densities (mass/volume ratios), which are generally taken to be 0.9 and 1.1 kg L-1, respectively. Clearly, assuming a bird’s body to consist of two compartments is a coarse approximation because it in fact consists of a great many compounds each having their own specific density. Nevertheless, when calibrated on a sample of birds using carcass analysis, overall density measurements would in principle allow for rather accurate estimates of fat contents in birds.
However, to be successful, one has to be able to measure volume with great accuracy because the differences in specific density of the fat and the non-fat component are small. Body volume may be calculated by subtracting the weight under water from the weight in air. Although underwater weighing is a much applied and highly reliable method to estimate the volume of human subjects (Behnke et al. 1942), a practical problem is that birds will not allow themselves to be weighed voluntarily under water. Moreover, this method causes problems in birds due to the presence of air between the feathers and difficulties in correcting for the dead-volume in the respiratory tract. Whereas the underwater weighing method makes use of Archimedes’ law, plethysmography is an alternative based on Boyle’s and Charles’ law (i.e. pressure times volume divided by temperature is constant). In this method, a large volume, containing an object with an unknown volume, is reduced by a fixed amount; the consecutive pressure increase is proportional to the size of the unknown object. This method has been applied with success in humans (e.g. Gundlach et al. 1980), but experiments with rats (Beynen & Gundlach 1986) and Brent Geese Branta bernicla (H. Korte pers. comm.) have failed, the main problem probably being the incapacity to control or measure temperature profiles in the system. At least currently, technical problems inhibit measurement of body composition through density measurements.
2. Isotopic water dilution space
Migratory fat is stored in expanding vacuoles in a fixed number of adipocytes or fat-cells (Griminger 1986), and for this reason very little water is attached to stored fat. This means that water percentages of the fat-free part of the body should not change when fat is stored. Indeed, considerable evidence shows that the water percentage of the total lean mass accounts for only limited variation between individuals and that the remaining variation cannot be accounted for by the size of the fat stores (Child & Marshall 1970; Piersma & van Brederode 1990; Ellis & Jehl 1991). An estimate of the water content of an animal would thus yield a prediction of the lean mass of that body (Pace & Rathbun 1945), assuming the bird is in water balance and full-grown (neonates have a relatively high water content that decreases to adult levels during development [e.g. Drent et al. 1992]).
There are two ways to estimate the water content of an intact animal: (1) total body electrical conductivity, based on the fact that the presence of water modifies a magnetic field (discussed under the next subheading); and (2) dilution space techniques, during which a known amount of inert marker is ingested or injected, given time to equilibrate with the body fluids, and then measured for the density or concentration in the blood (Gessaman 1999). Usually, unstable (tritium, 3H) or stable (deuterium, 2H) hydrogen isotopes in water are used to determine body water (e.g. Nagy & Costa 1980; Crum et al. 1985). Several technical problems require attention, however: (1) the time for the isotopic markers to reach equilibrium is variable; (2) water turnover during the equilibration period has to be taken into account; (3) the assumption of the animal being in water balance may be violated; and (4) isotopes may be lost due to incorporation in compounds other than water (e.g. Speakman 1997). Thus, we recommend that studies in which the dilution space method is applied are accompanied by a series of validation trials.
A recent summary by Speakman (1997, pp.134-135) of 79 studies on 35 bird and mammal species concluded that the hydrogen dilution space usually exceeded the total body water pool determined by desiccation, on average by 4.7% in birds (range is -2.7% to +18%, 8 studies, 4 species). Thus, if calibration trials show the discrepancies (that may be due to incorporation of hydrogen isotopes in compounds other than water) to be consistent among measurements, estimates of water content, and henceforth predictions of lean body mass, should be corrected for this bias (Bowen & Iverson 1998).
3. Total body water electrical conductivity (TOBEC)
The total body water electrical conductivity technique (usually abbreviated as TOBEC) to estimate total body water, and hence lean body mass, is based on the measurement of electrical conductivity from the change in the phase relation of voltage and current in a 5 Mhz-frequency oscillating magnetic signal passed through a coil (Walsberg 1988). Birds, alive or dead (but note that body temperature may affect readings, unpubl. data), constrained in their movements in various ways and stuck in a plastic tube in a standardised way, are briefly positioned in the circular ‘pigeon hole’ of the machine to obtain a reading of the electrical conductivity. A similar reading is then made of the empty tube, and this procedure may be replicated a number of times to enhance precision (Conway et al. 1994). Up to now, published TOBEC studies have uniquely relied on devices produced by a single company (EM-SCAN 1991). Calibrating studies focused on the prediction of fat stores in live birds, relying on TOBEC to accurately predict lean mass, and subtracting lean mass from total body mass to get an estimate of fat mass (Walsberg 1988; Castro et al. 1990; Roby 1991; Scott et al. 1991; Skagen et al. 1993; Conway et al. 1994; Meijer et al. 1994). TOBEC instruments are portable.
Although these studies report high correlations between the size of the measured fat store and the TOBEC reading, especially in interspecific comparisons, only the most recent studies by Skagen et al. (1993), Conway et al. (1994), and Meijer et al. (1994) address the question of whether TOBEC adds any predictive power to the traditional more straightforward estimates of fat content, such as visual fat scores, or regressions of fat mass on body mass taking structural size variation into account (Wishart 1979; Piersma 1984). Based on an intraspecific study of Wood Thrushes Hylocichla mustelina, Conway et al. (1994) conclude that TOBEC readings were neither accurate in predicting lipid mass of individual birds, nor did they substantially improve the predictive ability of the traditional body fat prediction models mentioned above. Skagen et al. (1993) studying lipid contents in Semipalmated Calidris pusilla and White-rumped Sandpipers C. fuscicollis, Meijer et al. (1994) studying European Starlings Sturnus vulgaris, and T. Piersma et al. (unpubl. data) studying Red Knots Calidris canutus all came to similar conclusions considering the accuracy and usability of the TOBEC method. If, however, body temperature is kept constant (or that body temperature variations are suitably accounted for), TOBEC can help to obtain useful independent predictions of total body water, and thus of lean body mass (with r² values sometimes exceeding 0.9; see e.g. Roby 1991, but note that in this paper a group of non-fitting birds were excluded from the analyses). For studies on overall water content and water balance, TOBEC may still be of some use.
4. Energy balance
The energy balance method is a reliable tool to estimate the composition of body stores and reserves. After accounting for faecal energy loss, the net or metabolisable energy intake rate (
I, Watt) balances the rate of energy expenditure (E, Watt) for birds that are in mass balance. If the bird is not in mass balance and tissue deposition or catabolism takes place (M, g s-1), the energy budget can be written as:![]()
where e (J g-1) is the energy density of the anabolised or catabolised tissue if body mass change is positive or negative, respectively. Thus, dividing the difference between metabolisable, energy intake and energy expenditure by body mass change yields an estimate of the energy density of the body mass change:
![]()
Body stores typically consist of fat and protein. Protein is normally associated with 77% water (Blaxter 1989); however, fat is often thought not to be associated with any water. Indeed, energy density measurements of adipose tissue indicate a water content of only 4% (Johnston 1970). The energy densities of fat and wet protein are very different (Table 1), and the energy density of body stores may thus yield an estimate of the composition of the body stores in terms of protein and fat content.
The number of parameters to be measured for the compilation of an energy budget and the estimation of the energy density of body stores is considerable, including body mass change, rates of oxygen consumption and/or carbon dioxide production, food intake, and faecal energy loss. Fig. 1 shows the set-up that Klaassen & Biebach (1994) used to compile the energy budgets of Garden Warblers Sylvia borin and Thrush Nightingales Luscinia luscinia allowing for the estimation of all these parameters.
Body mass change is often a discontinuous process, for one as a result of irregular defecation. The start and the end of an energy budget trial has to be chosen such that the bird is in a comparable physiological state (e.g. with respect to filling of the digestive tract, water balance) except for its fuel store level. When body mass change is small as a result of changes in the amount of fuel stores, measurement errors and small stochastic fluctuations in body mass have a large impact on the energy density and composition estimate of the stores. Energy balance, in combination with starvation trials, are therefore most appropriately applied when body mass changes are large. Furthermore, the measurement of food intake in starvation trials can be omitted, reducing research effort and measurement error. In Fig. 2 this superiority in accuracy of starvation trials (panel C) compared to food balance trials (panel B) is illustrated for two experiments with Thrush Nightingale during the migratory phase (Klaassen et al. 1997).
Not all protein is burned during catabolism, leaving uric acid. Thus, from the total energy content of protein, only part is freed during catabolism. This should be taken into account when energy balances are compiled. It is a fair assumption that protein content changes in line with total body mass increase or decrease. For an increase in protein content the anabolic energy equivalent for protein should be used, whereas for a decrease in protein content the catabolic equivalent should be used (Table 1).
Glycogen may also be part of the stores, but this is a relatively small store residing in the liver and muscles. If large body mass changes are involved, then, changes in glycogen stores over the period of measurement will hardly ever have an effect on the estimated energy density and fat/protein composition of the stores. Furthermore, glycogen is a quickly mobilised and re-established store typically kept at a constant and high level when birds are not taking part in active behaviour. Thus, if care is taken that the beginning and end of the period over which the energy balance is compiled, are preceded by a period of quiescent behaviour, net changes in the glycogen stores will never be large. Wet glycogen has a nearly identical energy content as wet protein (Table 1). Disregarding glycogen stores will yield an overestimate of the wet protein content of a magnitude equal to the true wet-glycogen content.
Energy expenditure is often measured by oxygen consumption and carbon dioxide production. Simultaneous measurement of oxygen consumption and carbon dioxide production yields information on the composition of the catabolised tissue and thus of body stores utilised during starvation trials. For uricotelics, such as birds, however, the respiratory quotient (carbon dioxide production divided by oxygen consumption) is very similar for protein and fat catabolism (Table 1) making this an unreliable estimator for fuel composition.
Energy balance trials have been applied in migratory warblers to assess the energy density of their fuel stores during migration (Klaassen & Biebach 1994; Klaassen et al. 1997). Many authors have also attempted to estimate the energy density of fuel stores by plotting metabolisable energy intake rate against body mass change (e.g. Owen 1970; Klaassen et al. 1990; Handrich et al. 1993). The intercept of this relationship is then assumed to reflect the rate of energy expenditure. However, this method relies heavily on the assumption that the rate of energy expenditure is independent of the rate of body mass change or food intake (i.e. the heat increment of feeding or specific dynamic action [e.g. Blaxter 1989] but also behavioural changes are mostly not taken into account).
To summarise: although the energy budget method is only applicable to birds in captivity that undergo a large body mass change under controlled conditions and requires a careful compilation of the bird’s energy budget, this method provides a reliable estimate of the composition of catabolised or anabolised tissue.
5. Matter balance
As for the energy balance method, compiling an animal’s nitrogen balance allows determination of protein contribution to body mass change, whether positive or negative. Each gram of nitrogen translates roughly to 6.25 gram of protein.
It appears to be difficult to make a balanced nitrogen budget. It is a rather general phenomenon that protein turnover is a continuous process and also continuous during starvation (Reeds & Fuller 1983). Protein that is being replaced is often (though not always; see Nelson et al. 1983) catabolised; even in protein balance, a considerable intake and excretion of nitrogen has to take place. Small systematic errors of measurement in either nitrogen input or output may thus lead to large errors in the nitrogen balance (i.e. the difference between input and output). Clearly, this typical type of error in nitrogen or energy balance studies becomes less of a problem with an increase in the difference between input and output. During starvation trials, for instance, the compilation of a nitrogen balance is reasonably accurate and has been applied with great success (e.g. Lindgård et al. 1992, and studies cited therein). The superiority of starvation nitrogen balance studies over food-balance nitrogen balance studies is exemplified in Fig. 2A where variation in starving thrush nightingales is clearly less than in Thrush Nightingales that were fed (Klaassen et al. 1997).
One of the problems in the compilation of nitrogen balance is that faecal nitrogen is easily lost due to bacterial activity, leading to conversion of urea and uric acid to ammonia prior to collection of faeces. Ammonia, which evaporates easily, should be fixed by acidifying the sample. Samples containing ammonia can only be analysed if liquid. Despite the fact that birds excrete nitrogen in the form of uric acid mainly, small amounts of ammonia may also be produced; recently, however it has been found in some hummingbirds that the contribution of ammonia to total nitrogen excretion may be substantial (Preest & Beuchat 1997). Again, in the light of the fact that small changes in nitrogen output may result in large changes in the difference between nitrogen input and output (see above), one should be wary to take this alternative route of nitrogen excretion into account.
In summary, estimates of the protein content of catabolised or anabolised tissues by the nitrogen balance method can only be determined in captive birds under controlled conditions undergoing large changes of body mass. The measurements to be taken are simple, but in order to increase accuracy great care has to be taken to avoid nitrogen loss.
6. Nuclear magnetic resonance imaging (MRI) and computer tomography (CT)
Nuclear magnetic resonance imaging (MRI) makes use of the fact that protons emit radio signals when placed in a changing magnetic field. Computer tomography (CT) is a sophisticated kind of X-raying. Both techniques offer great promise for the visualisation of body organs in live birds. MRI is particularly promising because of its lack of radiation exposure and its greater potential to visualise different structures. Both techniques allow cross-sectional views of variable width through live animals. By completely scanning a subject by a series of contiguous thin axial slices, a three-dimensional impression of the animal can thus be generated (Roberts et al. 1993). Repeated measurement of individual birds will provide accurate insight in body compositional changes. However, most instruments designed to provide excellent and detailed images of humans, may not be suitable for use in small birds. Furthermore, the method requires that subjects lay still, and birds may therefore have to be anaesthetized to enable successful application of the method.
Validation studies for body composition using MRI or CT have been conducted on humans (e.g. Roberts et al. 1993; Sohlström et al. 1993; Abate et al. 1994; Ross et al. 1994), domestic mammals (e.g. Sørensen 1992; Scholz et al. 1993), and fish (e.g. Rye 1991). Body composition of poultry has also been studied using these techniques (Mitchell et al. 1991; Svihus & Katle 1993; Scollan et al. 1998). Osa et al. (1993) also scanned a dead Emperor Penguin Aptenodytes forsteri and a dead Adélie Penguin Pygoscelis adeliae, but no calibration was performed. In all cases, the validation experiments showed high correlations between true organ and tissue volumes, as measured by carcass analysis or other established techniques, and the MRI and CT results. To exemplify the potential of these scanning techniques, Fig. 3 shows the results of a MRI validation experiment for heart, liver, gizzard and breast muscle size using carcasses of Bewick’s Swans Cygnus columbianus bewickii. Organ volumes were estimated by computer-aided analysis of sequences of sagittal views ranging from head to tail and compared with true organ volumes measured in carcass analyses.
In addition to MRI, whole-body nuclear magnetic spectroscopy can also be used to obtain gross measures of total body water, protein, and lipid content (Mitchell et al. 1991). This methodology does not require the subjects to be as immobile as in MRI and the time involvement is substantially smaller.
In short, MRI and CT are promising non-invasive techniques providing a host of information on the subject studied. However, access to these sophisticated devices is generally logistically complicated and potentially costly, that is, 'hard to get.' Furthermore, depending on the system one can get access to, one should take into account that one may have to invest some time and money into acquiring the desired quality of scans (i.e. one needs time for the optimization process). Despite these discouraging factors, MRI and CT remain the only methods allowing detailed size analysis of internal organs.
7. Ultrasound
The first applications of ultrasound technology to study changes in the lean components of free-living birds used devices from the animal husbandry (meat production) industry (Sears 1988; Newton 1993). These devices relied on the ‘pulse-echo’ method, in which the time between the input signal and the reflected output signal is visualised on a small screen to indicate the tissue thickness. More recent instruments relying on reflected ultrasound waves have the major advantage in making the ‘landscape’ of internal organs and the skeleton visible. Even though it is usually necessary to apply a scanning gel for good images (that can easily be removed by washing with water), ultrasonographic imaging is quick, causing little distress to experimental animals. The technique is now commonly used for diagnostic purposes in the medical and veterinary practices (Grooters et al. 1994; Lambertz et al. 1995) and also in the context of animal production (Perkins et al. 1992; Herring et al. 1994). This wide spectrum of applications has led several companies (e.g. Aloka, Hitachie, and Pie Medical) to develop affordable and transportable equipment that can be used in field studies.
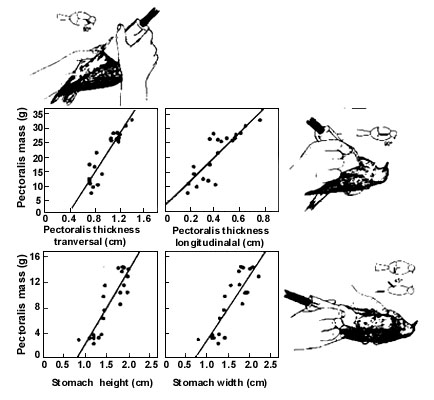
Recent studies have demonstrated remarkable variability in the size of muscles and internal organs in long-distance migrating shorebirds (Piersma et al. 1996; Piersma & Lindström 1997; Piersma 1998; T. Piersma, G.A. Gudmundsson & K. Lilliendahl submitted). In some mollusk-eating species, the stomach especially appears to undergo large changes in size in relation to long flights and dietary changes (Piersma et al. 1993). We recently explored the use of ultrasonographic imaging to measure the size of the pectoral muscle block and the stomach of Red Knots and of Greater Golden Plovers Pluvialis apricaria (Dietz et al. 1999). With errors of individual measurements of 20-25% for pectoral muscle and 26-44% for stomach, the accuracy of ultrasound measurements was adequate for the purpose of detecting changes of this order of magnitude in individuals and larger samples, especially if several independent measurements are carried out for each individual. In view of the fair correlations between the ultrasonographic measurement of organ thickness and the fresh mass of that organ (Fig. 4), the technique can usefully be applied to measure rapid changes in organ size of individual birds, even in species with a small stature (birds weighed 100-200 g in this example). The promise of this technique was illustrated by documenting individual changes in pectoral muscle and stomach mass in captive Red Knots. These birds showed the predicted decrease in stomach size that should accompany a change from a diet of hard-shelled molluscs to protein-rich food pellets (Piersma et al. 1993), and the birds also showed predicted hypertrophy of the pectoral muscle block in conjunction with migratory fattening (M.W. Dietz et al. in prep.; see Evans et al. 1992)
Dietz et al. (1999) conclude that ultrasound imaging provides a simple, reasonably accurate technique to measure organs of individual medium sized birds, provided that the focal organs are compact and are not hidden from ultrasonic view by air sacs and skeletal elements. In practice, this means that only pectoral muscle, stomach and perhaps, developing egg follicles, are at present open for scrutiny.
DISCUSSION
Throughout this review, we have taken as our implicit starting point the suggestion that carcass analyses always yield the best unbiased description of organ composition and stores, the problems with this technique being its invasiveness and its non-individually repeatable character (Lindström & Piersma 1993). Of course, gross morphological changes and variations in size at the organ level are relevant in the context of weight-savings to reduce metabolic costs and maximize flight performance. Morphological changes are thus relevant in studies on the ecophysiology of avian foraging (e.g. Klaassen 1998), movement and migration (e.g. Piersma & Lindström 1997); however, there is much more than size only to the functioning of an organ in an ecological context. For example, there may be changes in functionally important enzymatic concentrations (e.g. Lundgren & Kiessling 1985), and the (sub-) microscopic details may vary with time and with function (e.g. Starck 1996).
We have been struck by the fact that quite a few detailed investigations of the methodology of quantifying facets of body composition, usually carried out at great expense on the part of the avian subjects and the scientists involved, never had a follow up in an ecological application. Perhaps the funding dried up before the methodology could be applied; and it is also possible that the application of the particular methodological study had not been thought through in sufficient detail. Whatever the reason for the discrepancy between calibration and application, the detailed reviews of past methodological work (notably Brown 1996) save the general results contained in these studies for use in the design of new research projects where measurements of body compositional traits appear necessary.
In summary (Table 2), to study the contributions of fat and protein to changes in individual body mass in clear ecological contexts such as long-distance migration, the use of energy- and matter-balance studies has great potential. This is especially true in cases where the focal species shows little synchronization with respect to fattening and flight, and ‘longitudinal’ carcass studies are therefore of little use (Lindström & Piersma 1993, Van der Meer & Piersma 1994). In order to study size-changes at the level of organs, the use of nuclear magnetic resonance imaging, computer tomography and ultrasonographic imaging techniques has considerable potential. The many applications outside the fields of ornithology and ecophysiology have already led to a small growth industry, and enable the commercial production of devices that are not only ‘reasonably priced’ but that can also be taken into the field. Given the many methodological avenues and the many outstanding ecophysiological questions (Carey 1996; Klaassen 1996; Weibel et al. 1998), we expect that over the next decade ornithologists will make great strides in understanding adaptive body flexibility, that is, in understanding many of the functional aspects of body composition and organ size.
ACKNOWLEDGMENTS
We thank Maurine Dietz and Jim Gessaman for comments on a draft. TP is supported by a PIONIER-grant of the Netherlands Organization for Scientific Research (NWO). This is NIOZ-publication 3304, and publication number 2434 of the Netherlands Institute of Ecology, Centre for Limnology, Nieuwersluis.
REFERENCES
Abate, N., Burns, D., Peshock, R.M., Garg, A. & Grundy, S.M. 1994. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. Journal of Lipid Research 35: 1490-1496.
Behnke, A.R., Feen, B.G. & Welham, W.C. 1942. The specific gravity of healthy men. Journal of the American Medical Association 118: 495-501.
Beynen, A.C. & Gundlach, B.L. 1986. A non-invasive, plethysmometric method for determination of body fat in rats. Zeitschrift für Versuchstierkunde 28: 235.
Blaxter, K. L. 1989 Energy Metabolism in Animals and Man; Cambridge; Cambridge University Press: 336 pp.
Blem, C.R. 1976. Patterns of lipid storage and utilization in birds. American Zoolologist 16: 671-684.
Blem, C.R. 1990. Avian energy storage. In: Power, D.M. (ed.) Current Ornithology, Vol. 7; New York; Plenum Press: 59-113.
Bowen, W.D. & Iverson, S.J. 1998. Estimation of total body water in pinnipeds using hydrogen-isotope dilution. Physiological Zoology 71: 329-332.
Brown, M.E. 1996. Assessing body condition in birds. In: Nolan, V., Jr. & Ketterson, E. (eds.) Current Ornithology, Vol. 13; New York; Plenum Press: 67- 135.
Carey, C. (ed.) 1996. Avian Energetics and Nutritional Ecology; New York; Chapman & Hall: 543 pp.
Castro, G., Wunder, B.A. & Knopf, F.L. 1990. Total body electrical conductivity (TOBEC) to estimate total body fat of free-living birds. Condor 92: 496-499.
Child, G.I. & Marshall, S.G. 1970. A method of estimating carcass fat and fat-free weights in migrant birds from water content of specimens. Condor 72: 116-119.
Conway, C.J., Eddleman, W.R. & Simpson, K.L. 1994. Evaluation of lipid indices of the Wood Thrush. Condor 96: 783-790.
Crum, B.G., Williams, J.B. & Nagy, K.A. 1985. Can tritiated water-dilution space accurately predict total body water in Chukar Partridges? Journal of applied Physiol. 59: 1383-1388.
Diamond, J. 1993. Evolutionary physiology. In: Boyd, C.A.R. & Noble, D. (eds) The Logic of Life. The Challenge of Integrative Physiology; Oxford; Oxford University Press: 89-111.
Dietz, M.W., Dekinga, A., Piersma, T. & Verhulst, S. 1999. Estimating organ size in small migrating shorebirds with ultrasonography: an intercalibration exercise. Physiological Zoology 72: in press.
Dobush, G.R., Ankney, C.D. & Krementz, D.G. 1985. The effect of apparatus, extraction time, and solvent type on lipid extractions of Snow Geese. Canadian Journal of Zoology 63: 1917-1920.
Drent, R. H., Klaassen, M. & Zwaan, B. 1992. Predictive growth budgets in terns and gulls. Ardea 80: 5-17.
Driedzic, W.R., Crowe, K.L., Hicklin, P.W. & Sephton, D.H. 1993. Adaptations in pectoralis muscle, heart mass, and energy metabolism during premigratory fattening in Semipalmated Sandpipers (Calidris pusilla). Canadian Journal of Zoology 71: 1602-1608.
Ellis, H.I. & Jehl, J.R., Jr. 1991. Total body water and body composition in phalaropes and other birds. Physiological Zoology 64: 973-984.
EM-SCAN. 1991. EM-SCAN Model SA-2 small research animal body composition analyzer operation manual; Springfield, Ill.; EM-SCAN, Inc.
Evans, P.R., Davidson, N.C., Uttley, J.D. & Evans, R.D. 1992. Premigratory hypertrophy of flight muscles: an ultrastructural study. Ornis Scandinavica 23: 238-243.
Gessaman, J.A. 1999. Evaluation of some nonlethal methods of estimating avian body fat and lean mass. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2-16. Johannesburg: BirdLife South Africa.
Gessaman, J.A. & Nagy, K.A. 1988. Energy metabolism: Errors in gas-exchange conversion factors. Physiological Zoology 61: 507-513.
Griminger, P. 1986. Lipid metabolism. In: Sturkie, P.D. (ed.) Avian Physiology, Fourth Edition; New York; Springer-Verlag: 345-358.
Grooters, A.M., Biller, D.S., Ward, H., Miyabayashi, T. & Couto, C.G. 1994. Ultrasonographic appearance of feline alimentary lymphoma. Veterinary Radiology & Ultrasound 35: 468-472.
Gundlach, B.L., Nijkrake, H.G.M. & Hautvast, J.G.A.J. 1980. A rapid and simplified plethysmometric method for measuring body volume. Human Biology 52: 23-33.
Handrich, Y., Nicolas, L. & Le Maho, Y. 1993. Winter starvation in captive Common Barn Owls: bioenergetics during refeeding. Auk 110: 470-480.
Herring, W.O., Miller, D.C., Bertrand, J.K. & Benyshek, L.L. 1994. Evaluation of machine, technician, and interpreter effects on ultrasonic measurers of backfat and longissimus muscle area in beef Cattle. Journal of Animal Science 72: 2216-2226.
Hume, I.D. & Biebach, H. 1996. Digestive tract function in the long-distance migratory Garden Warbler, Sylvia borin. Journal of Comparative Physiology B 166:388-395.
Jehl, J.R., Jr. 1997. Cyclical changes in body composition in the annual cycle and migration of the Eared Grebe (Podiceps nigricollis). Journal of Avian Biology 28: 132-142.
Johnston, D.W. 1970 Caloric density of avian adipose tissue. Comparative Biochemistry and Physiology 34: 827-832.
Karasov, W.H. 1996. Digestive plasticity in avian energetics and feeding ecology. In: Carey, C. (ed.) Avian Energetics and Nutritional Ecology; New York; Chapman & Hall: 61-84.
Kerr, D.C., Ankney, C.D. & Millar, J.R. 1982. The effect of drying temperature on extraction of petroleum ether soluble fat of small birds and mammals. Canadian Journal of Zoology 60: 470-472.
King, J.R. & Murphy, M.E. 1985. Periods of nutritional stress in the annual cycles of endotherms: fact or fiction? American Zoologist 25: 955-964
Klaassen, M. 1996. Metabolic constraints on long-distance migration in birds. Journal of Experimental Biology 199: 57-64.
Klaassen, M. 1998. Physiological flexibility and its impact on energy metabolism and foraging behaviour in birds. In: Olff, H., Brown, V.K. & Drent, R.H. (eds.) Herbivores, Plants and Predators. Oxford; Blackwell Science.
Klaassen, M. & Biebach, H. 1994. Energetics of fattening and starvation in the long-distance migratory garden warbler, Sylvia borin, during the migratory phase. Journal of Comparative Physiology B 164: 362-371.
Klaassen, M., Kersten, M. & Ens, B.J. 1990. Energetic requirements for maintenance and premigratory body mass gain of waders wintering in Africa. Ardea 78: 209-220.
Klaassen, M., Lindström, Å. & Zijlstra, R. 1997. Composition of fuel stores and digestive limitations to fuel deposition rate in the long-distance migratory Thrush Nightingale Luscinia luscinia. Physiological Zoology 70:125-133.
Kleiber, M. 1975 The Fire of Life, An Introduction to Animal Energetics; Malabar; Robert E. Krieger Publishing Company: 453 pp.
Lambertz, H., Menzel, T. & Stellwaag, M. 1995. New miniaturized versus conventional biplane transesophageal transducers: recent clinical experience in adults. Journal of the American Society of Echocardiography 8: 527-535.
Lindgård, K., Stokkan, K.A., Le Maho, Y. & Groscolas, R. 1992. Protein utilization during starvation in fat and lean Svalbard Ptarmigan (Lagopus mutus hyperboreus). Journal of Comparative Physiology B 162: 607-613.
Lindström, Å. & Piersma, T. 1993. Mass changes in migrating birds: the evidence for fat and protein storage re-examined. Ibis 135: 70-78.
Lundgren, B.O. & Kiessling, K.-H. 1985. Seasonal variation in catabolic enzyme activities in breast muscle of some migratory birds. Oecologia 66: 468-471.
Meijer, T., Möhring, F.J. & Trillmich, F. 1994. Annual and daily variation in body mass and fat of Starlings Sturnus vulgaris. Journal of Avian Biology 25: 98-104.
Mitchell, A.D., Wang, P.C., Rosebrough, R.W., Elsasser, T.H. & Schmidt, W.F. 1991. Assessment of body composition of poultry by nuclear magnetic resonance imaging and spectroscopy. Poultry Science 70: 2494-2500.
Murton, R.K. & Westwood, N.J. 1977. Avian breeding cycles; Oxford; Clarendon Press: 594 pp.
Nagy, K.A. & Costa, D.P. 1980. Water flux in animals: analysis of potential errors in the tritiated water method. American Journal of Physiology 238: R454-R465.
Nelson, R.A., Steiger, D.L. & Beck, T.D.I. 1983. Neuroendocrine and metabolic interactions in the hibernating Black Bear. Acta Zoologica Fennici 174: 137-141.
Newton, S.F. 1993. Body condition of a small passerine bird: ultrasonic assessment and significance in overwintering survival. Journal of Zoology, London 229: 561-580.
Olsson, K.-E. & Saltin, B. 1970 Variation in total body water with muscle glycogen in Man. Acta Physiologica Scandinavica 80: 11-18.
Osa, Y., Kuramochi, T., Watanuki, Y., Naito, Y., Murano, M., Hayama, S.I., Orima, H. & Fujita, M. 1993. Application of computed tomography to morphological study of Emperor and Adelie Penguins. Auk 110: 651-653.
Owen, R. B., Jr. 1970. The bioenergetics of captive Blue-winged Teal under controlled and outdoor conditions. Condor 72: 153-163.
Pace, N. & Rathbun, E.N. 1945. Studies on body composition: III. the body water and chemically combined nitrogen content in relation to body fat. Journal of Biological Chemistry 158: 685-691.
Pennycuick, C.J. 1978. Fifteen testable predictions about bird flight. Oikos 30: 165-176.
Pennycuick, C.J. 1998. Computer simulation of fat and muscle burn in long-distance bird migration. Journal of theoretical Biology 191: 47-61.
Perkins, T.L., Green, R.D., Hamlin, K.E., Shepard, H.H. & Miller, M.F. 1992. Ultrasonic prediction of carcass merit in beef Cattle: evaluation of technician effects on ultrasonic estimates of carcass fat thickness and longissimus muscle area. Journal of Animal Science 70: 2758-2765.
Piersma, T. 1984. Estimating energy reserves of Great Crested Grebes Podiceps cristatus on the basis of body dimensions. Ardea 72: 119-126.
Piersma, T. 1998. Phenotypic flexibility during migration: optimization of organ size contingent on the risks and rewards of fueling and flight? Journal of Avian Biology 29: 511-520.
Piersma, T. & van Brederode, N.E. 1990. The estimation of fat reserves in coastal waders before their departure from northwest Africa in spring. Ardea 78: 221-236.
Piersma, T. & Lindström, Å. 1997. Rapid reversible changes in organ size as a component of adaptive behaviour. Trends in Ecology and Evolution 12: 134-138.
Piersma, T. & Gill, R.E., Jr. 1998. Guts don’t fly: small digestive organs in obese Bar-tailed Godwits. Auk 115: 196-203.
Piersma, T., Koolhaas, A. & Dekinga, A. 1993. Interactions between stomach structure and diet choice in shorebirds. Auk 110: 552-564.
Piersma, T., Bruinzeel, L., Drent, R., Kersten, M., Van der Meer, J. & Wiersma, P. 1996. Variability in basal metabolic rate of a long-distance migrant shorebird (Red Knot Calidris canutus) reflects shifts in organ sizes. Physiological Zoology 69: 191-217.
Preest, M.R. & Beuchat, C.A. 1997 Ammonia excretion by hummingbirds. Nature 386: 561-562.
Reeds, P.J. & Fuller, M.F. 1983. Nutrient intake and protein turnover. Proceedings of the Nutritional Society 42: 463-471.
Roberts, N., Cruzorive, L.M., Reid, N.M.K., Brodie, D.A., Bourne, M. & Edwards, R.H.T. 1993. Unbiased estimation of human body composition by the Cavalieri's method using magnetic resonance imaging. Journal of Microscopy, Oxford. 171: 239-253.
Roby, D.D. 1991. A comparison of two nonivasive techniques to measure total body lipid in live birds. Auk 108: 509-518.
Ross, R., Shaw, K.D., Rissanen, J., Martel, Y., Deguise, J. & Avruch, L. 1994. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. American Journal of Clinical Nutrition 59: 1277-1285.
Rye, M. 1991. Prediction of carcass composition in Atlantic Salmon by computerized tomography. Aquaculture 99: 35-48.
Scholz, A., Baulain, U. & Kallweit, E. 1993. Multivariate statistical analysis of images from magnetic-resonance-tomography on living Pigs. Züchtungskunde 65: 206-215.
Scollan, N.D., Caston, L.J., Liu, Z. Zubair, A.K., Leeson, S. & McBride, B.W. 1998. Nuclear magnetic resonance imaging as a tool to estimate the mass of the pectoralis muscle of chickens in vivo. British Poultry Science 39: 221-224.
Scott, I., Grant, M. & Evans, P.R. 1991. Estimation of fat-free mass of live birds: Use of total body electrical conductivity (TOBEC) measurements in studies of single species in the field. Functional Ecology 5: 314-320.
Sears, J. 1988. Assesssment of body condition in live birds; measurements of protein and fat in Mute Swan, Cygnus olor. Journal of Zoology, London 216: 295-308.
Skagen, S.K., Knopf, F.L. & Cade, B.S. 1993. Estimation of lipids and lean mass of migrating sandpipers. Condor 95: 944-956.
Sohlström, A., Wahlund, L.O. & Forsum, E. 1993. Adipose tissue distribution as assessed by magnetic resonance imaging and total body fat by magnetic resonance imaging, underwater weighing, and body-water dilution in healthy women. American Journal of Clinical Nutrition 58: 830-838.
Sørensen, M.T. 1992. In vivo prediction of Goat body composition by computer tomography. Animal Production 54: 67-73.
Speakman, J.R. 1997. Doubly labelled water. Theory and practice; London; Chapman & Hall: 399 pp.
Starck, J.M. 1996. Phenotypic plasticity, cellular dynamics, and epithelial turnover of the intestine of Japanese quail (Coturnix coturnix japonica). Journal of Zoology, London 238: 53-79.
Svihus, B. & Katle, J. 1993. Computerized tomography as a tool to predict composition traits in broilers - comparisons of results across samples and years. Acta Agricultura Scandinavica, Section A-Animal Science 43: 214-218.
Van der Meer, J. & Piersma, T. 1994. Physiologically inspired regression models for estimating and predicting nutrient stores and their composition in birds. Physiological Zoology 67: 305-329.
Walsberg, G.E. 1988. Evaluation of a nondestructive method for determining fat stores in small birds and mammals. Physiological Zoology 61: 153-159.
Walsberg, G.E. & Wolf, B.O. 1995. Variation in the respiratory quotient of birds and implications for indirect calorimetry using measurements of carbon dioxide production. Journal of Experimental Biology 198: 213-219.
Weibel, E.R., Taylor, C.R. & Bolis, L. (eds) 1998. Principles of Animal Design. The Optimization and Symmorphosis Debate; Cambridge; Cambridge University Press: 314 pp.
Wishart, R.A. 1979. Indices of structural size and condition of American wigeon (Anas americana). Canadian Journal of Zoology 57: 2369-2374.
Table 1. Respiratory quotient (RQ, according to Walsberg & Wolf 1995), energy gain (kJ/g dry, according to Gessaman & Nagy 1988 and Kleiber 1975 and kJ/g fresh according to Johnston 1970, Olsson & Saltin 1970 and Blaxter 1989) for a uricotelic animal catabolising or anabolising lipids, glycogen and protein.
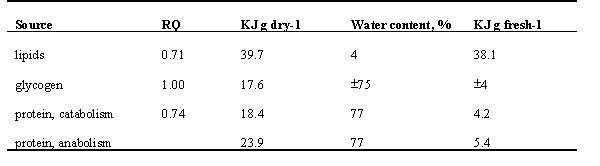
Table 2. Summary of the available methods to determine (changes in) the size of (parts of) the active metabolic machinery of birds.
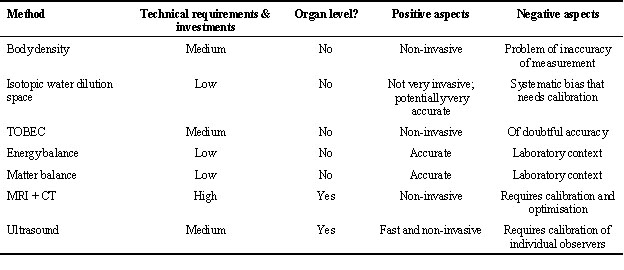
Fig. 1. Example of an experimental set up that can be used for the compilation of energy and material balances. The set up shown allows for a continuous registration of the rate of energy expenditure (via analysis of oxygen consumption and/or carbon dioxide production), body mass, feeding activity and locomotor activity. Furthermore interval estimates of food intake rate and faeces production can be made. (From Klaassen & Biebach 1994.)
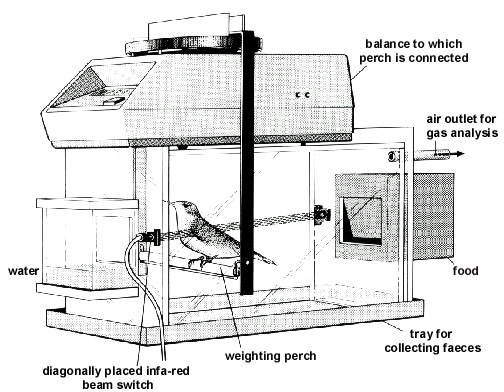
Fig. 2. (A) Protein balance, (B) tissue energy balance from food balance trials (i.e. daily metabolisable energy intake minus daily energy expenditure), and (C) tissue energy balance for starving thrush nightingales as a function of daily body mass change. Straight line denotes reduced major axes, forced through the origin, from which tissue composition was calculated. (From Klaassen et al. 1997.)
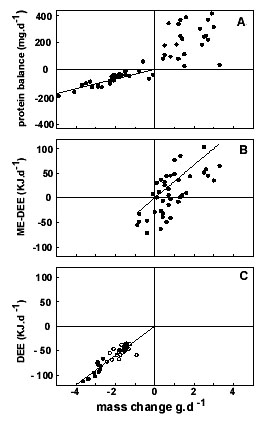
Fig. 3. Results of a calibration study to estimate sizes of different organs of Bewick’s Swans using non-invasive nuclear magnetic resonance imaging (MRI). A volume index measured by MRI is plotted against volume measurements using water displacement after carcass dissection. Regression lines were forced through the origin (M. Klaassen, K. Nicolaij & M. Vogel unpubl.). In the background a sagittal view through a Bewick’s Swan laying on its back is shown. On the left and the right primary wing feathers are visible. The sternum (white) points upwards and is flanked by the breast muscles (grey). At the centre, the heart is visible (black).
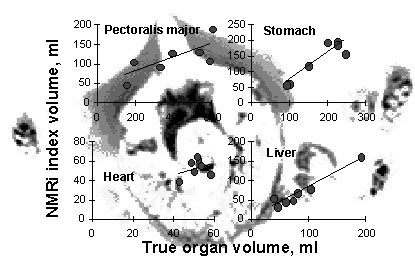
Fig. 4. Relationships between true fresh organ masses (y-axis, determined by dissection) and the appropriate ultrasonographic scanning values (x-axis) in Red Knots (after Dietz et al. 1999). The positioning of the ultrasound probes to make measurements of pectoral muscle and stomach sizes are indicated by the small drawings.